Abstract
AIMS:
The current study was to assess the protective role of resveratrol in cypermethrin-induced reproductive toxicity in male Wistar rats.
MATERIALS AND METHODS:
Rats were exposed to cypermethrin (3.83 mg/kg bw) for 14 days. Pre- and post-treatment of resveratrol (20 mg/kg bw for 14 days) was given to cypermethrin exposed rats. At the end of the experiment, rats were sacrificed, testis and epididymis were removed, sperm characteristics, sex hormones, and various biochemical parameters were studied.
RESULTS:
Cypermethrin exposure resulted in a significant decrease in weight of testis and epididymis, testicular sperm head counts, sperm motility and live sperm counts and increase in sperm abnormalities. Serum testosterone (T), follicle stimulating hormone (FSH), luteinizing hormone (LH), reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), glutathione S-transferase (GST), glutathione reductase (GR), glutathione peroxidase (GPx) and total protein (TP) content were decreased and lipid peroxidation (LPO) level was increased on cypermethrin exposure. Pre- and post-treatment of resveratrol increased sperm head counts, sperm motility, live sperm counts, T, FSH, LH, GSH, CAT, SOD, GST, GR, GPx and TP contents and decreased LPO. Treatment with resveratrol alone has improved sperm parameters and testicular antioxidant defence system.
CONCLUSION:
The study concluded that resveratrol ameliorated cypermethrin-induced testicular damage by reducing oxidative stress and by enhancing the level of sex hormones.
KEY WORDS: Cypermethrin, oxidative stress, resveratrol, sex hormones, sperm parameters
INTRODUCTION
Synthetic pyrethroids are one of the most commonly used insecticides. In recent years, the use of these insecticides have increased over organochlorines, organophosphates and carbamates because of their high effectiveness against a wide range of insects, rapid biodegradation, low mammalian toxicity and target oriented mechanism of action. Cypermethrin is common synthetic pyrethroid used in agriculture, forestry as well as in public and animal health programmes. Although considered nontoxic to mammals, recent studies have shown the adverse effect of cypermethrin on the nervous system,[1] hepatic and renal system[2] and male reproductive system[3,4,5] in laboratory animals. The reports of reproductive toxicity of cypermethrin are a major concern because human spermatogenesis may be vulnerable to chronic exposure to chemicals at very low exposure. Hence, this study was designed to determine the reproductive toxicity associated with cypermethrin in male Wistar rats.
The testicular tissue contains an elaborate array of antioxidant enzymes and free radical scavenger to ensure that the spermatogenic and steroidogenic functions are not disturbed by chronic exposure of xenobiotics. These antioxidant defence systems are of major importance because peroxidative damage is regarded as the most important cause of impaired testicular function. Although testes are having plenty of endogenous antioxidants for scavenging of free radicals generated, chronic exposure of xenobiotics such as cypermethrin may cause excessive lipid peroxidation (LPO) and oxidative injury. Hence, there is a need for exogenous antioxidants to decrease oxidative stress in testes as well as to positively regulate the spermatogenic cycle and steroidogenic function.
The use of natural plant products is increasing day by day to address human health issues. Phytochemicals have good medicinal properties and are used to cure so many diseases without producing side-effects. Resveratrol (3,5,4’-trihydroxy-trans-stilbene) is a naturally occurring dietary polyphenolic compound in red wine. It is quite abundant in grape seed extract and in grape juice and grape skin. Resveratrol has been reported to have several biologic effects such as a potent antioxidative effect via prevention of LPO[6,7] antiplatelet activity,[8] an antiinflammatory activity.[9] Resveratrol triggers a variety of established cellular and molecular effectors, the most remarkable of which is the estrogen response systems.[10,11] Resveratrol modulates the estrogen-response systems and may therefore be involved in male reproduction. This study was planned to evaluate the possible role of resveratrol in cypermethrin induced reproductive toxicity in male Wistar rats.
MATERIALS AND METHODS
Chemicals
Technical grade cypermethrin or alpha-cypermethrin (97%) was obtained from Garda Chemicals, Mumbai, India. Resveratrol was purchased from the Sigma Aldrich (USA). All other chemicals were of analytical grade.
Animals
Male Wistar rats weighing 250-300 g were used in this study. The animals were maintained in controlled temperature (22 ± 2°C) with a 12 h light-dark cycle and given food and water ad libitum.
Experimental schedule
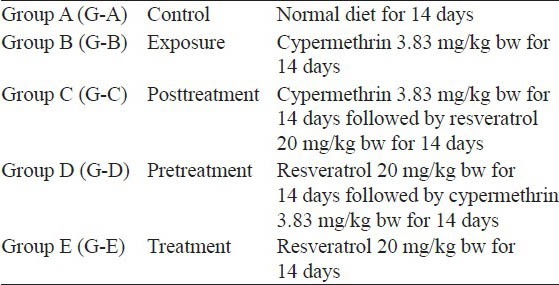
Thirty rats were divided into five groups of six each as per following experimental schedule [Table 1]. α-cypermethrin and resveratrol were dissolved in dimethylsulfoxide (DMSO) and administered orally by gavaging. The maximum volume of DMSO given to each rat was 0.5 ml. The animal experiments were carried out as per approval of Institutional Animal Ethical Committee (IAEC) vide proposal reference no (BU/Pharma/IAEC/10/028).
Table 1.
Experimental schedule
At the end of the experiment rats were sacrificed by ether inhalation, testes and epididymis were removed and weighted. One testis was used for sperm head counts and other testis was used for estimation of LPO, enzymatic and nonenzymatic antioxidants and total protein (TP) content. Epididymis was used for sperm motility and sperm morphology study. Blood samples were taken from the heart and serum was separated for estimation of reproductive hormones.
Sperm parameters
Sperm head counts were performed using hemocytometer as described by Choi[12] with necessary modifications. The testis was removed and weighted. Tunica albuginea (outer covering) was removed and the testis was minced and homogenized in 0.9% NaCl and 0.05% triton X solution for 2 min at highest speed. On an average, 10-15 μl of homogenate was placed on hemocytometer. After 5 min sperm heads were counted in red blood cell chamber at × 40 magnification.
A segment from the distal end of epididymis was removed and minced in 2 ml of Dulbecco's phosphate buffer saline maintained at 36-38°C. Minced cauda was placed in a water bath to disperse the sperm for 1-5 min and gently mixed using pasture pipette. Mixture (5-10 μl) was loaded in hemocytometer chamber and the number of nonmotile sperms was counted in white blood cell chamber. Hemocytometer was kept at 40 50°C for 1 min (to kill the sperms) and then total number of dead sperms were counted.[13] Other segment of epididymis was minced in 1 ml of 0.9% saline mixed with 1 ml of 10% neutral buffer saline. The suspension was diluted with water to suitable volume for performing the assay. 1-2 ml of eosin (1%) was added to 20 ml of above suspension and incubated at room temperature for 1 h. One drop of suspension was taken on the slide and a smear was prepared. The slide was viewed under light microscope at ×40 magnification for evaluation of head and tail abnormalities. A total of 200 sperms were examined on each slide and head and tail abnormalities were expressed as a percentage.[14]
Hormone assay
Serum testosterone (T), luteinizing hormones (LH) and follicle stimulating hormone (FSH) were assayed by rat specific ELISA Kits (Qayee-Bio Life Science, Shanghai, China), based on the principle of competitive binding and according to the manufacturer's instructions.
Biochemical estimations
Preparation of homogenate
Testis was homogenized in 10% (w/v) ice cold buffer (0.1 M phosphate buffer, pH 7.4 + 150 mM KCl). A part of homogenate was used for LPO and reduced glutathione (GSH) estimations and the other part was centrifuged at 9000 rpm for 20 min. The supernatant (S) so obtained was used for superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GST), glutathione peroxidase (GPx), glutathione reductase (GR) and TP estimations.
Lipid peroxidation
Lipid peroxidation was estimated as described by Ohkawa et al.[15] One milliliter homogenate was incubated at 37°C for 10 min. One milliliter of 10% (w/v) chilled trichloroacetic acid (TCA) was added to it and centrifuged at 2500 rpm for 15 min at room temperature. One milliliter ml of 0.67% thiobarbituric acid (TBA) was added to 1 ml of supernatant and kept in boiling water bath for 10-15 min. The tubes were cooled under tap water, followed by the addition of 1 ml of distilled water. Absorbance was recorded at 530 nm and the results were expressed as nmoles MDA/h/g tissue.
Reduced glutathione
Reduced GSH was estimated by the method of Ellman.[16] One milliliter of 10% homogenate was mixed with 1 ml of 5% TCA (w/v), the mixture was allowed to stand for 30 min. and centrifuged at 2500 rpm for 15 min. 0.5 ml of supernatant was taken and 2.5 ml of 5′5′-dithionitrobenzoic acid (DTNB) was added, mixed thoroughly and absorbance was recorded at 412 nm. The results were expressed as μmoles/g tissue.
Superoxide dismutase
Superoxide dismutase was estimated as described by Kakkar et al.[17] 650 μl of sodium pyrophosphate buffer, 50 μl phenazine methasulphate, 150 μl of nitroblue tetrazolium chloride and 100 μl NADPH were added to 50 μl of supernatant ‘S’. The mixture vortexed thoroughly, incubated for 90 s and 500 μl glacial acetic acid was added to stop the reaction. 2.0 ml of n-butanol was added to the mixture, vortexed thoroughly and kept at room temperature for 10 min. Absorbance was measured at 560 nm and the results were expressed as μmoles/min/mg protein.
Catalase
Catalase was estimated by the method of Sinha et al.[18] 1 ml of phosphate buffer and 0.4 ml water was added to 0.1 ml of ‘S’. Reaction was started by adding 0.5 ml H2O2 and the mixture was incubated at 37°C for 1 min. Reaction was stopped by adding 2 ml of dichromate: Acetic acid reagent and kept at boiling water bath for 15 min. The mixture was cooled and absorbance was read at 570 nm. CAT activity was calculated in terms of μmol/min/mg protein.
Glutathione S-transferase
Glutathione S-transferase was estimated as per method of Habig et al.[19] The reaction mixture consisting of 1.425 ml phosphate buffer (0.1 M, pH 6.5) 1.475 ml GSH (1.0 mM), 20 μl 1-chloro-2,4-dinitrobenzene (CDNB, 1 mM) and 60 μl water were added to 20 μl of ‘S’ to give 3.0 ml of the reaction mixture. Absorbance was recorded at 340 nm and the GST activity was calculated as μmoles CDNB conjugate formed/min/mg protein using molar extinction coefficient of 9.6 × 103/M/cm.
Glutathione peroxidase
Glutathione peroxidase was estimated by the method of Rotruck et al.[20] 0.4 ml tris HCl buffer, 0.2 ml GSH, 0.1 ml water, 0.2 ml H2O2 and were added to 0.1 ml ‘S’. The mixture was incubated at 37°C for 15 min and 0.5 ml TCA (10%) was added. The mixture was centrifuged at 2000 rpm for 15 min, 0.5 ml of supernatant was taken and 2 ml di-sodium hydrogen phosphate buffer and 0.5 ml Ellman's Reagent were added. Absorbance was read at 420 nm. The results were expressed as nmoles/min/mg protein.
Glutathione reductase
Glutathione reductase was estimated by the method of Carlberg and Mannervik.[21] 2.5 ml buffer, 0.2 ml NADPH, 0.2 ml GSSG and 0.1 ml “S” were mixed and allowed to stand for 30 s. Absorbance was recorded at 340 nm for 3 min at 30 s intervals. GR activity was calculated in terms of nmoles/min/mg protein.
Total protein
Protein was estimated by the method of Lowry et al.[22]
Statistical analysis
Results were expressed as mean ± standard error of the mean data was subjected to one-way analysis of variance. The treatment groups were compared with the control group using Dunnett's test. All the statistics were carried out in GraphPad InStat Software Inc., v. 3.06, San Digeo, USA.
RESULTS
Organ weight
Significant (P < 0.01) decrease in the weight of testis (11.66%) and epididymis (22.72%) was observed in G-B, indicating the deleterious effect of cypermethrin. In post- and pre-treatment Group C and D the decrease in testis weight was 4.13%, where as in epididymis it was 18.18 and 21.7%, respectively. The improvement in testis and epididymis weight in G-C and G-D compared with G-B suggested the ameliorating effect of resveratrol. The weight improvement was more pronounced in testis compared to epididymis. Nonsignificant (P > 0.05) increase in the weight of testis (2.47%) and significant (P < 0.05) increase in weight of epididymis (4.54%) was observed in G-E [Figure 1].
Figure 1.
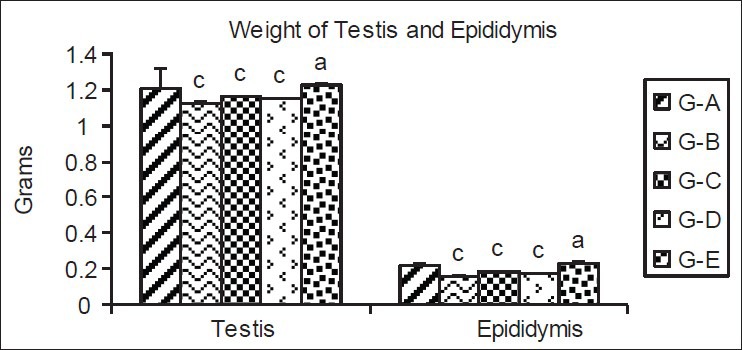
Effect of cypermethrin and resveratrol on the weight of testis and epididymis. The values represent as Mean ± SEM for 6 rats each. c=P < 0.01 a=P > 0.05 as compared with control value. G-A=Control, G-B=Cypermethrin; G-C=Post-treatment group, G-D=Pre-treatment group and G-E=Resveratrol treatment groups
Sperm parameters
The significant (P < 0.01, 38.90%) decrease in sperm head count was reported in G-B on cypermethrin exposure, where as 6.85% and 6.30% decrease was observed in post- and pre-treatment resveratrol Groups C and D, respectively. The results showed that resveratrol treatment increased the sperm head counts in cypermethrin exposed rats. Treatment of control rats (G-A) with resveratrol (G-E) showed significant (P < 0.01, 7.82%) increase in testicular sperm head count [Table 2 and Figure 2].
Table 2.
Effect of cypermethrin and resveratrol on sperm head count, morphology, motility and live sperm count
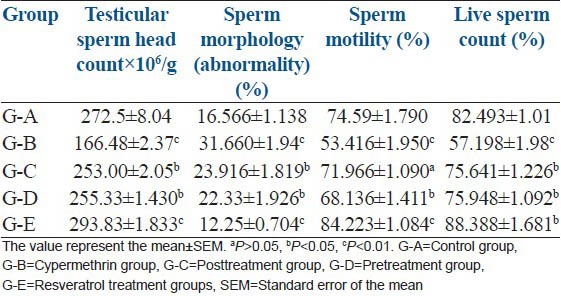
Figure 2.
Sperm counts in different groups on cypermethrin exposure and resveratrol treatment. a= Control, b= Cypermethrin, c= Post-treatment group, d= Pre-treatment group and e= Resveratrol treatment groups
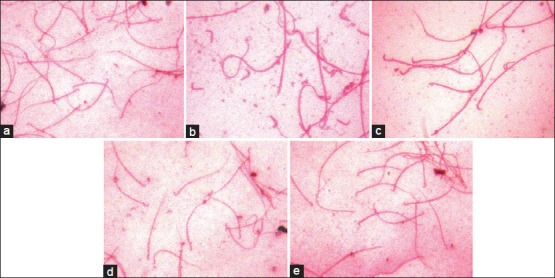
The results showing an increase in cypermethrin-induced Sperm morphological abnormalities and protective role of resveratrol is summarized in the Table 2. Sperm abnormalities significantly increased in G-B (91%, P < 0.01). In post- and pre-treatment of resveratrol, the percentage of sperm morphological abnormalities was low (G-C, 44%, P < 0.05 and G-D, 35%, P < 0.05). However, sperm abnormalities were significantly (P < 0.01) reduced (26%) in G-E as compared to G-A. Increase in headless tail (7%), broken tail (1.5%), coiled tail (1.5%), bent tail (3%), detached head (3%) was observed in G-B. In G-C and D, headless tail (5, 4%), broken tail (1, 1%), coiled tail (1.5, 1%), bent tail (2, 2.5%) and detached head (2, 2.5%) have been reported [Table 3 and Figure 3]. All comparison were made with control G-A.
Table 3.
Effect of cypermethrin and resveratrol on sperm morphology
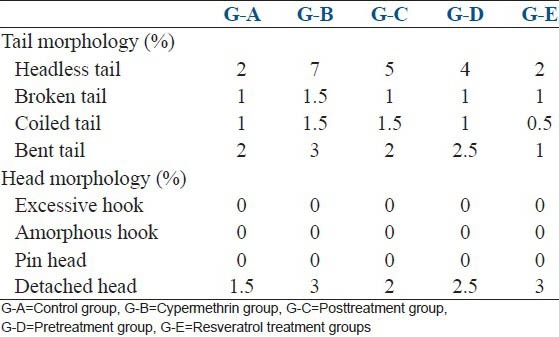
Figure 3.
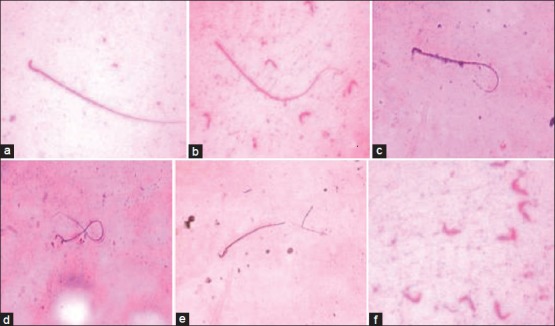
Abnormal sperm tail and head morphology after cypermethrin exposure. a-Normal sperm, b-Headless tail, c-Bent tail, d-Coiled tail, e-Broken tail, f-Detached head
Group B showed significant (P < 0.01) decrease in motile sperm (28.38%) indicating that cypermethrin exposure adversely affects the fertility. The decrease in sperm motility was nonsignificant (P > 0.05) decrease in G-C (3.57%) and significant (P < 0.05) decrease in G-D (8.65%). However, significant (P < 0.01) increase in sperm motility was depicted in G-E (12.9%) as compared with G-A [Table 2].
Significant decrease in percentage of live sperm was observed in G-B (30.73%, P < 0.01), but on post- and pre-treatment with resveratrol in G-C and G-D the percentage decrease was 8.30% and 7.93%, respectively. However, G-E showed significant (P < 0.05) increase (6.67%) as compared to G-A [Table 2].
Sex hormone assay (testosterone, follicle stimulating hormone, luteinizing hormone)
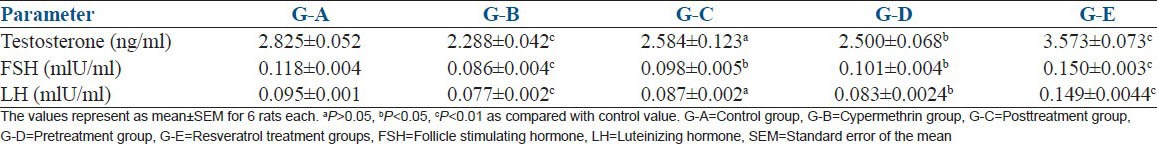
Rats in G-B showed significant (P < 0.01) decrease in T (19%), FSH (27.11%), LH (18.94%) on cypermethrin exposure. Posttreatment with resveratrol in G-C showed a nonsignificant (P > 0.05) decrease in T (8.53%), LH (8.42%) and significant (P < 0.05) decrease in FSH (16.94%). A significant (P < 0.05) decrease in T (11.48%), FSH (14.40%) and LH (12.63%) was reported in resveratrol pretreatment group G-D. The results showed that post- and pre-treatment with resveratrol increased the level of reproductive hormones compared to cypermethrin exposure group. On the other hand significant (P < 0.01) increase in T (26.47%), FSH (27.11%) and LH (56.84%) was observed in G-E [Table 4].
Table 4.
The effect of resveratrol against cypermethrin induced reproductive hormone alterations in Wistar rats
Oxidative stress parameters
Lipid peroxidation
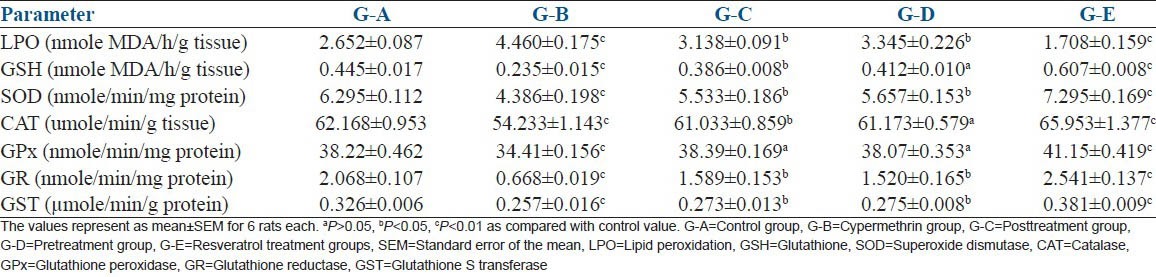
Lipid peroxidation level was increased by 65.706% in cypermethrin exposed Group-B as compared to Group-A. The significant (P < 0.01) increase in LPO level indicates deleterious effect of cypermethrin. LPO level was 18.41% and 26.22% high in G-C and G-D respectively, compared to G-A. The findings showed that resveratrol treatment markedly decreased LPO level compared to G-B indicating beneficial effect of post- and pre-treatment. The pretreatment showed better LPO lowering effect compared to posttreatment. Significant (P < 0.01) decrease was seen in G-E (35.54%) as compared with G-A [Table 4].
Nonenzymatic antioxidant
Glutathione level increased significantly in G-B (47.19%, P < 0.01), G-C (13.25%, P < 0.05) and G-D (7.4%, P < 0.05). The treatment G-E showed significant (P < 0.01) increase (36.40%) as compared to G-A [Table 5].
Table 5.
The effect of resveratrol against cypermethrin induced oxidative stress in Wistar rats
Enzymatic antioxidants
The significant (P < 0.01) decrease in SOD (69.67%), CAT (12.75%), GPx (9.96%), GR (65.70%) and GST (21.16%) level was observed in G-B as compared to G-A. G-C and G-D showed significant (P < 0.05) decrease in SOD (12.10, 10.13%,), CAT (1.81, 1.58%, P > 0.05), GPx (23.16, 26.49%), GST (16.25,15.6%) and nonsignificant (P > 0.05) in GPx (0.04,0.39%) level respectively as compared to group A. On the contrary, significant (P < 0.01) increase in SOD (15.88%), CAT (6.10%), GPx (7.66%), GR (56.72%) and GST (16.87%) was observed in G-E as compared to G-A [Table 5]. The observations suggest that pre- and post-treatment with resveratrol improved the antioxidant defense of the animal and showed ameliorating effect on cypermethrin exposure. The antioxidant defense was improved even when there was no potential xenobiotic threat (G-E).
DISCUSSION
Exposure to environmental toxicants including pesticides is a proven factor in impairment of male reproductive system and infertility. Cypermethrin initially thought to be safe for household application, a number of recent reports showed its reproductive toxicity in mammalian and nonmammalian laboratory and wildlife animal species.[3,23] Testicular and epididymis weight is a valuable index of reproductive health. The decrease in weight of testis weight on xenobiotics exposure may be due to reduced tubule size, decrease number of germ cells and elongated spermatids.[24] The decrease in organ weight may be due to decrease in level of serum T, FSH and LH as observed in the study which is in consistence with findings of others.[4] Pyrethroids and other pesticides have exhibited antiandrogenic effect largely due to the accumulation in testis.
Accumulation of cypermethrin in testis and other reproductive organs may have accelerated oxidative stress leading to accelerated death of spermatogenic cells associated with sperm abnormalities.[25] Oxidative stress may also be responsible for decrease in testis and epididymis weight observed is this study. Reduction in sperm head count on cypermethrin exposure may be due to decrease in serum T level observed in this study. Decreased T might have suppressed the male spermatogenesis. The inhibition in spermatogenesis may also be due to low level of LH and FSH which are required for normal spermatogenesis in pubertal rats. The decrease in sperm count is in agreement with the findings of others, where cypermethrin treatment was associated with the inhibition of T, LH and FSH level.[23,26,27] Decrease in sperm motility, live sperm and increase in the number of the abnormal sperm may be due to enhanced ROS production by cypermethrin in the testis and epididymis as observed in this study. Pesticide induced ROS production is known to adversely affect sperm motility, live sperm, and increased sperm abnormality.[26,28,29] A significant elevation in the number of abnormal shape of sperm head was noticed in cypermethrin exposed rats.[30] It is well known that testosterone plays a key role in the development of male reproductive tissues such as the testis and prostate. Testosterone is needed for the continued production of different generation of germ cells in the seminiferous tubules. Therefore, reduction of testosterone level may lead to the separation of germ cells from the epithelium of the seminiferous tubules.[31]
We observed decrease in serum testosterone level in the cypermethrin exposed rats. The decrease in serum T level is possibly due to direct effect of cypermethrin on testicular tissue. It is clear from the findings of this study that accumulation of cypermethrin in testicular tissue enhanced oxidative stress. The high oxidative stress has resulted in decreased cell viability of all types of cells in testicular tissue. The low cell viability and accelerated cell death not only resulted in decreased cell and tissue mass but also various abnormalities in sperm structure and function.
Reduction in pituitary gonadotrophins (FSH and LH) secretions on pesticide exposure have been previously reported.[32,33] We also observed decrease in FSH and LH level on cypermethrin exposure. Concurrent decrease in T, FSH and LH level also advocates extra testicular targets of cypermethrin. Reduction in T as well FSH and LH suggests that apart from testicular tissue, cypermethrin may also be affecting hypothalamus-pituitary axis. LH stimulates Leydig cells to produce T, hence decrease in LH may also be contributing factor for low level of T. Low level of T, FSH and LH inhibit effective spermatogenesis and development of seminiferous tubule, resulting in low number of functional sperms and low fertility.[34]
In present study, cypermethrin exposure increased LPO level in testis, indicating increase in oxidative stress in the tissue. Accumulation of cypermethrin in testicular tissue leads to membrane degeneration and excessive free radical formation. The excessive free radicals further damage the membranes as well as the antioxidant defense of the tissue. The compromised antioxidant defense system and excessive free radicals accelerates oxidative stress, which is evident in form of increased LPO on cyoermethrin exposure. Pesticide induced increase in LPO has previously been reported.[26,35,36]
Glutathione an important antioxidant molecules, which in conjugation with GPx plays a significant role in protecting cells against oxidative stress of xenobiotics by scavenging ROS.[37,38] In this study, reduction in GSH was observed on cypermethrin exposure. The decrease in GSH may be due to enhanced utilization of GSH for detoxification of cypermethrin-induced free radicals.[39,40]
Superoxide dismutase is considered the first line of defense against deleterious effects of ROS in the cell by catalyzing dismutation of superoxide radicals to form H2O2. Decrease in testicular SOD activity was observed in cypermethrin exposed group B as compared to the control group A. The oxidative stress induced by cypermethrin exposure may have depleted cellular SOD level. Decrease in SOD level have been reported on pesticide exposure in previous studies.[36,39,41] CAT is another important enzymatic antioxidants that act against toxic oxygen free radicals such as superoxide (O2−) and hydroxyl ions (OH−) in biological systems.[42] CAT alleviates oxidative stress by catalyzing the formation of H2O and O2 from H2O2.[43] In the present study, we observed a decrease in CAT activity on cypermethrin administration. This decrease in CAT activity might be due to accumulation of hydrogen peroxide in testis. Accumulated H2O2 is known to inhibit CAT activity.[44] Another important enzyme of the series is GPx. This enzyme is nonspecific for H2O2 and it catalyzes metabolism of a number of substrates ranging from H2O2 to organic hydroperoxides.[45] Therefore, an excess of H2O2 and lipid peroxides are efficiently scavenged by GPx activity. GPx activity was decreased on cypermethrin exposure in present study. Decrease in GPx activity on pesticide exposure has been reported earlier by Nasuti et al.[46]
Decrease in GR activity was observed on cypermethrin exposure as compared to control. GR is a member of the pyridine-nucleotide disulfide oxidoreductase family of flavo enzymes that catalyzes the reduction of glutathione disulfide (GSSG) to GSH in the presence of NADPH. In this study, significant decrease in the GR may be due to cypermethrin-induced damage to the tertiary structure of the enzyme.[44] Cypermethrin administration to rat showed reduced activity of GST. GST catalysis the conjugation of the reduced GSH to electrophiles and protects cellular components from oxidative damage.[47] GST is known to bind strongly to hydrophobic compounds like pyrethroids. Previous studies have reported decrease in GST level in rat testis on lindane exposure.[26]
The antioxidant activity of resveratrol has been reported. In present study, post- and pre-treatment with resveratrol showed increase in testis, epididymis weight, decreased sperm abnormalities and improved sperm head counts, sperm motility and live sperms as compared to cypermethrin treated rats. The results indicated that resveratrol improved the sperm parameters in cypermethrin induced damage. The improvement in sperm parameter may be due to the good antioxidant potential and facilitating the removal of ROS during posttreatment regimen. In the pretreatment regimen, resveratrol may have strengthened the antioxidant defense to minimize the damage caused by cypermethrin accumulation. The result showed that resveratrol treatment reduced cypermethrin induced increase in LPO level in post- and pre-treatment regimens. Resveratrol treatment increased GSH level and also enhanced the activity of antioxidant enzymes SOD, CAT, GR, Gpx and GST in rats exposed to cypermethrin. In the present study, resveratrol treatment enhanced T, FSH and LH level. The increase in testicular T may be due to increase cell viability and testicular mass and synchronism in all reproductive parameters. The increase in T may be direct effect of resveratrol on Leydig cells and steroidogenesis. Increase in FSH and LH may be due to direct effect of resveratrol on hypothalamus-pituitary axis.
CONCLUSION
The study concluded that resveratrol ameliorated cypermethrin-induced testicular damage by reducing oxidative stress and by enhancing level of sex hormones.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Singh AK, Tiwari MN, Prakash O, Singh MP. A current review of cypermethrin-induced neurotoxicity and nigrostriatal dopaminergic neurodegeneration. Curr Neuropharmacol. 2012;10:64–71. doi: 10.2174/157015912799362779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sushma N, Devasena T. Aqueous extract of Trigonella foenum graecum (fenugreek) prevents cypermethrin-induced hepatotoxicity and nephrotoxicity. Hum Exp Toxicol. 2010;29:311–9. doi: 10.1177/0960327110361502. [DOI] [PubMed] [Google Scholar]
- 3.Hu JX, Li YF, Li J, Pan C, He Z, Dong HY, et al. Toxic effects of cypermethrin on the male reproductive system: With emphasis on the androgen receptor. J Appl Toxicol. 2013;33:576–85. doi: 10.1002/jat.1769. [DOI] [PubMed] [Google Scholar]
- 4.Wang XZ, Liu SS, Sun Y, Wu JY, Zhou YL, Zhang JH. Beta-cypermethrin impairs reproductive function in male mice by inducing oxidative stress. Theriogenology. 2009;72:599–611. doi: 10.1016/j.theriogenology.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Wang Q, Zhao XF, Liu P, Meng XH, Yu T, et al. Cypermethrin exposure during puberty disrupts testosterone synthesis via downregulating StAR in mouse testes. Arch Toxicol. 2010;84:53–61. doi: 10.1007/s00204-009-0479-y. [DOI] [PubMed] [Google Scholar]
- 6.Gulcin I. Antioxidant properties of resveratrol: A structure-activity insight. Innov Food Sci Emerg Technol. 2010;11:210–8. [Google Scholar]
- 7.Collodel G, Federico MG, Geminiani M, Martini S, Bonechi C, Rossi C, et al. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod Toxicol. 2011;31:239–46. doi: 10.1016/j.reprotox.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Wu CC, Wu CI, Wang WY, Wu YC. Low concentrations of resveratrol potentiate the antiplatelet effect of prostaglandins. Planta Med. 2007;73:439–43. doi: 10.1055/s-2007-967173. [DOI] [PubMed] [Google Scholar]
- 9.de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol Nutr Food Res. 2005;49:405–30. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 10.Bhat KPL, Kosmeder JW, 2nd, Pezzuto JM. Biological effects of resveratrol. Antioxid Redox Signal. 2001;3:1041–64. doi: 10.1089/152308601317203567. [DOI] [PubMed] [Google Scholar]
- 11.Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: In vitro and in vivo studies and the underlying mechanisms (review) Int J Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- 12.Choi EK, Tsunekawa N, Kanai Y, Kurohmaru M. A new preparation protocol for measurement of testicular sperm production. J Reprod Dev. 2008;54:90–3. doi: 10.1262/jrd.19123. [DOI] [PubMed] [Google Scholar]
- 13.Williams J. Semen analysis and fertility assessment in rabbit. In: Chapin RE, Heindel JJ, editors. Methods in Toxicology. Part A. Vol. 3. San Diego: Academic Press; 1993. pp. 344–50. [Google Scholar]
- 14.Türk G, Atessahin A, Sönmez M, Yüce A, Ceribasi AO. Lycopene protects against cyclosporine A-induced testicular toxicity in rats. Theriogenology. 2007;67:778–85. doi: 10.1016/j.theriogenology.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 17.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 18.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 19.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 20.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 21.Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–90. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 23.Assayed ME, Salem HA, Khalaf AA. Protective effects of garlic extract and vitamin c against cypermethrin reproductive toxicity in male rats. Res J Vet Sci. 2008;1:1–15. [Google Scholar]
- 24.Choudhary N, Goyal R, Joshi SC. Effect of malathion on reproductive system of male rats. J Environ Biol. 2008;29:259–62. [PubMed] [Google Scholar]
- 25.Sharma P, Singh R. Protective role of curcumin on lindane induced reproductive toxicity in male Wistar rats. Bull Environ Contam Toxicol. 2010;84:378–84. doi: 10.1007/s00128-010-9942-y. [DOI] [PubMed] [Google Scholar]
- 26.Joshi SC, Bansal B, Jasuja ND. Evaluation of reproductive and developmental toxicity of cypermethrin in male male albino rats. Toxicol Environ Chem. 2011;93:593–602. [Google Scholar]
- 27.Liu L, Hu JX, Wang H, Chen BJ, He Z, Xu LC. Effects of beta-cypermethrin on male rat reproductive system. Environ Toxicol Pharmacol. 2010;30:251–6. doi: 10.1016/j.etap.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 28.el-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Role of alpha-tocopherol and beta-carotene in ameliorating the fenvalerate-induced changes in oxidative stress, hemato-biochemical parameters, and semen quality of male rats. J Environ Sci Health B. 2004;39:443–59. doi: 10.1081/pfc-120035929. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Gautam AK, Agarwal KR, Shah BA, Saiyad HN. Demonstration of sperm head shape abnormality and clastogenic potential of cypermethrin. J Environ Biol. 2004;25:187–90. [PubMed] [Google Scholar]
- 30.Li YF, Pan C, Hu JX, Li J, Xu LC. Effects of cypermethrin on male reproductive system in adult rats. Biomed Environ Sci. 2013;26:201–8. doi: 10.3967/0895-3988.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Elbetieha A, Da’as SI, Khamas W, Darmani H. Evaluation of the toxic potentials of cypermethrin pesticide on some reproductive and fertility parameters in the male rats. Arch Environ Contam Toxicol. 2001;41:522–8. doi: 10.1007/s002440010280. [DOI] [PubMed] [Google Scholar]
- 32.Biswas NM, Ghosh P. Effect of lead on male gonadal activity in albino rats. Kathmandu Univ Med J (KUMJ) 2004;2:43–6. [PubMed] [Google Scholar]
- 33.Pareek TK, Joshi AR, Sanyal A, Dighe RR. Insights into male germ cell apoptosis due to depletion of gonadotropins caused by GnRH antagonists. Apoptosis. 2007;12:1085–100. doi: 10.1007/s10495-006-0039-3. [DOI] [PubMed] [Google Scholar]
- 34.Monet-Kuntz C, Hochereau-de Reviers MT, Terqui M. Variations in testicular androgen receptors and histology of the lamb testis from birth to puberty. J Reprod Fertil. 1984;70:203–10. doi: 10.1530/jrf.0.0700203. [DOI] [PubMed] [Google Scholar]
- 35.Giray B, Gürbay A, Hincal F. Cypermethrin-induced oxidative stress in rat brain and liver is prevented by vitamin E or allopurinol. Toxicol Lett. 2001;118:139–46. doi: 10.1016/s0378-4274(00)00277-0. [DOI] [PubMed] [Google Scholar]
- 36.Vaithinathan S, Saradha B, Mathur PP. Methoxychlor-induced alteration in the levels of HSP70 and clusterin is accompanied with oxidative stress in adult rat testis. J Biochem Mol Toxicol. 2009;23:29–35. doi: 10.1002/jbt.20262. [DOI] [PubMed] [Google Scholar]
- 37.Michiels C, Raes M, Toussaint O, Remacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med. 1994;17:235–48. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 38.Meister A. Glutathione, metabolism and function via the gamma-glutamyl cycle. Life Sci. 1974;15:177–90. doi: 10.1016/0024-3205(74)90206-9. [DOI] [PubMed] [Google Scholar]
- 39.Raina R, Verma PK, Pankaj NK, Kant V. Ameliorative effect of alpha tocopherol on cypermethrin-induced oxidative stress and lipid peroxidation in Wistar rats. Int J Med Med Sci. 2009;1:396–9. [Google Scholar]
- 40.Raina R, Verma PK, Pankaj NK, Prawez S. Induction of oxidative stress and lipid peroxidation in rats chronically exposed to cypermethrin through dermal application. J Vet Sci. 2009;10:257–9. doi: 10.4142/jvs.2009.10.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yousef MI, el-Demerdash FM, Kamel KI, Al-Salhen KS. Changes in some hematological and biochemical indices of rabbits induced by isoflavones and cypermethrin. Toxicology. 2003;189:223–34. doi: 10.1016/s0300-483x(03)00145-8. [DOI] [PubMed] [Google Scholar]
- 42.Burton GW, Cheeseman KH, Ingold KU, Slater TF. Lipid antioxidants and products of lipid peroxidation as potential tumour protective agents. Biochem Soc Trans. 1983;11:261–2. doi: 10.1042/bst0110261. [DOI] [PubMed] [Google Scholar]
- 43.Rajeshkumar NV, Kuttan R. Modulation of carcinogenic response and antioxidant enzymes of rats administered with 1,2-dimethylhydrazine by picroliv. Cancer Lett. 2003;191:137–43. doi: 10.1016/s0304-3835(02)00203-3. [DOI] [PubMed] [Google Scholar]
- 44.Latchoumycandane C, Mathur PP. Induction of oxidative stress in the rat testis after short-term exposure to the organochlorine pesticide methoxychlor. Arch Toxicol. 2002;76:692–8. doi: 10.1007/s00204-002-0388-9. [DOI] [PubMed] [Google Scholar]
- 45.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 46.Nasuti C, Cantalamessa F, Falcioni G, Gabbianelli R. Different effects of Type I and Type II pyrethroids on erythrocyte plasma membrane properties and enzymatic activity in rats. Toxicology. 2003;191:233–44. doi: 10.1016/s0300-483x(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 47.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistances. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]


