Abstract
There is a growing health burden in developing countries due to recent trends of increasing incidence of neurodegenerative diseases. To reduce the healthcare cost and effective management of dementia illness, early diagnosis, accurate differentiation and their progression assessment is becoming crucially important. We are utilizing 99mTc-d, l-hexamethyl propyleneamine oxime (HMPAO) brain perfusion single photon emission computed tomography (SPECT) to characterize dementia on the basis of perfusion patterns and observed significant improvement in their management. Eleven patients (median age of 60 years range of 53-83 years) with clinical suspicion of dementia underwent 99mTc-HMPAO brain perfusion SPECT. SPECT-computed tomography acquisition done, data are reconstructed, reviewed in three view and further processed in “neurogam” to get voxel based analysis and the comparison with age based normal database and surface mapping. Final diagnosis was done with clinical correlation. Four patients are diagnosed as Alzheimer's disease, two as frontotemporal dementia, one as dementia of Lewy bodies, two as vascular dementia and two as pseudodementia. All imaging findings are well-correlated with clinical background. Brain perfusion SPECT with HMPAO was very helpful to us in characterizing the patients of dementia by its perfusion pattern.
Keywords: Brain perfusion, dementia, hexamethyl propyleneamine oxime, single photon emission computed tomography
Introduction
Dementia is a very common neurodegenerative disorders in the elderly and a growing concern all over the world including the developing countries like India. In 2005, it was estimated that 24.3 million worldwide and 1.8 million people in India and South Asia are affected with dementia. In India, the number of people with Alzheimer's disease (AD) and other dementias is increasing every year because of the steady growth in the older population and stable increment in life expectancy and it is expected to increase two-fold by 2030 and three-fold by 2050.[1,2] There are 400,000 new cases per year for India and South Asia.[2] According to the World Health Organization (WHO), the South-East Asia Region, the dementia mortality rate for India is 13.5/100,000 males and 11.1 for 100,000 females.[1] Compared with other chronic medical conditions (heart diseases, cancer, and stroke) AD is in fourth position for the leading cause of death in the Asia Pacific region.[3]
Almost, 10% of persons over age of 65 and up to 50% over 85 suffer from dementia. In approximately two-third cases of dementia patients in over 65 years are diagnosed as AD.[4] In addition to a lot of financial burden, the disease also exacts a heavy emotional toll on family members and caregivers.[5] Therefore, early and accurate diagnosis of AD is very important to reduce the disease burden and its progression to advanced stage. In low- and middle-income countries, diagnosis is often much delayed, and survival is much shorter than that in the developed world.[6] Around 10-37% of the elderly population with dementia in developing countries is classified as having potentially vulnerable living circumstances with requiring long-term and specialized care.[7]
Standard workup for AD is: Physical, neurologic, and psychiatric examination; psychometric testing; blood work (screening for B 12 deficiency and hypothyroidism); and brain imaging.[8] Although this approach is useful in detecting dementia, it is not entirely reliable for establishing a diagnosis of very early AD or predementia illness. Though, it is evident from different clinical research that, brain perfusion single photon emission computed tomography (SPECT) and metabolic positron emission tomography (PET) has the potential to detect the pathophysiological changes much before the clinical evidence of dementia, these techniques are very less utilized in clinical set up.
Functional imaging also helps to differentiate AD from other form of dementia by its specific pattern of defects, which is very important because, treatment will defer entirely for different causes. Brain perfusion SPECT is sensitive in detecting impairment of regional cerebral blood flow (rCBF) and different perfusion patterns have been associated with different types of dementia, which may well be utilized in the differential diagnosis of dementias.[9,10,11] Accurate classification of dementias is becoming crucially important because of recent advances in medical treatment.[12,13] The severity of the perfusion abnormality may increase with increasing clinical impairment, leading to an additional role of SPECT in disease staging.[14,15,16] SPECT has also an impact on therapeutic decisions by differentiating AD from depressive pseudo dementia, which presents with predominantly prefrontal perfusion impairment. Different researchers suggested that functional brain imaging with PET or SPECT has the potential to aid in identifying regional patterns of perfusion, metabolism, or both that are characteristic of different types of dementia.[17,18,19]
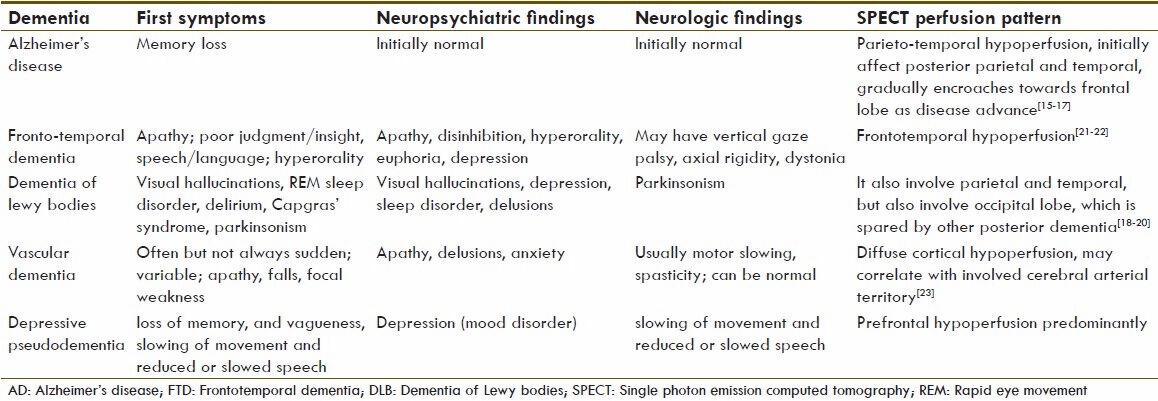
Temporoparietal hypoperfusion is associated with posterior dementia, such as Alzheimer's type, whereas frontal or frontotemporal hypoperfusion suggests frontal lobe dementia, such as Pick's disease. The radiotracer distribution pattern in AD is similar for SPECT and PET imaging. In general, the radiotracer activity is decreased in the posterior parietal lobes and the temporal lobes. In advanced stage, particularly those with a loss of executive function, hypometabolism/hypoperfusion in the frontal lobes is noted. The primary neocortical regions, including the sensorimotor regions, the visual cortex, and the subcortical gray matter, are usually relatively intact.[20,21,22] As shown in Table 1, major form of dementia and their perfusion patterns are shortlisted.
Table 1.
Clinical differentiation of the major differentia
Though the role of SPECT in dementia is well-established, it is very less utilized technique in clinical practice, particularly in developing countries. As nuclear medicine imaging as a whole is becoming available to our routine clinical practice in developing countries nowadays, the utilization of brain SPECT in management of this debilitating disease should also be explored. We are trying to implement the potentials of brain perfusion SPECT in clinical practice to diagnose and characterize dementia patients based on the perfusion patterns with Tc-99m exametazime(d, l-hexamethyl propyleneamine oxime/HMPAO). In this study, we have reviewed 11 patients of dementia and observed different perfusion patterns to help in differential diagnosis of dementia.
Materials and Methods
Patients
Eleven patients with clinical suspicion of dementia were studied. There were 6 male (55%) and 5 female (45%), with mean age of 63.6 ± 9.2 years (median age 60 years with range of 53-83 years). The baseline clinical characteristics of the studied patients were defined by experienced psychiatrist. The findings of standard workup such as history, neuropsychiatric, neurologic, and biochemical examination are noted to use in further analysis.
Radiopharmaceutical
d, l-hexamethyl propyleneamine oxime kit was imported from Medi-Radiopharma, Budapest, Hungary for radiopharmaceutical preparations. Freshly eluted 99mTc (<1 h old) was used, and Tc-99m-HMPAO, the radiotracer was prepared according to manufacturer's instruction. After a minimum storage of 10 min in room temperature, the radiopharmaceutical was injected intravenously to the patients through previously cannulated channel. Dose of radiopharmaceuticals to each patient ranged from 666 MBq-925 MBq (18-25 mCi). During the injection, subjects were lying comfortably in a semi darkened and silent room. The radiotracer was injected in a resting supine position. The gap between radiotracer injection and imaging time was 45-90 min.
Single photon emission computed tomography-computed tomography procedure
All cases were scanned with our dual-head SPECT-CT (Siemens Symbia T2) gamma camera equipped with low energy general purpose collimator, 45-90 min after injection using a special head holder for fixation in a complete symmetrical position. A 20% window centered at a 140 KeV photo peak for Tc-99m was used. The image acquisition was performed in a 360° step and shoot rotation (180° rotation for each head) with 25 s per view for a total 64 views in a 128 × 128 matrix with a zoom factor of 2. Consecutively, CT scan of head (slice thickness of 3 mm, matrices of 512 × 512) was acquired after SPECT in sequential mode with the X-ray tube operated at 130 kV and 30 mA.
Reconstruction and analysis
For each patient, all steps for image reconstruction and analysis were carried out by two observers (one of them was experienced nuclear medicine physician and the other was well trained technologist). Reconstruction was performed by Iterative reconstruction (subset-8 and iteration-4) in syngo flash three-dimensional algorithm. Gaussian filter were used. Attenuation correction was performed using CT attenuation map. The images were reoriented to obtain transverse, sagittal and coronal section. After proper reconstruction SPECT images are fused with CT image and images are reviewed as SPECT, CT, and SPECT-CT slices side by side.
The reconstructed SPECT data is further processed in “Neurogam” application to obtain the region wise quantification and comparative analysis in respect of standard normal database. With the help of the application, reconstructed tomo data was co-registered with a reference template (the Talairach atlas) and quantitative analysis was done on brain SPECT images of each patients. Mean rCBF was recorded for each region on both hemispheres. Mean rCBF is calculated automatically by the application itself by predefined algorithm; that is, the mean pixel value in each corresponding region of interest as a percentage of the maximum pixel value in the entire volume of brain. The application also compares the data with age based normal database within it and a surface mapping was done according to color scale depending on the standard deviation from normal.
Results
All of 11 patients studied were presented with some form of memory loss and evaluated for dementia. Eight patients had some form of psychological disturbances, two had history of cerebrovascular accident (CVA) with neurologic correlation, one had presented with muscle rigidity and akathesia. A master chart of the patients, their clinical history, perfusion pattern and differential diagnosis are tabulated [Table 2]. Five patients revealed perfusion pattern consistent with posterior dementia. One of them had typical posterior parietal and temporal hypoperfusion, which is sign of early AD. Three patients had also frontal hypoperfusion in addition to parietotemporal hypoperfusion, consistent with advanced AD pattern [Figure 1]. One of posterior dementia pattern had finding of occipital lobe hypoperfusion in addition to parietotemporal hypoperfusion, which is typical of Lewy body dementia [Figure 2]. Two patients had frontotemporal hypoperfusion, which is a typical pattern found in frontotemporal dementia (FTD) or Pick's disease [Figure 3]. Both of the patients with history of CVA displayed diffuse cerebral hypoperfusion, including the area of stroke, not consistent with any of typical pattern and diagnosed as vascular dementia [Figure 4]. Two patients with severe depression revealed on bilateral prefrontal hypoperfusion and are diagnosed as pseudodementia [Figure 5].
Table 2.
Master chart of patient's details
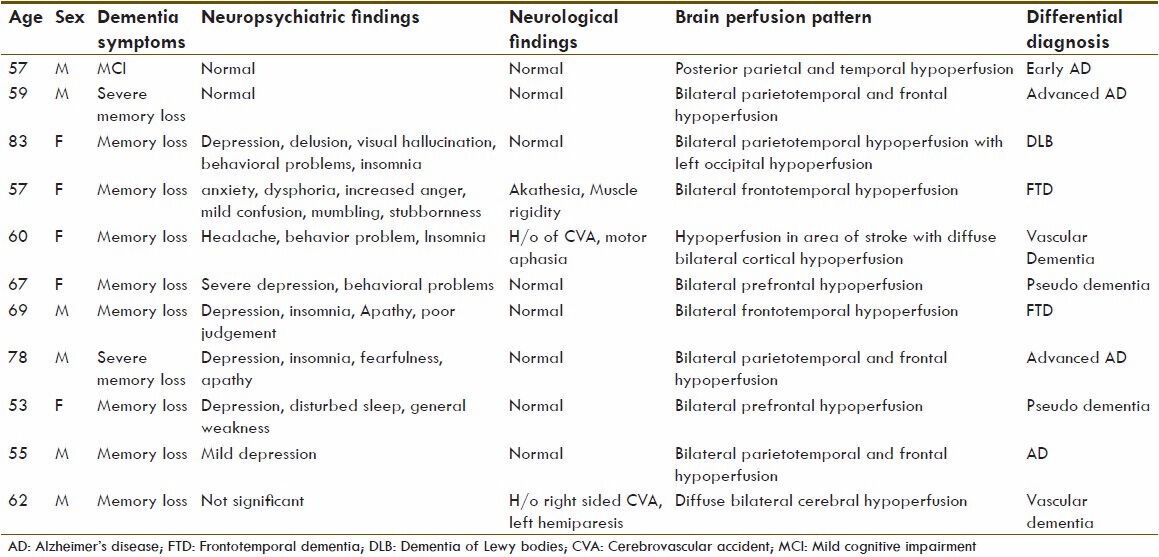
Figure 1.
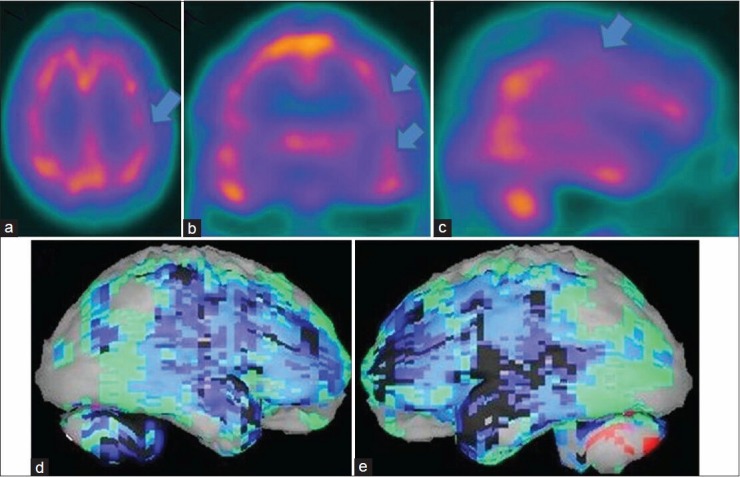
A 78-year-old male patient with history of significant memory loss and depression showing the brain perfusion pattern of an advanced Alzheimer's disease in 99mTc-hexamethyl propyleneamine oxime brain perfusion single photon emission computed tomography (SPECT) study. (a) Transaxial,b) coronal,c) sagittal view of SPECT images and (d) right lateral,e) left lateral surface projection views of “Neurogam” processed images, are showing bilateral parietotemporal and frontal hypoperfusion, the occipital pole is spared and there is increased cerebellar activity
Figure 2.
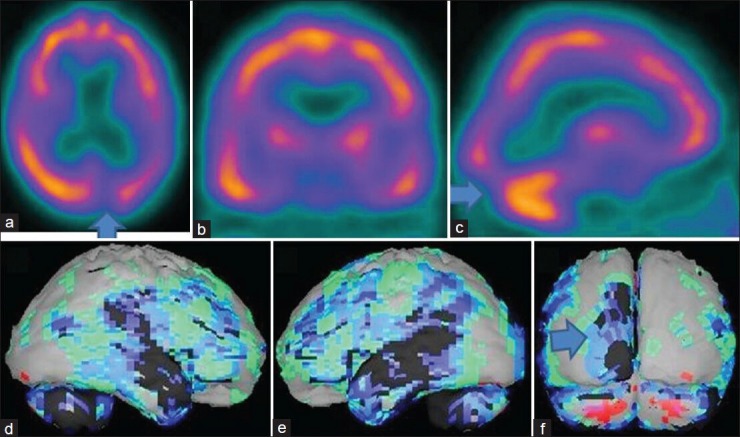
An 83-year-old female patient with history of memory loss, visual hallucination, delusion, sleep disorder, and depression showing the brain perfusion pattern of a Lewy body dementia in 99mTc-hexamethyl propyleneamine oxime brain perfusion single photon emission computed tomography (SPECT) study. (a) Transaxial,b) coronal,c) sagittal view of SPECT images and (d) right lateral,e) left lateral, (f) posterior surface projection views of “Neurogam” processed images, are showing bilateral parietotemporal hypoperfusion with, left occipital lobe hypoperfusion
Figure 3.
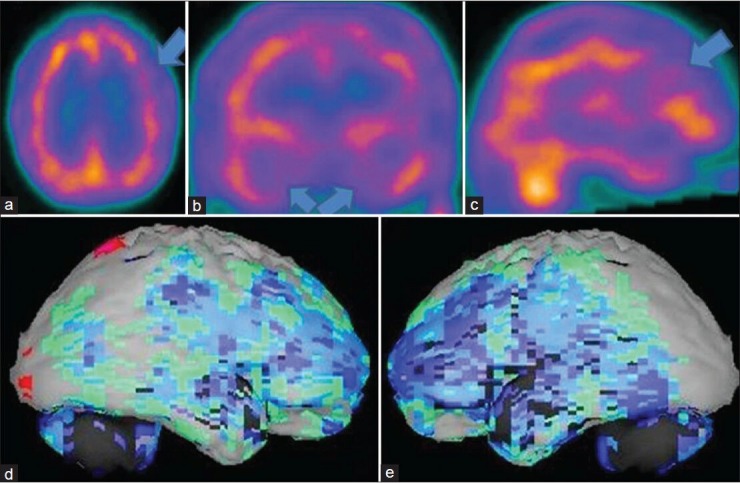
A 69-year-old male patient with memory loss, depression, insomnia, apathy, poor judgment is showing the brain perfusion pattern of a frontotemporal dementia in 99mTc-hexamethyl propyleneamine oxime brain perfusion single photon emission computed tomography (SPECT) study. (a) Transaxial,b) coronal,c) sagittal view of SPECT images and (d) right lateral,e) left lateral surface projection views of “Neurogam” processed images, are showing bilateral frontotemporal hypoperfusion
Figure 4.
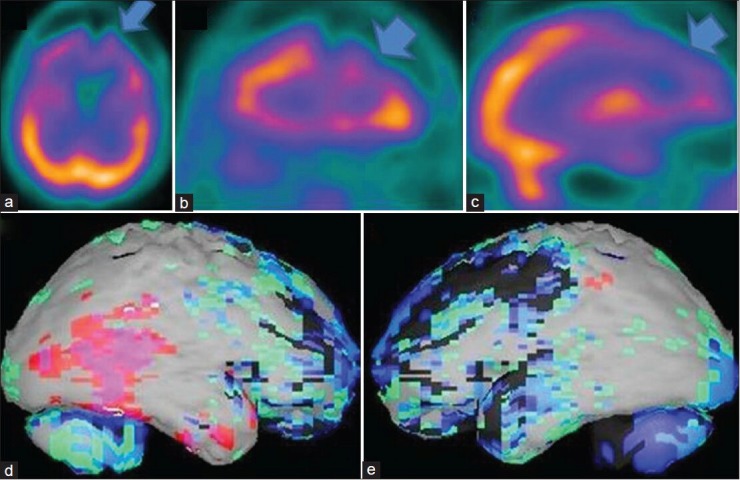
A 62-year-old male patient with history of right sided cerebral stroke and loss of memory problem is showing the brain perfusion pattern of a vascular dementia in 99mTc-hexamethyl propyleneamine oxime brain perfusion single photon emission computed tomography (SPECT) study. (a) Transaxial,b) coronal,c) sagittal view of SPECT images and (d) right lateral,e) left lateral surface projection views of “Neurogam” processed images, are showing bilateral diffuse cortical hypoperfusion including the area of stroke
Figure 5.
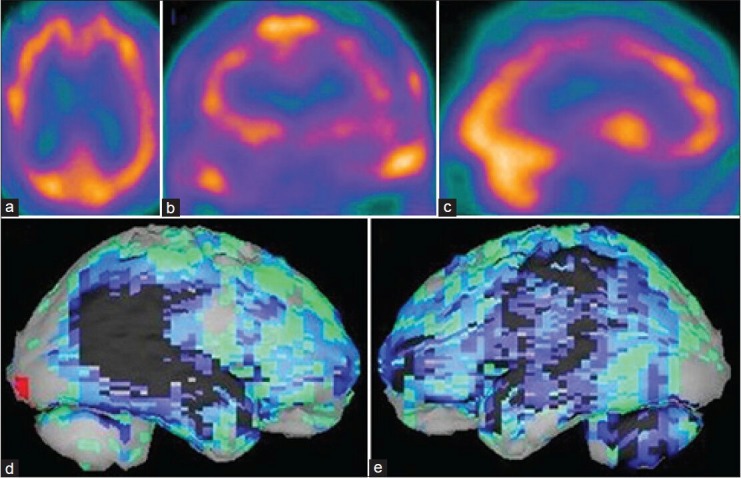
A 67-year-old female patient with history of depression and loss of memory problem is showing the brain perfusion pattern of a pseudodementia in 99mTc-hexamethyl propyleneamine oxime brain perfusion single photon emission computed tomography (SPECT) study. (a) Transaxial, (b) coronal,c) sagittal view of SPECT images and (d) right lateral,e) left lateral surface projection views of “Neurogam” processed images, are showing bilateral prefrontal cortex hypoperfusion
Discussion
According to the WHO Global Burden of Disease report 2006, dementia is the third leading cause contributors to years of life lost due to disability in the elderly in low- and middle-income countries.[23] Dementia is often associated with physical, mental and financial burden and Governments neither provide long-term care nor support carers.[7] Evidence suggests that elderly people with dementia in developing countries do not often utilize health services, and when they do, the health care system is often ill prepared to provide quality services for dementia.[24] Many need long-term care, currently provided by family carers, with primary care services not meeting their needs.[7] Carers of elderly people with dementia experienced greater psychological distress compared with carers of elderly people with other medical conditions.[25] According to AD international, the standard treatment goals of dementia management include: Early diagnosis; optimization of physical health, cognition, activity and well-being; detection and treatment of behavioral and psychological symptoms of dementia; Educating carer and providing long-term support to carer.[26] The early diagnosis is most important to optimize his cognition level/well-being and reduce the cost of cares by reducing the risk of progression to advanced disease. This is also a very crucial factor in the developing countries, where socioeconomic status is an important issue. Providing a well-established diagnostic tool to the clinicians for correctly identifying the disease at earlier stage is of great help in our countries.
The brain perfusion SPECT of all the patients in our study helped the referring physician to make an accurate differential diagnosis in dementia and to personalize their treatment to improve further management. Though it was found during analysis, the history is well correlated with the brain SPECT findings and consistent with final diagnosis in most of the patients, the referring psychiatrist was not sure of the diagnosis in most of them. The very 1st patient was diagnosed as mild cognitive impairment clinically revealed the changes related to early AD. The brain perfusion pattern typically involved the posterior parietal and temporal cortex. This patient's benefit is immense due to his early diagnosis, so that his treating doctor could start his treatment at very early stage. All other AD patients have also frontal lobe involvement signifying the advanced stage of disease. The patient diagnosed with dementia of Lewy bodies by perfusion pattern of occipital lobe hypoperfusion along with features of posterior dementia, when retrospectively reviewed, it is shown that his clinical symptoms of depression, delusion, visual hallucination, behavioral problems, and insomnia are well-correlated with symptoms found in such type of patients.[27,28,29,30] The clinical features of the patients diagnosed with FTD also revealed some form of motor rigidity along with behavioral problems and sleep disorders.[31,32] The need to rule out AD in the patients of vascular dementia was successfully carried out by our SPECT imaging.[33] Patients of pseudodementia with predominant prefrontal hypoperfusion are greatly benefitted from the brain perfusion SPECT, because of clinical presence of depression and memory loss is very common in both AD and depressive pseudodementia.[34]
Though it is evident from different clinical research that the brain perfusion SPECT have the potential to characterize the patients of dementia and help in differential diagnosis, it is our practical experience to utilize this technique in clinical practice, a unique experience from a developing country, where majority of neurodegenerative diseases are treated on basis of clinical symptoms only. Another important aspect of this study is though a limited number of dementia patients are examined, we have experienced almost all different varieties in differential diagnosis of dementia. The whole experience gave us huge confidence because of well clinical correlation with the image findings. Different perfusion patterns in a single paper can also be helpful to the readers to get an overall idea about it.
Conclusion
As per our experience, we found 99mTc-HMPAO brain perfusion SPECT to be very helpful in clinical practice to characterize the patients of dementia by its perfusion pattern and help in the differential diagnosis. The clinical significance of this experience in a developing country will be of very high importance to promote brain SPECT for a routine use in dementia in our clinical practice.
Acknowledgments
We are really thankful to all the technical staffs of the Brain Imaging Centre, Dakshi Diagnostics Services Pvt. Ltd. for carrying out the scans and helping in different phases of study. We also acknowledge the contribution of our junior colleagues, who helped to get the clinical details of the patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mathers C, Leonardi M. GBD. Geneva: World Health Organization; 2000. 2003.Global Burden of dementia in the year 2000. Summary of Methods and Data Sources. working paper. [Google Scholar]
- 2.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: A Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dementia in the Asian Pacific Region: The Epidemic is Here. Executive summary of a report by Access Economics Pty Limited for Asia Pacific members of Alzheimer's Disease International. 2006 [Google Scholar]
- 4.Albert MS, Drachman DA. Alzheimer's disease: What is it, how many people have it, and why do we need to know? Neurology. 2000;55:166–8. doi: 10.1212/wnl.55.2.166. [DOI] [PubMed] [Google Scholar]
- 5.Witthaus E, Ott A, Barendregt JJ, Breteler M, Bonneux L. Burden of mortality and morbidity from dementia. Alzheimer Dis Assoc Disord. 1999;13:176–81. doi: 10.1097/00002093-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, et al. Alzheimer's disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–26. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prince MJ. The 10/66 dementia research group-10 years on. Indian J Psychiatry. 2009;51(Suppl 1):S8–S15. [PMC free article] [PubMed] [Google Scholar]
- 8.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 9.Buttler CR, Costa DC, Walker Z, Katona CL. PET and SPECT imaging in the dementias. In: Murray IP, Ell PJ, editors. Nuclear Medicine in Clinical Diagnosis and Treatment. 2nd ed. Edinburgh Scotland: Churchill Livingstone; 1998. pp. 713–28. [Google Scholar]
- 10.Ryding E. SPECT measurements of brain function in dementia; a review. Acta Neurol Scand Suppl. 1996;168:54–8. doi: 10.1111/j.1600-0404.1996.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 11.Brooks DJ. Functional imaging techniques in the diagnosis of non-Alzheimer dementias. J Neural Transm Suppl. 1996;47:155–67. doi: 10.1007/978-3-7091-6892-9_10. [DOI] [PubMed] [Google Scholar]
- 12.Nordberg A. Functional studies of new drugs for the treatment of Alzheimer's disease. Acta Neurol Scand Suppl. 1996;165:137–44. doi: 10.1111/j.1600-0404.1996.tb05884.x. [DOI] [PubMed] [Google Scholar]
- 13.Skoog I, Marcusson J, Blennow K. Dementia. It's getting better all the time. Lancet. 1998;352(Suppl 4):SIV4. doi: 10.1016/s0140-6736(98)90266-5. [DOI] [PubMed] [Google Scholar]
- 14.Costa DC, Pilowsky LS, Ell PJ. Nuclear medicine in neurology and psychiatry. Lancet. 1999;354:1107–11. doi: 10.1016/s0140-6736(99)06095-x. [DOI] [PubMed] [Google Scholar]
- 15.Laatsch L, Jobe T, Sychra J, Lin Q, Blend M. Impact of cognitive rehabilitation therapy on neuropsychological impairments as measured by brain perfusion SPECT: A longitudinal study. Brain Inj. 1997;11:851–63. doi: 10.1080/026990597122927. [DOI] [PubMed] [Google Scholar]
- 16.Greene JD, Miles K, Hodges JR. Neuropsychology of memory and SPECT in the diagnosis and staging of dementia of Alzheimer type. J Neurol. 1996;243:175–90. doi: 10.1007/BF02444012. [DOI] [PubMed] [Google Scholar]
- 17.Tikofsky RS, Hellman RS, Parks RW. Single photon emission computed tomography and applications to dementia. In: Parks RW, Zec RF, Wilson RS, editors. Neuropsychology of Alzheimer's Disease and other Dementias. New York, NY: Oxford University Press; 1993. pp. 489–510. [Google Scholar]
- 18.Hellman RS, Tikofsky RS, Van Heertum R, Coade G, Carretta R, Hoffmann RG. A multi-institutional study of interobserver agreement in the evaluation of dementia with rCBF/SPET technetium-99m exametazime (HMPAO) Eur J Nucl Med. 1994;21:306–13. [PubMed] [Google Scholar]
- 19.Mayberg HS. Clinical correlates of PET-and SPECT-identified defects in dementia. J Clin Psychiatry. 1994;55(Suppl):12–21. [PubMed] [Google Scholar]
- 20.Van Heertum RL, Tikofsky RS, Rubens AB. Dementia. In: Van Heertum RL, Tikofsky RS, editors. Functional Cerebral SPECT and PET Imaging. 3rd ed. New York, NY: Lippincott Williams and Wilkins; 2000. pp. 127–88. [Google Scholar]
- 21.Talbot PR, Snowden JS, Lloyd JJ, Neary D, Testa HJ. The contribution of single photon emission tomography to the clinical differentiation of degenerative cortical brain disorders. J Neurol. 1995;242:579–86. doi: 10.1007/BF00868810. [DOI] [PubMed] [Google Scholar]
- 22.Varma AR, Adams W, Lloyd JJ, Carson KJ, Snowden JS, Testa HJ, et al. Diagnostic patterns of regional atrophy on MRI and regional cerebral blood flow change on SPECT in young onset patients with Alzheimer's disease, frontotemporal dementia and vascular dementia. Acta Neurol Scand. 2002;105:261–9. doi: 10.1034/j.1600-0404.2002.1o148.x. [DOI] [PubMed] [Google Scholar]
- 23.Mathers C, Lopez AD. Updated Projections of Gobal Mortality and Burden of Disease, 2002-2030: Data Sources, Methods and Results. Evidence and Information for 33 Policy Working Paper. World Health Organization. 2006 [Google Scholar]
- 24.Shaji KS, Arun Kishore NR, Lal KP, Prince M. Revealing a hidden problem. An evaluation of a community dementia case-finding program from the Indian 10/66 dementia research network. Int J Geriatr Psychiatry. 2002;17:222–5. doi: 10.1002/gps.553. [DOI] [PubMed] [Google Scholar]
- 25.Dias A, Samuel R, Patel V, Prince M, Parameshwaran R, Krishnamoorthy ES. The impact associated with caring for a person with dementia: A report from the 10/66 Dementia Research Group's Indian network. Int J Geriatr Psychiatry. 2004;19:182–4. doi: 10.1002/gps.1016. [DOI] [PubMed] [Google Scholar]
- 26.World Alzheimer Report 2009: Alzheimer's Diseases International. 2009 [Google Scholar]
- 27.Donnemiller E, Heilmann J, Wenning GK, Berger W, Decristoforo C, Moncayo R, et al. Brain perfusion scintigraphy with 99 m Tc-HMPAO or 99 m Tc-ECD and 123I-beta-CIT single-photon emission tomography in dementia of the Alzheimer-type and diffuse Lewy body disease. Eur J Nucl Med. 1997;24:320–5. doi: 10.1007/BF01728771. [DOI] [PubMed] [Google Scholar]
- 28.Ishii K, Yamaji S, Kitagaki H, Imamura T, Hirono N, Mori E. Regional cerebral blood flow difference between dementia with Lewy bodies and AD. Neurology. 1999;53:413–6. doi: 10.1212/wnl.53.2.413. [DOI] [PubMed] [Google Scholar]
- 29.Okamura N, Arai H, Higuchi M, Tashiro M, Matsui T, Hu XS, et al. [18F] FDG-PET study in dementia with Lewy bodies and Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:447–56. doi: 10.1016/s0278-5846(01)80005-1. [DOI] [PubMed] [Google Scholar]
- 30.Ishii K, Imamura T, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer's disease. Neurology. 1998;51:125–30. doi: 10.1212/wnl.51.1.125. [DOI] [PubMed] [Google Scholar]
- 31.Pagani M, Salmaso D, Ramström C, Jonsson C, Lundqvist R, Thurfjell L, et al. Mapping pathological (99m) Tc-d, l-hexamethylpropylene amine oxime uptake in Alzheimer's disease and frontal lobe dementia with SPECT. Dement Geriatr Cogn Disord. 2001;12:177–84. doi: 10.1159/000051255. [DOI] [PubMed] [Google Scholar]
- 32.Hodges JR. Frontotemporal dementia (Pick's disease): Clinical features and assessment. Neurology. 2001;56(11 Suppl 4):S6–10. doi: 10.1212/wnl.56.suppl_4.s6. [DOI] [PubMed] [Google Scholar]
- 33.Mori E, Ishii K, Hashimoto M, Imamura T, Hirono N, Kitagaki H. Role of functional brain imaging in the evaluation of vascular dementia. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S91–101. [PubMed] [Google Scholar]
- 34.Cho MJ, Lyoo IK, Lee DW, Kwon JS, Lee JS, Lee DS, et al. Brain single photon emission computed tomography findings in depressive pseudodementia patients. J Affect Disord. 2002;69:159–66. doi: 10.1016/s0165-0327(01)00301-9. [DOI] [PubMed] [Google Scholar]


