Abstract
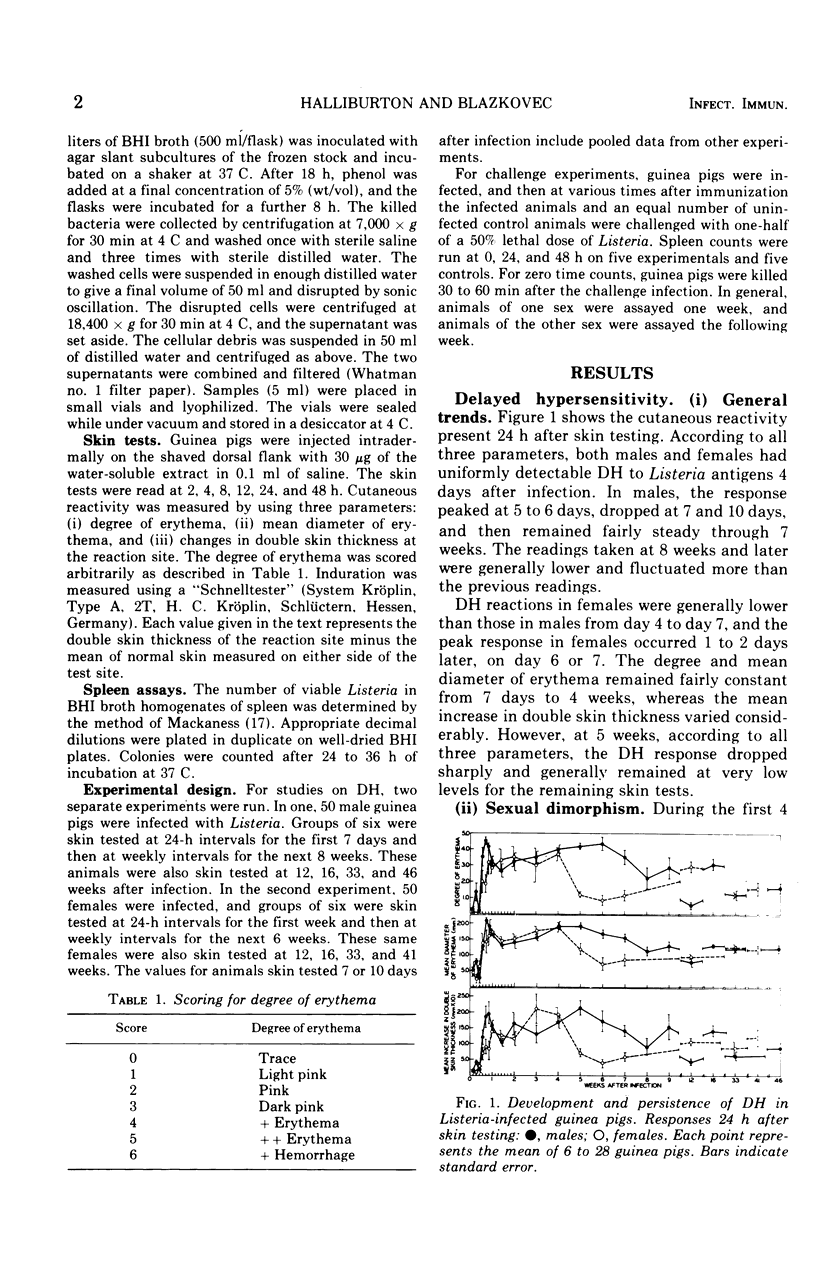
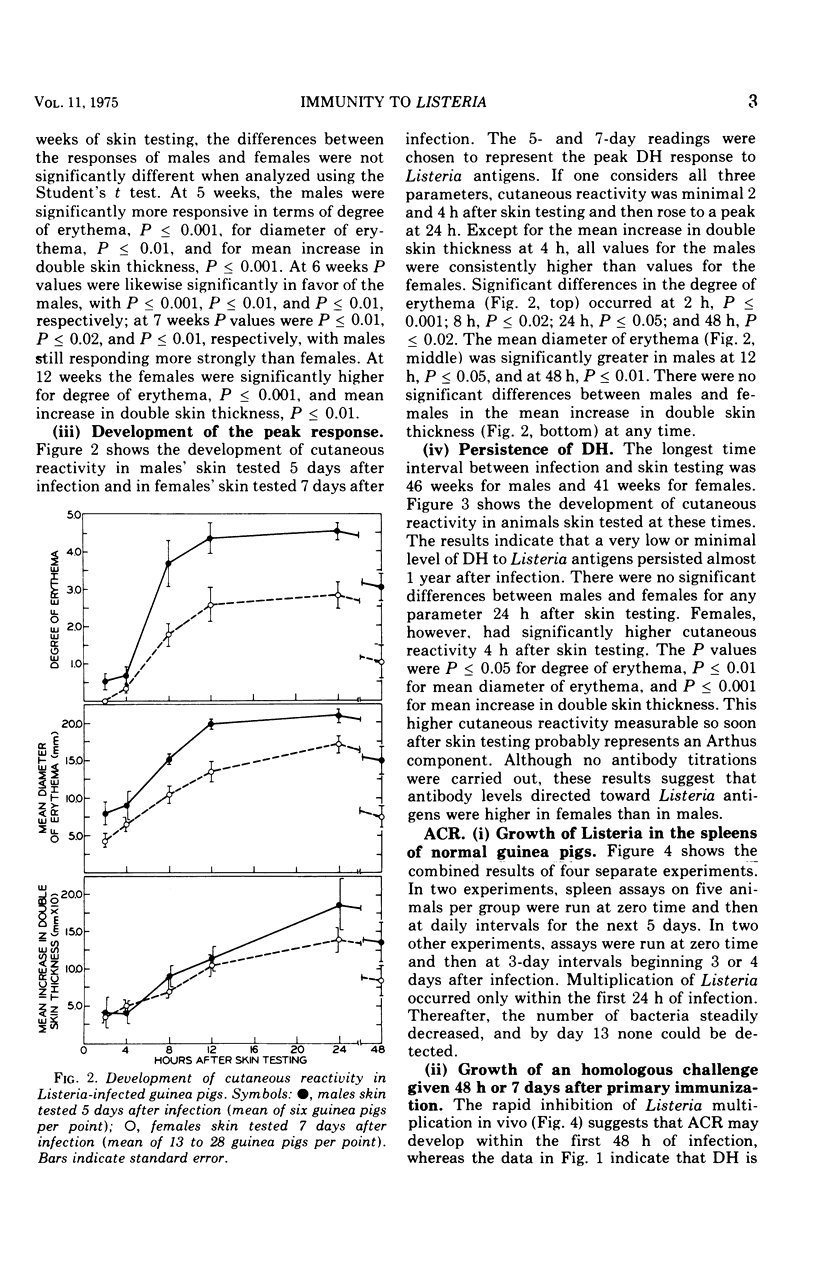
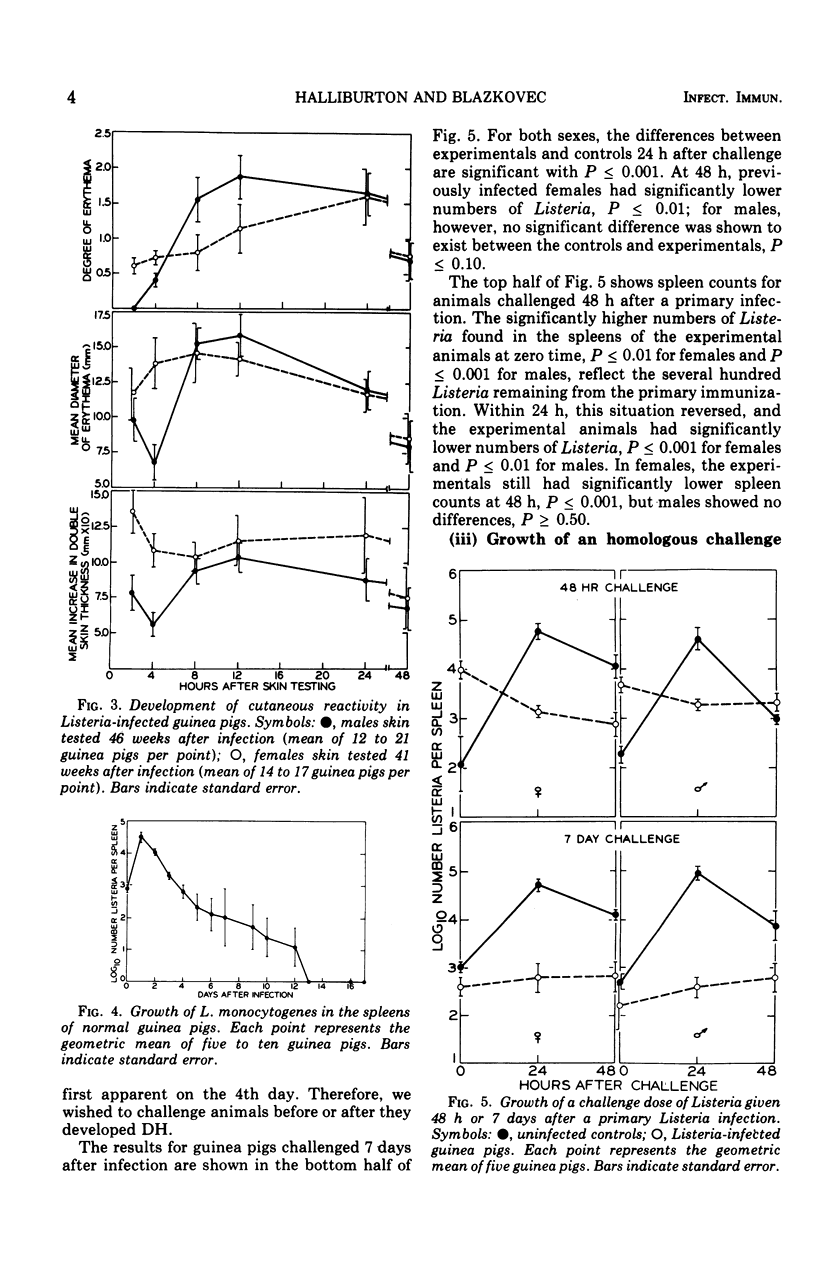
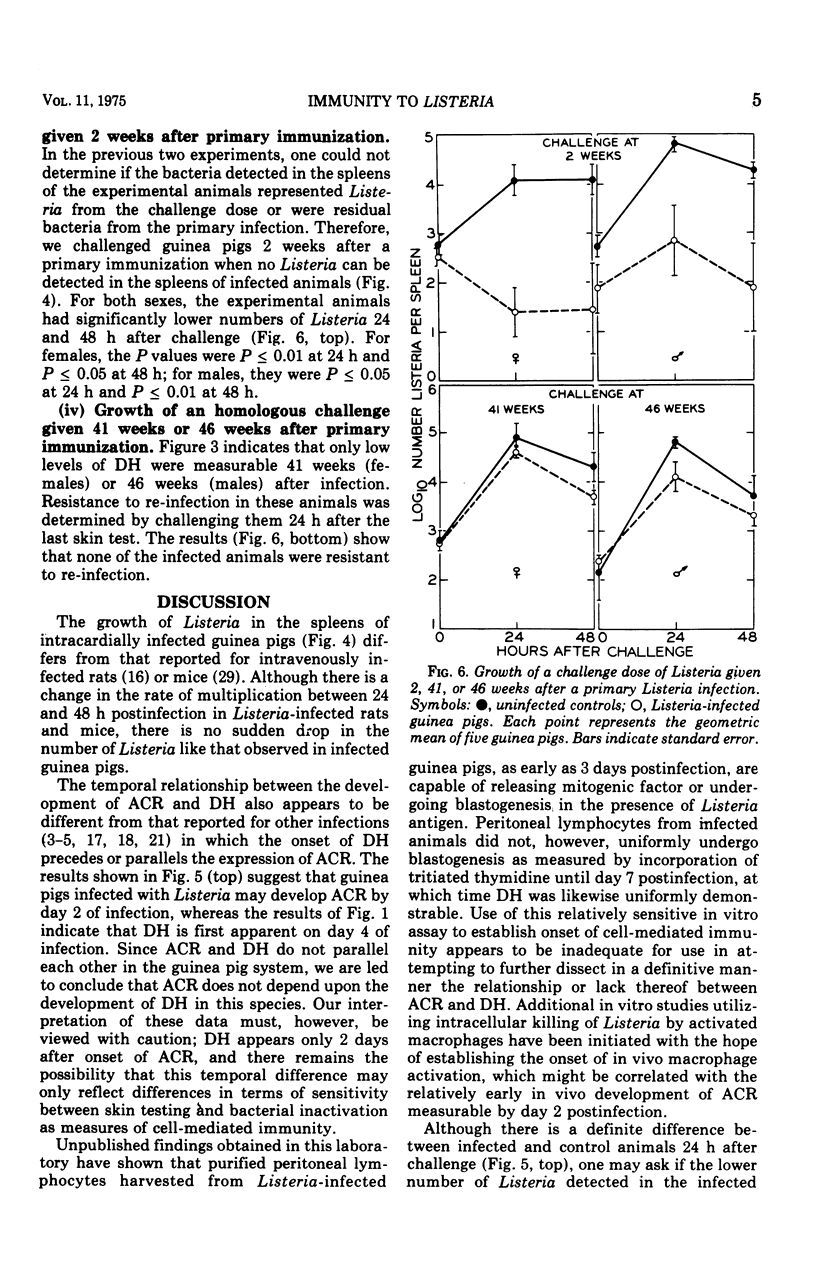
Randomly bred pigs of both sexes were injected intracardially with one-half of a 50% lethal dose of Listeria monocytogenes. When infected animals were skin tested with 30 mug of a water-soluble extract of sonically disrupted Listeria, both males and females had uniformly detectable levels of delayed hypersensitivity (DH) 4 days after infection. In males, cutaneous hypersensitivity to Listeria antigens reached a peak on day 5 or 6 of infection, and high levels of DH persisted through the 7th week. In females, DH reached a peak on day 6 or 7, remained at this level through the 4th week, and then dropped sharply. Cutaneous reactivity was usually higher for males than for females, and differences between the sexes were statistically significant 5, 6, and 7 weeks after infection. Low levels of DH were still present 41 weeks (females) or 46 weeks (males) after infection. Assays to determine the number of viable Listeria present in spleen homogenates indicated that bacterial multiplication occurred only during the first 24 hours of infection. The number of Listeria declined steadily thereafter, and by day 13 no bacteria could be recovered from the spleens of infected animals. Spleen assays indicated that Listeria-infected animals of both sexes were resistant to a small challenge dose of Listeria given 48 hours, 7 days, or 2 weeks after the primary infection. Resistance to re-infection was absent in females challenged at 41 weeks and in males challenged at 46 weeks.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B., Blanden R. V. Infection-immunity in experimental salmonellosis. J Exp Med. 1966 Oct 1;124(4):601–619. doi: 10.1084/jem.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. Delayed hypersensitivity and arthus reactivity in relation to host resistance in salmonella-infected mice. J Immunol. 1968 Nov;101(5):830–845. [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. II. Effect of adjuvant on the allergenicity and immunogenicity of heat-killed tubercle bacilli. Cell Immunol. 1970 Sep;1(3):266–275. doi: 10.1016/0008-8749(70)90048-1. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Mechanisms in antimicrobial immunity. J Reticuloendothel Soc. 1971 Jul;10(1):58–99. [PubMed] [Google Scholar]
- David J. R., David R. R. Cellular hypersensitivity and immunity. Inhibition of macrophage migration and the lymphocyte mediators. Prog Allergy. 1972;16:300–449. [PubMed] [Google Scholar]
- Dumonde D. C., Page D. A., Matthew M., Wolstencroft R. A. Role of lymphocyte activation products (LAP) in cell-mediated immunity. I. Preparation and partial purification of guinea-pig LAP. Clin Exp Immunol. 1972 Jan;10(1):25–47. [PMC free article] [PubMed] [Google Scholar]
- Halliburton B. L., Hinsdill R. D. Recall of acquired cellular resistance in mice by antigens from killed Brucella. Infect Immun. 1972 Jan;5(1):42–47. doi: 10.1128/iai.5.1.42-47.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. I. Chemical and immunoelectrophoretic characterization. J Bacteriol. 1967 Feb;93(2):544–549. doi: 10.1128/jb.93.2.544-549.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D., Mackaness G. B. The mediator of cellular immunity. II. Migration of immunologically committed lymphocytes into inflammatory exudates. J Exp Med. 1971 Feb 1;133(2):400–409. doi: 10.1084/jem.133.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The immunology of antituberculous immunity. Am Rev Respir Dis. 1968 Mar;97(3):337–344. doi: 10.1164/arrd.1968.97.3.337. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The relationship of delayed hypersensitivity to acquired cellular resistance. Br Med Bull. 1967 Jan;23(1):52–54. doi: 10.1093/oxfordjournals.bmb.a070516. [DOI] [PubMed] [Google Scholar]
- McGregor D. D., Hahn H. H., Mackaness G. B. The mediator of cellular immunity. V. Development of cellular resistance to infection in thymectomized irradiated rats. Cell Immunol. 1973 Feb;6(2):186–199. doi: 10.1016/0008-8749(73)90021-x. [DOI] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T., Mackaness G. B. The mediator of cellular immunity. I. The life-span and circulation dynamics of the immunologically committed lymphocyte. J Exp Med. 1971 Feb 1;133(2):389–399. doi: 10.1084/jem.133.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T. The mediator of cellular immunity. IV. Cooperation between lymphocytes and mononuclear phagocytes. Cell Immunol. 1971 Aug;2(4):317–325. doi: 10.1016/0008-8749(71)90066-9. [DOI] [PubMed] [Google Scholar]
- Neiburger R. G., Youmans G. P., Youmans A. S. Relationship between tuberculin hypersensitivity and cellular immunity to infection in mice vaccinated with viable attenuated Mycobacterial cells or with Mycobacterial ribonucleic acid preparations. Infect Immun. 1973 Jul;8(1):42–47. doi: 10.1128/iai.8.1.42-47.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The action of cortisone acetate on cell-mediated immunity to infection: histogenesis of the lymphoid cell response and selective elimination of committed lymphocytes. Cell Immunol. 1972 Mar;3(3):501–515. doi: 10.1016/0008-8749(72)90255-9. [DOI] [PubMed] [Google Scholar]
- Tolderlund K., Bentzon M. W., Bunch-Christensen K., Mackeprang B., Guld J., Waaler H. BCG-induced allergy and immunity in guinea-pigs during the first year after vaccination. Bull World Health Organ. 1967;36(5):747–758. [PMC free article] [PubMed] [Google Scholar]
- Tolderlund K., Bunch-Christensen K., Guld J. Duration of allergy and immunity in BCG-vaccinated guinea pigs. A five-year study. Bull World Health Organ. 1967;36(5):759–769. [PMC free article] [PubMed] [Google Scholar]
- Tripathy S. P., Mackaness G. B. The effect of cytotoxic agents on the primary immune response to Listeria monocytogenes. J Exp Med. 1969 Jul 1;130(1):1–16. doi: 10.1084/jem.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]



