Abstract
BACKGROUND:
There is substantial evidence that adult stem cell populations exist in human endometrium, and hence it is suggested that either endogenous endometrial stem/progenitor cells can be activated or bone marrow derived stem cells can be transplanted in the uterine cavity for endometrial regeneration in Asherman's syndrome (AS).
AIMS AND OBJECTIVES:
The objective was to evaluate the role of sub-endometrial autologous stem cell implantation in women with refractory AS in attaining menstruation and fertility.
SETTING:
Tertiary care referral center.
DESIGN:
Prospective case series.
MATERIALS AND METHODS:
Six cases of refractory AS with failed standard treatment option of hysteroscopic adhesiolysis in the past were included. Mononuclear stem cells (MNCs) were implanted in sub-endometrial zone followed by exogenous oral estrogen therapy. Endometrial thickness (ET) was assessed at 3, 6, and 9 months.
RESULTS:
Descriptive statistics and statistical analysis of study variables was carried out using STATA version 9.0. The mean MNC count was 103.3 × 106 (±20.45) with mean CD34+ count being 203,642 (±269,274). Mean of ET (mm) at 3 months (4.05 ± 1.40), 6 months (5.46 ± 1.36) and 9 months (5.48 ± 1.14) were significantly (P < 0.05) increased from pretreatment level (1.38 ± 0.39). Five out of six patients resumed menstruation.
CONCLUSION:
The autologous stem cell implantation leads to endometrial regeneration reflected by restoration of menstruation in five out of six cases. Autologous stem cell implantation is a promising novel cell based therapy for refractory AS.
KEY WORDS: Asherman's syndrome, autologous stem cells, bone marrow, endometrial regeneration
INTRODUCTION
Asherman's syndrome (AS) is the partial or complete obliteration of the uterine cavity and or cervical canal with synechiae, resulting in menstrual abnormalities hypomenorrhea, amenorrhea, infertility and recurrent pregnancy loss, depending on the severity. The prevalence of AS varies according to the population studied and the investigations used for its detection.[1] It's prevalence varies from 2% to 22% in infertile women.[2,3] The main etiology includes trauma to gravid (postpartum curettage - 21.5-40%)[4,5,6] and nongravid uterine cavity (diagnostic curettage - 1.6%, abdominal myomectomy - 1.3%, intrauterine contraceptive device [IUCD] insertion - 0.2%),[1] miscarriages (5-39%),[7,8,9,10,11] infections particularly genital tuberculosis (TB), schistosomiasis and genetic predisposition. Endometrial regeneration from the basal layer, postulated to have stem cells or progenitor cells, contributes to the replacement of the functional layer. The basal layer concomitant with the stem cells/progenitor cells of the endometrium, are often removed or destroyed during these conditions. The endometrial stroma is largely replaced by fibrous tissue and the functional layer is replaced by an epithelial monolayer which is nonresponsive to hormonal stimulation and fibrous synechiae forms across the cavity.[12] Adhesions involve different layers of endometrium, myometrium or connective tissue.
Treatment of choice of AS is hysteroscopic adhesiolysis followed by prevention of recurrence of adhesions after surgery. This can be done by IUCD insertion, Foley's balloon catheter, hyaluronic acid application or estrogen therapy, with varying results.[13,14,15] It has been reported that the recurrence rate for intrauterine adhesions (IUAs) ranges from 3.1% to 23.5%[13,14,15] among all cases of IUAs and 20-62.5%[13,14,15,16] in those with severe adhesions.
Patients with recurrence of adhesions or those not responding to the available treatment options are at a disadvantage and have to go for surrogacy or adoption.
Stem cells have long been used successfully to treat several hematopoietic disorders and malignancies such as multiple myeloma, leukemia, and lymphomas.[17]
There is ample evidence that adult stem cell populations exist in human endometrium, and hence it might be possible to activate endogenous endometrial stem/progenitor cells can in cases of atrophic or thin endometrium or to transplant bone marrow (BM) derived stem cells in the uterine cavity for endometrial regeneration in AS or severe cases of IUAs.[18] Low levels of circulating BM derived hemopoietic stem cells, mesenchymal stem cell and endothelial progenitor cells integrate into damaged tissues and trans-differentiate into host tissues, including endometrium.[19,20] Studies have shown that these cells incorporate into the endometrium in low numbers and trans-differentiate into endometrial epithelial, stromal and endothelial cells.[17,21,22,23]
Implanting autologous hemopoietic adult stem cells into the sub-endometrial zone (junction between myometrium and endometrium) with the opinion that implanted stem cells may trans-differentiate into the resident endometrial stem cells and result in endometrial regeneration, is a novel but promising approach.
The objective of this study was to evaluate the role of sub-endometrial autologous stem cell implantation in regeneration of the endometrium and attaining menstruation. This treatment would provide a new ray of hope for women with AS.
MATERIALS AND METHODS
The study was conducted for a period of 1 year.
The study was carried out on women of reproductive age between 25 and 35 years with primary or secondary infertility due to AS Grade III or IV (due to any cause), diagnosed hysteroscopically with hypomenorrhea/amenorrhea and in whom standard treatment option of hysteroscopic adhesiolysis followed by estrogen stimulation had failed.
Women with mild degree of IUA, active genital TB, chronic debilitating or hematopoietic diseases affecting the BM and those not willing to participate were excluded from the study.
An informed written consent was obtained from each participant after counseling and explaining the procedure in detail.
These women were a highly selective group, as only after they had failed hysteroscopic adhesiolysis and oral estrogen therapy in the past, were included in the study, hence acted as internal control group.
After history taking and examination to rule out other co-morbidities, a fresh sex hormone profile was done to rule out other causes of amenorrhea before stem cell implantation. This was followed by BM aspiration under local anesthesia in hematology day care center.
Under strict aseptic precaution, BM (30 ml) was aspirated from iliac crest using disposable BM aspiration needle (Jamshidi, 11 G) and collected in heparinized syringes. Samples were immediately transported to stem cell facility, All India Institute of Medical Sciences in sterile plastic tubes for adult stem cell harvesting.
Preparation of hematopoietic stem cell
The isolation of mononuclear cells (MNCs) was done by Ficoll density separation method. BM was diluted in 1:3 ratio with ×1 phosphate buffered saline (PBS) and layered over lymphocyte separation medium and centrifuged at a speed of 800 G for 25 min.[24] MNCs (buffy coat) was aspirated with 10 ml disposable pipette and washed thrice in heparinized normal saline (NS)/PBS to remove the traces of Ficoll.[25] All the procedures were performed in the stem cell laboratory. Finally, MNCs were suspended in 3 ml heparinized NS.
The harvested MNC were evaluated for:
Viability: Trypan blue dye exclusion test was done to know the percentage of live cells
Cell morphology: MNC were stained with Giemsa stain and observed under microscope
CD34+ counts: MNC were tagged with CD34 antibodies and assessed by flow cytometry to evaluate the hematopoietic stem cells[26,27]
Total cell count: Cell numbers were assessed by counting the cell in the Neubauer chamber under microscope.
Same day the patient was taken up for stem cell implantation.
Stem cell implantation
The procedure was conducted in opposing tooth under intravenous (IV) sedation and antibiotic cover (single dose of 1 g cefazolin IV).
Patient was laid in lithotomy position. A transvaginal probe was covered with sterile disposable probe cover and guide attached to it. After locating the sub-endometrial zone on ultrasound (Wipro GE Voluson) ovum pick up needle (Cook No. 17) was introduced vaginally via the lateral fornix and stem cells were implanted in the sub-endometrial zone transmyometrial. A volume of 3 ml of MNC were delivered at 2-3 sites (fundus, anterior and posterior part) of the myometrium.
Patient was discharged after 2 h and antibiotic were given, tablet taxim-O (cefexime, alkem pharma) 200 mg twice a day, for 5 days.
Postprocedure, patients were started on oral estradiol valerate (Progynova, Schering manufacturer), tablets 6 mg/day in three divided doses, the same day for 12 weeks and medroxy progesterone was given in the last 10 days.
Subjects were followed-up at 3, 6, and 9 months interval and were asked to maintain a menstrual calendar. At the follow-up, initiation and changes in menstrual blood flow and their endometrial thickness (ET) between 14 and 18 day by transvaginal ultrasound (TVS) was noted.
Women who were previously amenorrhoeic started having cyclical bleeding after the procedure, this was taken as a sign of endometrial regeneration and was further confirmed by TVS.
Patients who started menstruation were shifted to cyclical oral estrogen (estradiol valerate) 2 mg thrice daily for 21 days (day 1 to day 26) and oral progesterone (Medroxyprogesterone, Serum Institute of India Ltd., India) 10 mg once daily from (day 16 to day 25).
Ethical clearance
The study was approved by the Institutional Committee for Stem Cell Research and Therapy and Human Ethics Committee of the concerned institute. The procedure used in the study was in accordance with guidelines of the Helsinki Declaration of 2000 on human experimentation.
The trial was registered with CTRI - CTRI/2013/08/003896.
Statistical analysis
Though the sample size of this study was small; descriptive statistics and statistical analysis of study variables was carried out using STATA version 9.0.
Changes in ET values due to treatment were compared using paired t-test to see the significant increase in ET values at 3, 6 and 9 months. Bi-variate correlation coefficient between MNC count and CD34+ cell was computed. For all the statistical tests P < 0.05 was considered for statistical significance.
RESULTS
A total of six women were included in the study and all of them had secondary amenorrhea with primary infertility. All of them had undergone hysteroscopic adhesiolysis (2-3 times) and received oral estrogens (2-3 cycles) in the past, but had failed to respond.
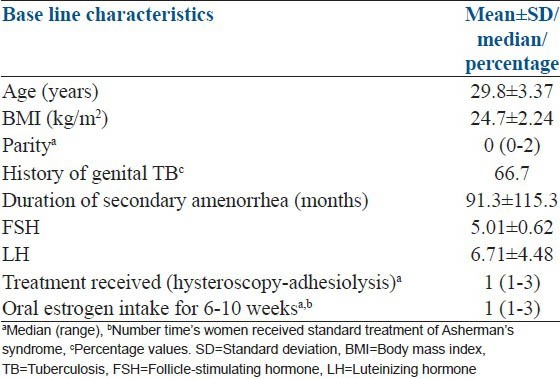
The mean age (years) of the study subjects were 29.8 ± 3.37 and all were in the reproductive age group with a mean body mass index (kg/m2) of 24.4 ± 2.24. The mean duration of amenorrhea was of 91.3 ± 115.35 months. Five out of six patients had a past history of genital TB (treated) and one had a history of D and C prior to the onset of amenorrhea [Table 1].
Table 1.
Base line characteristics of the study subjects (n=6)
The mean (±standard deviation [SD]) of MNC counts was 103.3 × 106 (±20.45) and ranged from 76 × 106 to 128 × 106. Similarly the mean (±SD) of CD34+ cells among the MNC's was 203,642 (±269,274) and ranged between 10,640 and 640,090 with a median value 58,800. The average percentage of CD4 positive cells in the MNCs was 0.197% [Table 2].
Table 2.
Correlation of ET with CD34 count
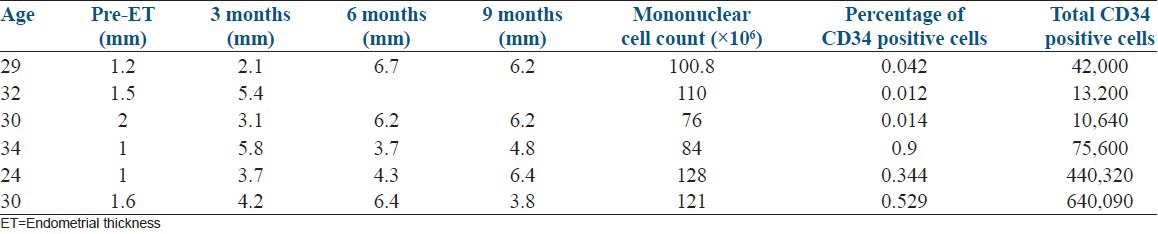
The mean preimplantation ET (mm) was 1.38 ± 0.39 and there was a statistically significant increase (t = 4.28, P = 0.008) in the growth of the endometrium to 4.05 ± 1.40 mm at 3 months [Figures 1-3]. Although the maximum ET attained by any patient was 6.7 mm, the growth of endometrium was found to be static during 6 months (5.46 ± 1.36; t = 8.15; P = 0.001) and 9 months (5.48 ± 1.14; t = 7.40; P = 0.002) follow-up [Table 2].
Figure 1.
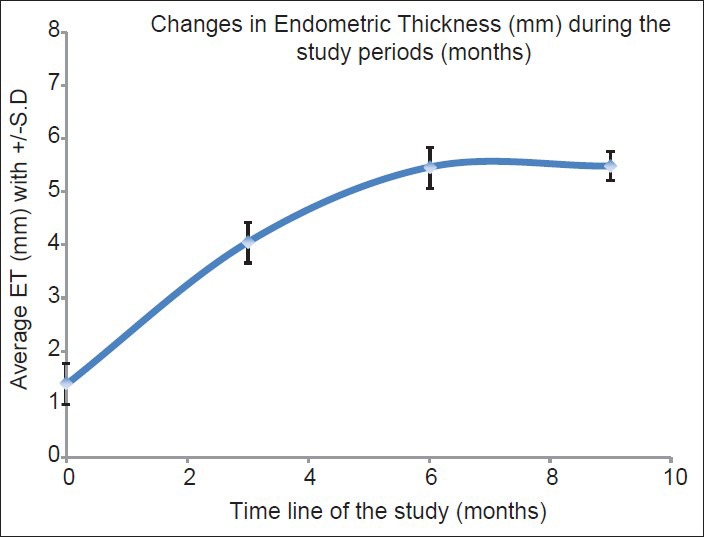
Endometrial growth over 9 months follow-up
Figure 3.
Posttransplant endometrial thickness of the same patient
Figure 2.
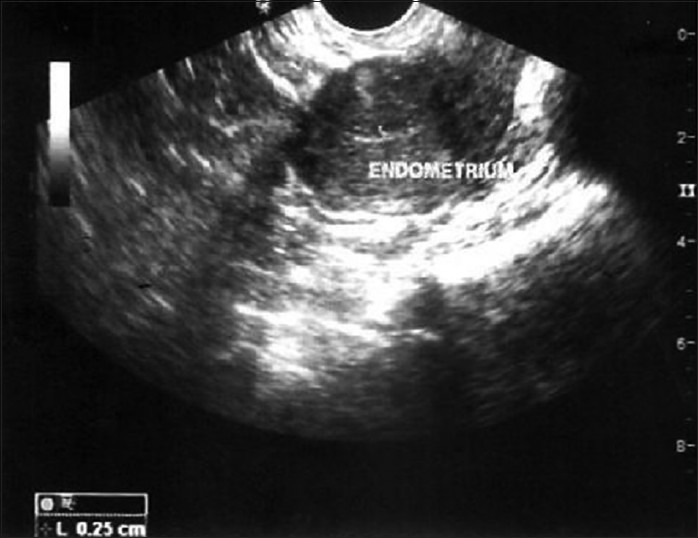
Pretransplant endometrial thickness of one patient
The correlation between the number of MNC count or CD34 positive cells with the increase in the ET was not significant (P > 0.05) [Table 2].
Five women started menstruating at 3 months posttransplant and continued having cyclical bleeding until 9 months follow-up. One woman who had resumed menses, lost to follow-up after 3 months. Three women had a loss of appetite, mild symptoms of gastritis, abdominal cramps, vomiting at the beginning of the estrogen intake, which resolved subsequently.
DISCUSSION
In this pilot study, six women with severe AS with secondary amenorrhea and infertility who had failed to respond to the standard treatment of hysteroscopic adhesiolysis followed by estrogen therapy were recruited as study subjects. The only case report published so far on this novel therapy is by Nagori et al.[28] Based on this case report and various reports supporting the presence of resident endometrial stem or progenitor cells in the endometrium, which can be activated and the possibility of transplanting BM derived stem cells into the uterine cavity for regeneration, prompted us to perform this study.
In our study, MNCs (3 ml) containing CD34+ cells were implanted in the sub-endometrial zone rather than the uterine cavity. Also it was not preceded by any curettage. Five out of six patients resumed menstruation after 3 months. Though the mean ET postimplantation had significantly increased and the cases were desirous of conception, it was not enough to begin IVF treatment. The maximum ET achieved by any patient was 6.7 mm. Majority of the patients had a past history of treated genital tuberculosis (n = 5) and only one had a history of D and C. The patient selected in their case by Nagori et al. had primary infertility with hypomenorrhea possibly due to previous curettage and premature ovarian failure, indicating that she was not a classical case of infertility due to AS.[28] This is the 1st time where adult stem transplant has been tried in patients of Asherman's due to genital TB. We do not know whether the inadequate growth of endometrium seen on our study is due to past endometrial TB or whether the results would have been better in patients with traumatic AS. Though, the only patient with history of D and C in the past did not show any difference in the results.
The regeneration seen in our patients could be due to the incorporated cells trans-differentiating into endometrial epithelium and stroma or the BM cells activating the remaining resident endometrial progenitor cells by providing growth factors, to proliferate and replace the lost cells. Further, exogenous oral estrogen given might have promoted the proliferation of a repaired receptive endometrium.
We chose CD34+ marker as they are the endothelial progenitor cells that promotes angiogenesis and tissue repair. Studies in the past have contemplated the mechanism of action of transplanted BM MNCs, CD34+ as being the secretion of certain bioactive molecules that promote angiogenesis and tissue repair, inhibit scarring, modulate inflammatory and immune reactions and activate specific progenitor cells.[29,30] Though CD34+ cells were quantified, all the MNCs were transplanted and hence which cell type got implanted cannot be commented on.
Taylor detected donor derived endometrial cells in the endometrial biopsy samples of the BM recipients and concluded that BM is the most likely candidate as a potential source of endometrial epithelial and stromal cells.[17] Furthermore, Blau et al. in 2001 found that BM stem cells circulate, albeit in low numbers and populate various organs including uterine endometrium.[31]
The potential role of endometrial injury in provoking endometrial regeneration and its favorable effect on implantation has been studied by various investigators.[32,33] Furthermore, BM derived stem cells participate in endometrial regeneration in the setting of cellular turnover and inflammatory stimuli.[17] The fact that we did not precede stem cell implantation with endometrial injury might have resulted in not so encouraging results, as far as fertility is concerned. Nagori et al. combined curettage with intrauterine BM transplant and ultrasound monitoring, which resulted in a successful IVF pregnancy. However, this innovative therapy holds promise for many women who have completed their families and suffer from secondary amenorrhea due to Asherman's/IUA. The limitation of our study was the small sample size. This was a pilot study and we plan to do a larger study where more number of patients will be recruited and specific marker positive stem cells will be separated and transplanted, which will enlighten us further. A well-designed large trial is the need of the hour to substantiate the role of this novel therapy in helping these patients resume menses and more importantly conceive.
CONCLUSION
Autologous stem cell implantation is an innovative therapy for endometrial regeneration in patients with AS. The fact that women resumed menstruation after implantation in our study holds promise for future fertility in patients with infertility due to this dreaded condition.
Footnotes
Source of Support: Research grant from All India Institute of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Schenker JG, Margalioth EJ. Intrauterine adhesions: An updated appraisal. Fertil Steril. 1982;37:593–610. doi: 10.1016/s0015-0282(16)46268-0. [DOI] [PubMed] [Google Scholar]
- 2.Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome – one century later. Fertil Steril. 2008;89:759–79. doi: 10.1016/j.fertnstert.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 3.Panayiotides I, Weyers S, Bosteels J, Van Herendae B. Intrauterine adhesion (IUA): Has there been progress in understanding and treatment over last 20 years? Gynecol Surg. 2009;6:197–211. [Google Scholar]
- 4.Westendorp IC, Ankum WM, Mol BW, Vonk J. Prevalence of Asherman's syndrome after secondary removal of placental remnants or a repeat curettage for incomplete abortion. Hum Reprod. 1998;13:3347–50. doi: 10.1093/humrep/13.12.3347. [DOI] [PubMed] [Google Scholar]
- 5.Rochet Y, Dargent D, Bremond A. The obetetrical outcome of women with surgically treated uterine synechiae. J Gynecol Obstet Biol Reprod. 1979;8:723–6. [PubMed] [Google Scholar]
- 6.Parent B, Barbot J, Dubuisson J. Uterine synechiae. Encycl Med Chir Gynecol. 1988;140A(Suppl):10–2. [Google Scholar]
- 7.Dmowski WP, Greenblatt RB. Asherman's syndrome and risk of placenta accreta. Obstet Gynecol. 1969;34:288–99. [PubMed] [Google Scholar]
- 8.Kodaman PH, Arici A. Intra-uterine adhesions and fertility outcome: How to optimize success? Curr Opin Obstet Gynecol. 2007;19:207–14. doi: 10.1097/GCO.0b013e32814a6473. [DOI] [PubMed] [Google Scholar]
- 9.Rabau E, David A. Intrauterine adhesions: Etiology, prevention, and treatment. Obstet Gynecol. 1963;22:626–9. [PubMed] [Google Scholar]
- 10.Toaff R, Ballas S. Traumatic hypomenorrhea-amenorrhea (Asherman's syndrome) Fertil Steril. 1978;30:379–87. doi: 10.1016/s0015-0282(16)43568-5. [DOI] [PubMed] [Google Scholar]
- 11.Ventolini G, Zhang M, Gruber J. Hysteroscopy in the evaluation of patients with recurrent pregnancy loss: A cohort study in a primary care population. Surg Endosc. 2004;18:1782–4. doi: 10.1007/s00464-003-8258-y. [DOI] [PubMed] [Google Scholar]
- 12.Foix A, Bruno RO, Davison T, Lema B. The pathology of postcurettage intrauterine adhesions. Am J Obstet Gynecol. 1966;96:1027–33. doi: 10.1016/0002-9378(66)90452-2. [DOI] [PubMed] [Google Scholar]
- 13.Valle RF, Sciarra JJ. Intrauterine adhesions: Hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol. 1988;158:1459–70. doi: 10.1016/0002-9378(88)90382-1. [DOI] [PubMed] [Google Scholar]
- 14.Pabuçcu R, Atay V, Orhon E, Urman B, Ergün A. Hysteroscopic treatment of intrauterine adhesions is safe and effective in the restoration of normal menstruation and fertility. Fertil Steril. 1997;68:1141–3. doi: 10.1016/s0015-0282(97)00375-0. [DOI] [PubMed] [Google Scholar]
- 15.Preutthipan S, Linasmita V. Reproductive outcome following hysteroscopic lysis of intrauterine adhesions: A result of 65 cases at Ramathibodi Hospital. J Med Assoc Thai. 2000;83:42–6. [PubMed] [Google Scholar]
- 16.Capella-Allouc S, Morsad F, Rongières-Bertrand C, Taylor S, Fernandez H. Hysteroscopic treatment of severe Asherman's syndrome and subsequent fertility. Hum Reprod. 1999;14:1230–3. doi: 10.1093/humrep/14.5.1230. [DOI] [PubMed] [Google Scholar]
- 17.Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–5. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 18.Gargett CE, Ye L. Endometrial reconstruction from stem cells. Fertil Steril. 2012;98:11–20. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Kørbling M, Estrov Z. Adult stem cells for tissue repair-a new therapeutic concept? N Engl J Med. 2003;349:570–82. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 20.Du H, Taylor HS. Stem cells and reproduction. Curr Opin Obstet Gynecol. 2010;22:235–41. doi: 10.1097/GCO.0b013e328338c152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikoma T, Kyo S, Maida Y, Ozaki S, Takakura M, Nakao S, et al. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. (e1-8).Am J Obstet Gynecol. 2009;201:608. doi: 10.1016/j.ajog.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Mints M, Jansson M, Sadeghi B, Westgren M, Uzunel M, Hassan M, et al. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum Reprod. 2008;23:139–43. doi: 10.1093/humrep/dem342. [DOI] [PubMed] [Google Scholar]
- 23.Cervelló I, Gil-Sanchis C, Mas A, Faus A, Sanz J, Moscardó F, et al. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS One. 2012;7:e30260. doi: 10.1371/journal.pone.0030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neagu M, Sicui E, Ordodi V, Paunescu V. Human mesenchymal stem cell as basic tools for tissue engineering: Isolation and culture. Rom J Biophys. 2012;15:29–34. [Google Scholar]
- 25.Gilmore MJ, Prentice HG, Blacklock HA, Janossy G, Hoffbrand AV. A technique for rapid isolation of bone marrow mononuclear cells using Ficoll-Metrizoate and the IBM 2991 blood cell processor. Br J Haematol. 1982;50:619–26. doi: 10.1111/j.1365-2141.1982.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 26.Gajkowska A, Oldak T, Jastrzewska M, Machaj EK, Walewski J, Kraszewska E, et al. Flow cytometric enumeration of CD34 + hematopoietic stem and progenitor cells in leukapheresis product and bone marrow for clinical transplantation: A comparison of three methods. Folia Histochem Cytobiol. 2006;44:53–60. [PubMed] [Google Scholar]
- 27.Mackie AR, Losordo DW. CD34-positive stem cells: In the treatment of heart and vascular disease in human beings. Tex Heart Inst J. 2011;38:474–85. [PMC free article] [PubMed] [Google Scholar]
- 28.Nagori B, Panchal S, Patel H. Endometrial regeneration using autologous adult stem cell followed by conception by in vitro fertilization in a patients of severe Asherman's syndrome. J Hum Reprod Sci. 2011;4:43–8. doi: 10.4103/0974-1208.82360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): Controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–46. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caplan AI. Why are MSCs therapeutic? New data: New insight. J Pathol. 2009;217:318–24. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: Entity or function? Cell. 2001;105:829–41. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 32.Barash A, Dekel N, Fieldust S, Segal I, Schechtman E, Granot I. Local injury to the endometrium doubles the incidence of successful pregnancies in patients undergoing in vitro fertilization. Fertil Steril. 2003;79:1317–22. doi: 10.1016/s0015-0282(03)00345-5. [DOI] [PubMed] [Google Scholar]
- 33.Li R, Hao G. Local injury to the endometrium: Its effect on implantation. Curr Opin Obstet Gynecol. 2009;21:236–9. doi: 10.1097/GCO.0b013e32832a0654. [DOI] [PubMed] [Google Scholar]


