Summary
Dilated cardiomyopathy (DCM) is a progressive heart disease characterized by left ventricular dilation and contractile dysfunction. Although many candidate genes have been identified using mouse models, few of them have been shown to be associated with DCM in humans. Germline depletion of Ncoa6, a nuclear hormone receptor coactivator, leads to embryonic lethality including heart defects. However, it is unclear whether Ncoa6 mutations cause heart diseases in adults. Here, we report that two independent mouse models of NCOA6 dysfunction develop severe DCM with decreased mitochondrial number and impaired mitochondrial function and reduced levels of both activity and target gene expression of peroxisome proliferator-activated receptor-δ (PPARδ), an NCOA6 target critical for normal heart function. Sequencing of NCOA6 coding regions revealed three independent non-synonymous mutations present in 5 of 50 (10%) idiopathic DCM (iDCM) patients. These data suggest that malfunction of NCOA6 can cause DCM in humans.
Introduction
Dilated cardiomyopathy (DCM), characterized by cardiac enlargement and systolic dysfunction, is the most common form of cardiomyopathy, accounting for up to 30–40% of all heart failure cases (Towbin and Bowles, 2002). Although DCM has diverse causes, approximately half of cases are idiopathic (iDCM). Clinical investigations have shown that approximately 40% of iDCM patients exhibit the patterns of autosomal-dominant inheritance, implying that genes play an important role in DCM pathogenesis (Hershberger et al., 2010). Hence, the search for novel susceptibility loci is a major challenge in DCM research.
Nuclear receptor coactivator 6 (or NCOA6, also known as ASC-2, NRC, TRBP, PRIP, and RAP250) is ubiquitously expressed in diverse tissues including the heart and stimulates the transcriptional activity of various transcription factors and nuclear hormone receptors (NRs) by coordinating with cofactors (Mahajan and Samuels, 2008). NCOA6 possesses two LXXLL motifs required for interaction with NRs. The first LXXLL motif of NCOA6 can interact with almost all liganded NRs while more restricted NRs can bind to second LXXLL motif. In mice, germ-line mutation of Ncoa6 induces growth retardation and developmental defects in the heart, liver, brain, and placenta resulting in embryonic lethality between embryonic days 8.5-12.5 (Antonson et al., 2003; Kuang et al., 2002; Mahajan et al., 2004; Zhu et al., 2003). Overexpression of the dominant negative version of the gene (DN1) incompletely suppresses endogenous NCOA6 activity, allowing mice to survive, but developed several defects including cataract, atrial thrombosis, and hypertrophy at the later stage (Kim et al., 2002).
PPARs, peroxisome proliferator-activated receptors, are the members of the NR superfamily that regulate fatty acid metabolism and mitochondrial function (Barger and Kelly, 2000; Wang et al., 2010). The transcriptional activity of PPARs is elevated or induced upon ligand binding and is regulated further by coactivators and corepressors (Guan et al., 2005; Viswakarma et al., 2010). PPARδ (also called PPARβ) is expressed at high levels in the heart and is essential for normal heart function (Cheng et al., 2004). In a mouse model, cardiomyocyte-specific PPARδ knockout leads to DCM, however, whether an association between PPARδ polymorphisms and DCM exists in humans remains unknown.
Results
Overexpression of DN1 Causes DCM in Mice
Germ-line Ncoa6 deficiency results in embryonic lethality with several developmental defects (Antonson et al., 2003; Kuang et al., 2002; Mahajan et al., 2004; Zhu et al., 2003). We produced transgenic (Tg) mouse lines overexpressing DN1 (849-929 residues containing an N-terminal LXXLL-1 motif of NCOA6, Figure S1A and S1B) that are able to reach adulthood although these animals are prone to diverse defects (Kim et al., 2002). Among nine DN1-Tg lines, four founder mice (#71, #84, #87, and #104) expressed DN1 in their hearts (representative expression of DN1 is shown for founder #87 in Figure S1C). The progenies of founder #87 died prematurely at 20-week-old (Figure S1D). Anatomical examination revealed cardiac enlargement and increased heart-to-body weight ratios with frequent pericardial effusion (Figure S1E-S1H). The progenies of founders #71, #84, and #104 exhibited similar phenotypes, which were absent in wild type (WT) and DN1/m-Tg mice in which the LXXLL-1 motif had been mutated to LXXAA (Kim et al., 2002). These phenotypes closely resembled those of dilated cardiomyopathy (DCM) (Towbin and Bowles, 2002). Given that DN1 would be expected to compete with the binding of other coactivators for liganded NRs and multi-organ defects were found in DN1-Tg mice, it is unclear whether DN1 specifically blocks the activity of endogenous NCOA6 and causes DCM through cardiac cells only. For instance, we found multiple phenotypes such as fatty liver, thymic and spleen atrophy, lung hypoplasia, kidney hyperplasia, and brain defects in DN1-Tg mice (Kim et al., 2002).
Cardiomyocyte-specific Ablation of NCOA6 Leads to DCM in Mice
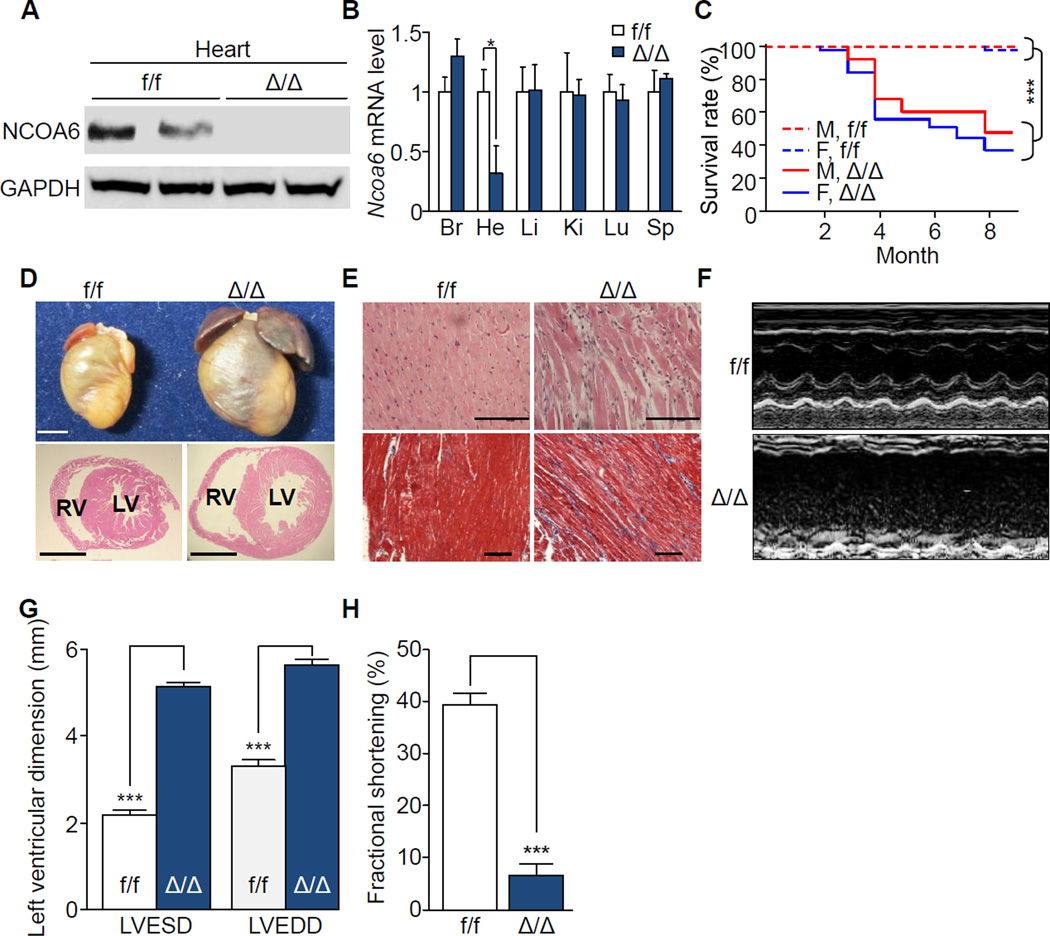
To confirm the heart-specific function of NCOA6, we generated cardiomyocyte-specific Ncoa6-deficient (Δ/Δ) mice by employing floxed Ncoa6 alleles (f/f) and a Cre recombinase transgene specific for differentiated cardiomyocytes under the control of the α-myosin heavy chain promoter (α-MHC-Cre-Tg) (Agah et al., 1997; Zhu et al., 2003). Compared to control mice (f/f), NCOA6 mRNA and protein levels were significantly decreased in a heart-restrictive manner in Δ/Δ mice (Figure 1A and 1B). These mice demonstrated Mendelian inheritance and showed no significant cardiac developmental defects. Consistent with the phenotypes observed in DN1-Tg mice, Δ/Δ mice showed premature death, increased heart weight-to-body weight ratios, and cardiac dilatation (Figure 1C and 1D, and S2A–S2D). The increased heart weight was caused by atrial thrombi, whereas there was no alteration in the weight of the ventricle (Figure S2C). Histological examination of the hearts of Δ/Δ mice revealed common features of DCM, including enlarged ventricular cavities with thin walls and reactive myocardial fibrosis (Figure 1D and 1E, and S2D). Notably, Ncoa6 heterozygous knockout mice (Δ/+) also exhibited the characteristics of DCM with late onset (Figure S2E-S2I), indicating that Ncoa6 haploinsufficiency is sufficient to precipitate DCM in mice. Consistent with the previous reports (Agah et al., 1997; Cheng et al., 2004), we could not find any significant differences of cardiac functions in α-MHC-Cre-Tg mice, compared to the f/f mice (data not shown). These results demonstrate that loss or insufficiency of NCOA6 function directly causes DCM.
Figure 1. Premature death and impaired cardiac function in cardiomyocyte-specific Ncoa6 knockout mice.
(A) Western blot analysis of NCOA6 in the hearts of one-month-old f/f and Δ/Δ male mice. GAPDH was used as a loading control.
(B) RT-qPCR analysis of Ncoa6 mRNA in various organs of one-month-old f/f and Δ/Δ male mice. Br, Brain; He, Heart; Li, Liver; Ki, Kidney; Lu, Lung; Sp, Spleen. Ncoa6 mRNA levels were normalized to those of Gapdh. Graphs show means ± s.d.; *P < 0.05.
(C) Kaplan-Meier survival curves of f/f (male, n = 34; female, n = 34) and Δ/Δ (male, n = 21; female, n = 39) mice. Genders and genotypes are labeled inside the plot. M, male; F, female. ***P < 0.001.
(D) Gross morphology (top) and histological examinations (bottom, H&E) of hearts from 4-month-old female f/f and Δ/Δ mice. LV, left ventricle; RV, right ventricle. Scale bars, 2.5 mm.
(E) H&E (top) and Masson’s trichrome staining (bottom) of left ventricles from 4-month-old female mice. Scale bars, 100 ìm.
(F–H) Representative profiles of M-mode echocardiographic analyses (F) and quantitative representations of LVESD and LVEDD (G) and percent fractional shortening (H) of f/f (male, n = 3; female, n = 4), and Δ/Δ (male, n = 3; female, n = 3) mice. Graphs show means ± s.d.; ***P < 0.001. See also Figure S1 and S2.
Comparative transthoracic echocardiographic analysis revealed severe dilatation of the left ventricle and decreased contractility without a significant alteration in heart rate in Δ/Δ mice at 4 months of age (Figure 1F). Left ventricular end-systolic dimensions and end-diastolic dimensions were enlarged 1.72-fold and 2.35-fold, respectively, compared to control mice (Figure 1G). Ejection fraction (EF) and fractional shortening, two direct measures of cardiac contractile function, were profoundly diminished compared to control mice (Figure 1H, and S2J). Impaired cardiac function was also evident in Δ/+ and DN1-Tg mice (Figure S1I and S1J, S2K and S2L). Increased expression of cardiac hypertrophy markers, including atrial natriuretic peptide (Anp) and α- to β-MHC isoform switching (Reiser et al., 2001), were found in 12- and 20-week-old, but not 4-week-old, Δ/Δ mice (Figure S2M–S2O). Furthermore, the heart of 12-week-old mice displayed hypertrophic phenotype (Figure S2P), suggesting that concentric hypertrophy transiently proceeds prior to the onset of DCM phenotype. Thus, Ncoa6 defects progressively compromise overall cardiac function in mice.
Impaired Cardiac Ultrastructure and Mitochondrial Function in NCOA6-deficient mice
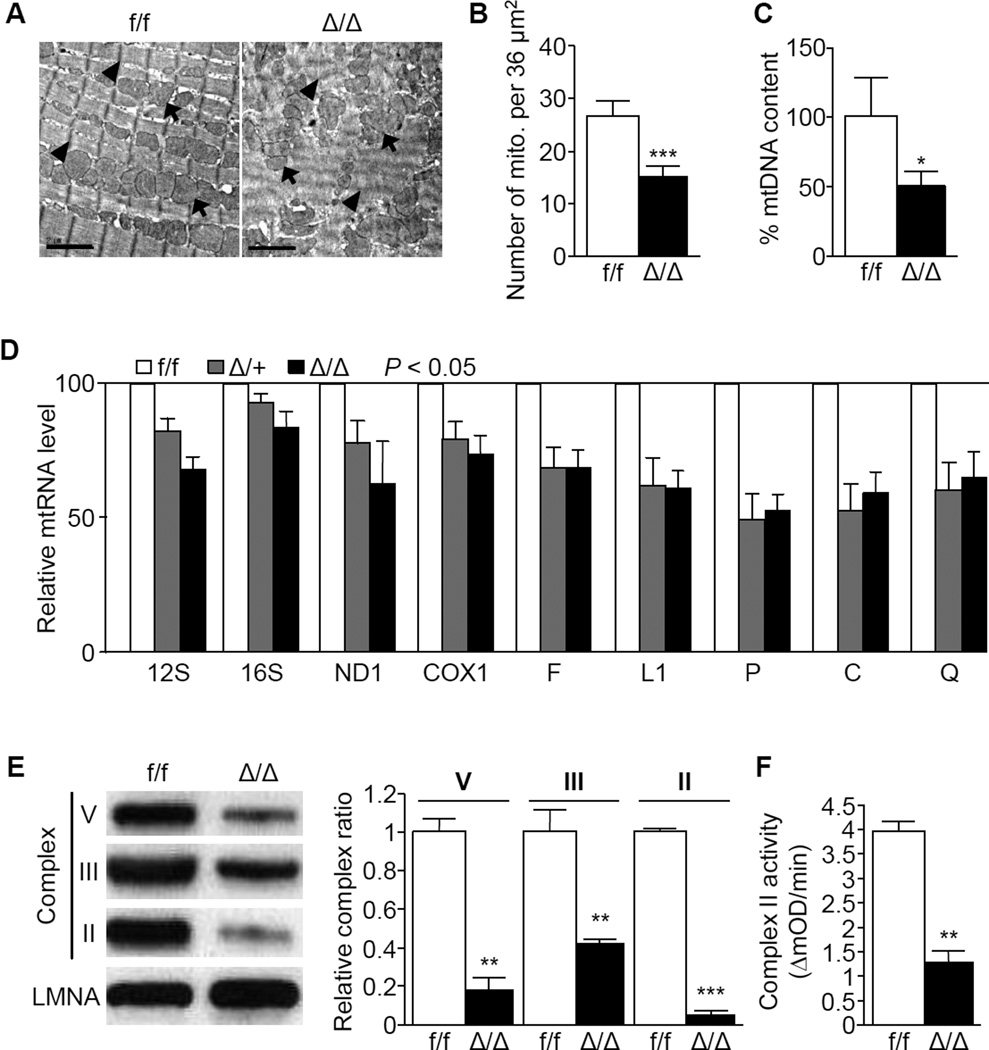
To examine potential mechanisms in the development of DCM, ultra-structural examination of cardiac tissues with transmission electron microscopy (TEM) was conducted and showed disarray of sarcomeres and mitochondria in the hearts of Δ/Δ mice (Figure 2A). The number of mitochondria was significantly decreased in the hearts of Δ/Δ mice (Figure 2B). In addition, reduced mitochondrial content was confirmed by measuring DNA, RNA, respiratory chain protein levels, and impaired activity of mitochondrial complex II was evident in Δ/Δ and Δ/+ mice (Figure 2C–2F). These results suggest that NCOA6 is essential for the maintenance of normal mitochondrial function in cardiomyocytes.
Figure 2. Cardiomyocyte-specific Ncoa6 deficiency impairs cardiac ultrastructures and mitochondrial function in mice.
(A) TEM analysis of left ventricles from 3-month-old f/f and Δ/Δ female mice. Scale bars, 2 ìm; arrowheads, Z-discs; arrows, mitochondria.
(B and C) Quantification of the number of mitochondria per 36 ìm2 calculated from three independent TEM images (B) and mtDNA contents (C) by Southern blot analyses in the hearts of 3- month-old male and female mouse ventricles (n = 3 for each genotype). Graphs show means ± s.d.; *P < 0.05; ***P < 0.001.
(D) Analyses of mitochondrial rRNAs (12S and 16S), mRNAs (ND1 and COXI), and tRNAs (F, L1, P, C, and Q) by Northern blot. P < 0.05 for all mtRNA. Left ventricles of 3- to 5-month-old male and female mice (n = 3 for each genotype) were used.
(E) Analysis of mitochondrial respiratory chain complexes II, III, and V by Western blot (left) and its quantitative ratios (right) of each complex to Lamin A (LMNA). Left ventricles of 3- to 5-month-old male and female mice (n = 3 for each genotype) were used. LMNA was used as a loading control. Graphs show means ± s.d.; **P < 0.01; ***P < 0.001.
(F) Analysis of mitochondrial complex II activity in 2- to 3-month-old mice (male, n = 3; female, n = 4 for each genotype). mOD, mitochondrial optical density. Graphs show means ± s.d.; **P < 0.01.
NCOA6 Deficiency Impairs PPARδ Activity in Cardiomyocytes
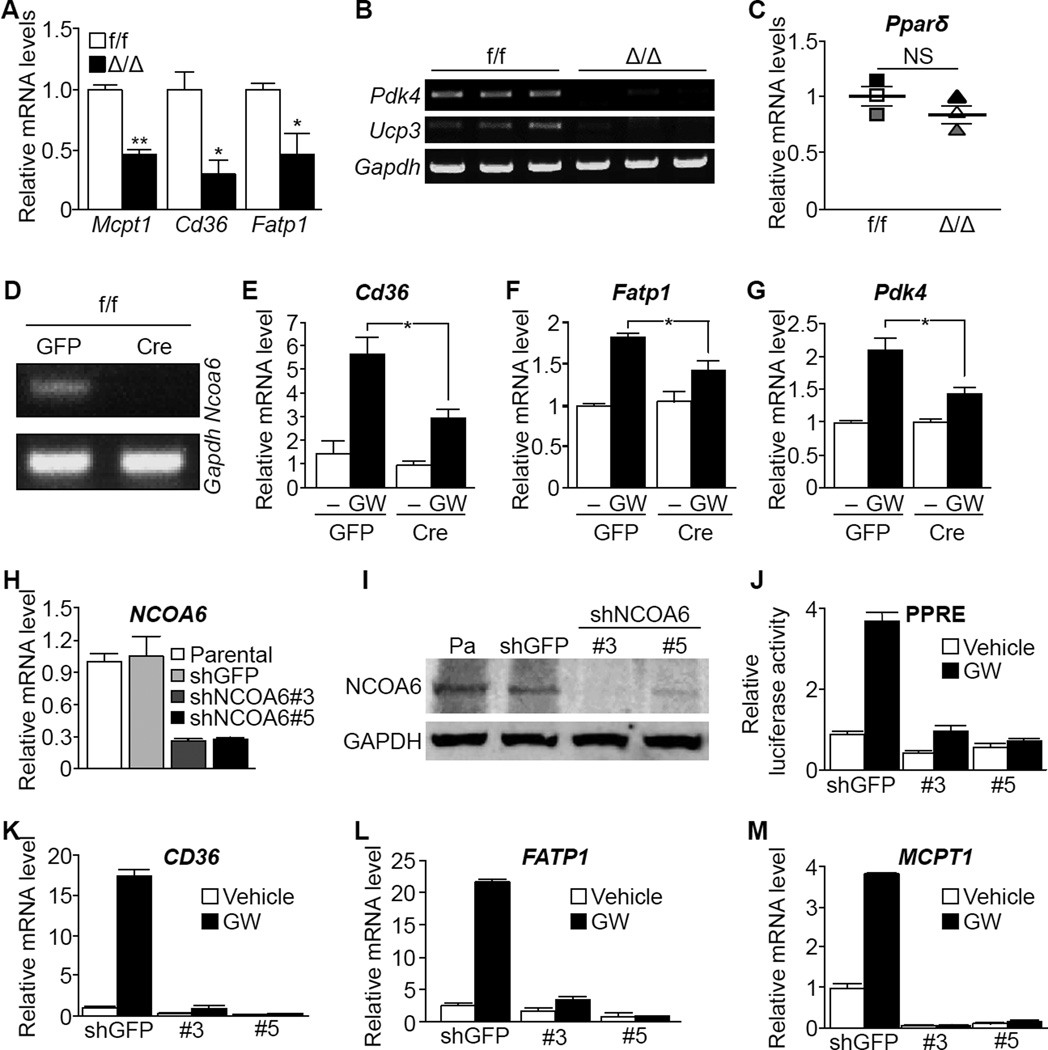
Since PPARδ, one of a major target of NCOA6, is crucial for mitochondrial biogenesis and cardiomyocyte-specific knockout of Pparδ causes spontaneous DCM in mice (Cheng et al., 2004; Wang et al., 2010), we hypothesized that depletion of NCOA6 alters PPARδ activity. To further explore the role of NCOA6 in PPARδ-mediated transactivation, we measured transcript levels of PPARδ targets in the mouse heart. Levels of key molecules involved in fatty acid oxidation (Cheng et al., 2004), such as muscle-type carnitine palmitoyltransferase-1 (Mcpt-1), Cd36, Fatp1, pyruvate dehydrogenase lipoamide kinase, isozyme 4 (Pdk4), and uncoupling protein 3 (Ucp3), were considerably decreased even though no significant difference in Pparδ transcript levels was observed between Δ/Δ and f/f hearts (Figure 3A-3C). Similar to cardiomyocyte-specific knockout of Pparδ (Cheng et al., 2004), Δ/Δ mouse showed cardiac lipid accumulation (Figure S3A). Collectively, these data suggest that NCOA6 deficiency results in dysfunctional activity of PPARδ and fatty acid metabolism. Interestingly, increased the expressions of estrogen receptor alpha (ER) and its target genes were found in the Δ/Δ. mouse hearts (Figure S3B-S3D).
Figure 3. NCOA6 is required for normal transcriptional activity of PPARδ.
(A and B) Transcript levels of PPARδ targets in the left ventricles of f/f (white; male, n = 1; female, n = 2) and Δ/Δ (black; male, n = 1; female, n = 2) mice. *P < 0.05; **P < 0.01.
(C) mRNA levels of PPARδ in the hearts of f/f and Δ/Δ mice (n = 3 per each genotype). Gapdh was used as a loading control. NS, not statistically significant.
(D) Semi-quantitative PCR analyses of Ncoa6 in primary cardiomyocytes, transduced with lentivirus encoding GFP or Cre constructs, isolated from the f/f mouse heart.
(E–G) Transcript levels of Cd36 (E), Fatp1 (F), and Pdk4 (G) in f/f primary cardiomyocytes containing GFP or Cre constructs. Treatment with GW501516 lasted 48 h. *P < 0.05.
(H-M) Transcript and protein levels of NCOA6 were examined by RT-qPCR (H) and Western blot (I). Parental, no transfection; shGFP, shRNA against GFP; shNCOA6 #3 and #5, independent shRNA constructs against NCOA6. (J) Relative PPRE- luciferase activity. Treatment with GW501516 lasted 24 h. (K-M) Transcript levels of CD36 (K), FATP1 (L), and MCPT-1 (M) were measured by RT-qPCR analyses after treatment with GW501516 for 48 h. Graphs show means ± s.d. See also Figure S3.
We also found that Cre-mediated Ncoa6 deficiency attenuated the induction of PPARδ targets by the selective PPARδ agonist GW501516 (GW) in neonatal primary cardiomyocytes (Figure 3D–3G). In addition, in the AC16 human cardiomyocyte cell line, shRNA-mediated NCOA6 knockdown (Figure 3H and 3I) reduced GW-induced activation of PPARδ and its target genes (Figure 3J–3M), indicating that PPARδ requires NCOA6 for optimal transactivation of its target genes.
Non-synonymous Mutations of NCOA6 in iDCM Patients
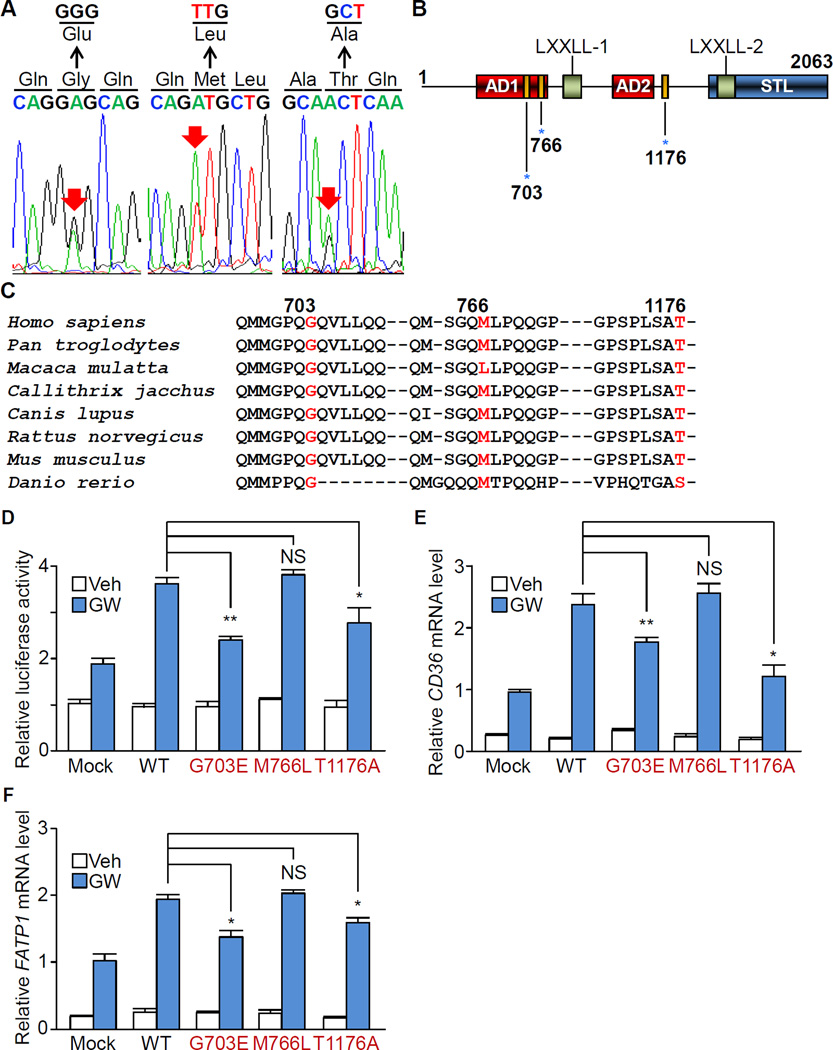
To find the relevance of NCOA6-mediated DCM pathogenesis to human iDCM, we screened the entire NCOA6 coding sequence in iDCM patients, and identified four non-synonymous substitutions in 8 out of 50 iDCM patients (c.1038C > G, P239R; c.2430G > A, G703E; c.2618A > T, M766L; and c.3848A > G, T1176A). P239R was found in both non-DCM and iDCM patients, indicating that this is not a DCM-causing mutation. Rather, it appears to be an SNP that is specific to the Korean population. This SNP is not found among american populations (Table S1) (Fu et al., 2013). G703E and M766L mutations were found in one patient each and three patients were harboring the T1176A mutation (Total 10%, Table S1). These three mutations were absent in 403 Korean healthy subjects and only one subject showed T1176A mutation among the 6,502 American populations (Figure 4A and 4B; Table S1) (Fu et al., 2013), suggesting that three variants are potent DCM-related mutations in humans. G703E and T1176A were located in regions of the NCOA6 gene that are highly conserved across species (Figure 4C) while Macaca mulatta encodes leucine rather than methionine at human M766 position, suggesting that M766L substitution might not be harmful. All five patients possessed one wild-type allele.
Figure 4. Non-synonymous mutations of NCOA6 in human iDCM patients result in impaired PPARδ activity.
(A) DNA sequences of NCOA6 exons that were obtained from iDCM patients. Red arrows indicate mutation sites.
(B) Schematic representation of the human NCOA6 protein. AD, activation domain; STL, serine, threonine and leucine rich region; blue asterisks, mutation sites.
(C) NCOA6 amino acid sequence alignment across 8 species. Numerals indicate the positions of the amino acid mutations (red) in patients.
(D–F) Relative luciferase activity of PPRE (D) and gene expression levels of CD36 (E) and FATP1 (F) in WT and mutant NCOA6-transfected AC16 cells with or without treatment with GW501516 for 48 h. Graphs show the means of triplicate experiments ± s.d.; NS, not statistically significant; *P < 0.05; **P < 0.01. See also Figure S4 and Table S1.
The effects of each NCOA6 mutation on the protein conformation were estimated with the protein structure prediction softwares (Buchan et al., 2010; Cheng et al., 2005). The G703E substitution appeared to induce remarkable structural deformations by transforming coiled-coil and β- sheet structures into an α-helix (Figure S4A). Furthermore, in silico evaluation with PolyPhen-2 and SIFT softwares showed a potentially deleterious effect of G703E on NCOA6 function (PolyPhen score of 0.999; SIFT score of 0.006) (Adzhubei et al., 2010; Ng and Henikoff, 2003). Although M766L and T1176A substitutions were predicted to induce no structural change and benign (Polyphen-2 score of 0 in both, and SIFT score of 0.039 and 0.289 in M766L and T1176A, respectively), we could not exclude the possibility that M766L and T1176A substitutions are also deleterious for NCOA6 functions. T1176A transcript level was similar to those of the WT and other mutant NCOA6, but its protein level was significantly low (Figure S4B and S4C) though its mechanism is inconclusive.
As depletion of Ncoa6 leads to decreased transcriptional activity of PPARδ in mice, we examined whether NCOA6 variants alters the activity PPARδ. In fact, overexpression of G703E and T1176A significantly suppressed the activation of a PPAR and its targets including CD36 and FATP1 in the AC16 parental and NCOA6 knockdown cells upon the treatment with GW (Figure 4D–4F, and S4D). However, no remarkable difference was observed between WT and M766L mutant NCOA6, implying that M766L may not be a pathogenic mutation for DCM though further investigation is required to clarify its physiological effect. Taken together, these data suggest that two substitutions (G703E and T1176A, if M766L is not) perturb NCOA6-PPAR signaling via distinct mechanisms: G703E causes structural and functional defects, whereas T1176A reduces the expression of PPARδ targets possibly by destabilizing NCOA6 proteins.
Discussion
Ncoa6 deficiency reduces the number of mitochondria in cardiac tissue. Cardiac abnormality of mitochondrial function is closely associated with DCM pathogenesis (Marin-Garcia et al., 1995). In addition, impaired expression of Ncoa6 has been found in muscle fibers with a dysfunctional electron transport system (Herbst et al., 2013). Thus, Ncoa6 mutations appear to induce loss of or suppress biogenesis of mitochondria in the heart. NCOA6 is a critical component of the steady-state ASC-2 complex (ASCOM), which is essential for proper NR transactivation (Mahajan and Samuels, 2008). Because NCOA6 stimulates NR transactivation activity, it is reasonable to expect altered downstream signaling of NRs to play a role in the mechanism for DCM. Here, we provide the potent evidence linking abnormal NR signaling to human DCM pathogenesis. Consistent with previous studies (Mahajan and Samuels, 2008), our results indicate that depletion of NCOA6 expression results in remarkably reduced PPARδ activity both in vitro and in vivo.
Germline-loss of Ncoa6 leads to embryonic lethality with multiple organ dysfunction including the placenta (Antonson et al., 2003; Kuang et al., 2002; Zhu et al., 2003). The homozygous null mutation of Ncoa6 precipitates cardiac developmental abnormalities at E10.5, suggesting that NCOA6 might still be essential for early cardiac development before this stage (Kuang et al., 2002). Since αMHC-Cre starts to function at E14.5, αMHC-Cre-mediated depletion of Ncoa6 might not be sufficient for development of early cardiac developmental defects.
It appears that NCOA6 mutations may disrupt diverse NR transactivation via an effect on ASCOM. In fact, another observation suggests that NR pathways other than PPARδ may be affected by NCOA6 mutations (Mahajan and Samuels, 2008). Our findings imply that additional NRs might be influenced by NCOA6 impairment. In support of these findings, it has been shown that mutations in mixed-lineage leukemia 2/4 (MLL2/4) and WD repeat domain 5 (WDR5), which are also components of ASCOM, are likely to be involved in congenital heart disease (Zaidi et al., 2013). This evidence indicates that Ncoa6 deficiency significantly alters the activity of the NRs, resulting in DCM.
A recent study in mice revealed that NCOA6 promotes ubiquitination-mediated degradation of ERα while Ncoa6 deficiency causes ERα accumulation in uterine stromal cells during the pre- implantation period (Kawagoe et al., 2012). Consistent with these findings, we observed that the expression of ERα and its target genes was significantly increased in Ncoa6 Δ/Δ mice prior to DCM pathogenesis. Interestingly, it is common for ERα expression to increase in end-stage DCM patients (Mahmoodzadeh et al., 2006). Based on these lines of evidence, we hypothesized that the expression and/or function of NCOA6 may be suppressed prior to the onset of clinical DCM manifestations, possibly accelerating the progression of the disease. Taken together, our findings will manifest stepping forward to more accurate diagnosis of human DCM.
Experimental procedures
Animals
All animal experiments were performed in accordance with Korean Food and Drug Administration guidelines. Protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Yonsei University (YLARC 2008-0014). Mice that had been backcrossed to the FVB/NTac strain for at least nine generations were used and maintained in the specific pathogen-free facility of the Yonsei Laboratory Animal Research Center (YLARC).
Primary cardiomyocyte culture
Primary cardiomyocytes were isolated as previously described (Crone et al., 2002).
Human Genomic DNA Mutation Analysis
Exon scanning of all NCOA6 (NM_014071) coding regions and splice junction sites was performed by direct sequencing.
Echocardiogram
Echocardiography was performed with the echocardiographic system (Vivid 7, GE Medical Systems, USA) equipped with a 12-MHz transducer. Mice with shaved chests were anesthetized with isoflurane (Hana Pharm. Co., Ltd.) and placed on a platform. End-systolic and end- diastolic dimensions were measured using M-mode echocardiogram imaging which were obtained at the papillary muscle level for the measurement of LV wall thickness and LV dimensions. We captured these images over more than 10 cardiac cycles, and the data shown are averages from at least 3 cardiac cycles per image acquisition. These procedures were performed at least three times and average values were determined for each mouse.
Statistical Analyses
Statistical significance was determined using the two-tailed Student’s t-test. Data were analyzed with GraphPad Prism (GraphPad Software). P values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by National Research Foundation (NRF) funded by Ministry of Education, Science and Technology (MEST) of the Korean government (2009-0081177, 2010-0020878, 2012R1A1A2009607); Bio-industry Technology Development Program, MAFRA (311054232- HD1102), Korea; a Korea Healthcare Technology R&D Project from the Ministry of Health & Welfare (A085136); a grant (14182MFDS978) from Korean Ministry of Food and Drug Safety in 2014; by Canadian Institutes of Health Research (CIHR, MOP-125933), Canada and National Research Foundation of Korea (GRN-2013S1A2A2035348). C. C. is Chercheur-Boursier Junior of Fonds de recherché du Québec-Santé (FRQS) and CIHR New Investigator; and J.W. L. is supported by NIH (DK064678).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
J.I.R., C.C., and J.L. performed in vitro and in vivo experiments; J.O., D.K.K., B.S.L., and S.M.K. performed cardiac phenotyping; C.B.P. performed mitochondria-related experiments; J.E.L., and J.H.L. analyzed human mutations; Y.S.G. performed TEM analysis; J.I.R., C.C., Y.H.S., and H.W.L. wrote the manuscript. H.W.L. designed and supervised the project.
We have no conflicts of interest.
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nature methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac- restricted, site-specific rearrangement in adult ventricular muscle in vivo. The Journal of clinical investigation. 1997;100:169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonson P, Schuster GU, Wang L, Rozell B, Holter E, Flodby P, Treuter E, Holmgren L, Gustafsson JA. Inactivation of the nuclear receptor coactivator RAP250 in mice results in placental vascular dysfunction. Molecular and cellular biology. 2003;23:1260–1268. doi: 10.1128/MCB.23.4.1260-1268.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends in cardiovascular medicine. 2000;10:238–245. doi: 10.1016/s1050-1738(00)00077-3. [DOI] [PubMed] [Google Scholar]
- Buchan DW, Ward SM, Lobley AE, Nugent TC, Bryson K, Jones DT. Protein annotation and modelling servers at University College London. Nucleic acids research. 2010;38:W563–W568. doi: 10.1093/nar/gkq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Randall AZ, Sweredoski MJ, Baldi P. SCRATCH: a protein structure and structural feature prediction server. Nucleic acids research. 2005;33:W72–W76. doi: 10.1093/nar/gki396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nature medicine. 2004;10:1245–1250. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nature medicine. 2002;8:459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- Fu W, O'Connor TD, Jun G, Kang HM, Abecasis G, Leal SM, Gabriel S, Rieder MJ, Altshuler D, Shendure J, et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature. 2013;493:216–220. doi: 10.1038/nature11690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan HP, Ishizuka T, Chui PC, Lehrke M, Lazar MA. Corepressors selectively control the transcriptional activity of PPARgamma in adipocytes. Genes & development. 2005;19:453–461. doi: 10.1101/gad.1263305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst A, Johnson CJ, Hynes K, McKenzie D, Aiken JM. Mitochondrial biogenesis drives a vicious cycle of metabolic insufficiency and mitochondrial DNA deletion mutation accumulation in aged rat skeletal muscle fibers. PloS one. 2013;8:e59006. doi: 10.1371/journal.pone.0059006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger RE, Morales A, Siegfried JD. Clinical and genetic issues in dilated cardiomyopathy: a review for genetics professionals. Genetics in medicine : official journal of the American College of Medical Genetics. 2010;12:655–667. doi: 10.1097/GIM.0b013e3181f2481f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe J, Li Q, Mussi P, Liao L, Lydon JP, DeMayo FJ, Xu J. Nuclear receptor coactivator-6 attenuates uterine estrogen sensitivity to permit embryo implantation. Developmental cell. 2012;23:858–865. doi: 10.1016/j.devcel.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Cheong C, Sohn YC, Goo YH, Oh WJ, Park JH, Joe SY, Kang HS, Kim DK, Kee C, et al. Multiple developmental defects derived from impaired recruitment of ASC-2 to nuclear receptors in mice: implication for posterior lenticonus with cataract. Molecular and cellular biology. 2002;22:8409–8414. doi: 10.1128/MCB.22.24.8409-8414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang SQ, Liao L, Zhang H, Pereira FA, Yuan Y, DeMayo FJ, Ko L, Xu J. Deletion of the cancer-amplified coactivator AIB3 results in defective placentation and embryonic lethality. The Journal of biological chemistry. 2002;277:45356–45360. doi: 10.1074/jbc.C200509200. [DOI] [PubMed] [Google Scholar]
- Mahajan MA, Das S, Zhu H, Tomic-Canic M, Samuels HH. The nuclear hormone receptor coactivator NRC is a pleiotropic modulator affecting growth, development, apoptosis, reproduction, and wound repair. Molecular and cellular biology. 2004;24:4994–5004. doi: 10.1128/MCB.24.11.4994-5004.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan MA, Samuels HH. Nuclear receptor coactivator/coregulator NCoA6(NRC) is a pleiotropic coregulator involved in transcription, cell survival, growth and development. Nuclear receptor signaling. 2008;6:e002. doi: 10.1621/nrs.06002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler E, Huber O, Martus P, Weiske J, Pregla R, Hetzer R, Regitz-Zagrosek V. Estrogen receptor alpha up-regulation and redistribution in human heart failure. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:926–934. doi: 10.1096/fj.05-5148com. [DOI] [PubMed] [Google Scholar]
- Marin-Garcia J, Goldenthal MJ, Pierpont ME, Ananthakrishnan R. Impaired mitochondrial function in idiopathic dilated cardiomyopathy: biochemical and molecular analysis. Journal of cardiac failure. 1995;1:285–291. doi: 10.1016/1071-9164(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic acids research. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser PJ, Portman MA, Ning XH, Schomisch Moravec C. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. American journal of physiology Heart and circulatory physiology. 2001;280:H1814–H1820. doi: 10.1152/ajpheart.2001.280.4.H1814. [DOI] [PubMed] [Google Scholar]
- Towbin JA, Bowles NE. The failing heart. Nature. 2002;415:227–233. doi: 10.1038/415227a. [DOI] [PubMed] [Google Scholar]
- Viswakarma N, Jia Y, Bai L, Vluggens A, Borensztajn J, Xu J, Reddy JK. Coactivators in PPAR-Regulated Gene Expression. PPAR research 2010. 2010 doi: 10.1155/2010/250126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Liu J, Li Y, Wu S, Luo J, Yang H, Subbiah R, Chatham J, Zhelyabovska O, Yang Q. Peroxisome proliferator-activated receptor {delta} is an essential transcriptional regulator for mitochondrial protection and biogenesis in adult heart. Circulation research. 2010;106:911–919. doi: 10.1161/CIRCRESAHA.109.206185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, Overton JD, Romano-Adesman A, Bjornson RD, Breitbart RE, Brown KK, et al. De novo mutations in histone-modifying genes in congenital heart disease. Nature. 2013;498:220–223. doi: 10.1038/nature12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YJ, Crawford SE, Stellmach V, Dwivedi RS, Rao MS, Gonzalez FJ, Qi C, Reddy JK. Coactivator PRIP, the peroxisome proliferator-activated receptor-interacting protein, is a modulator of placental, cardiac, hepatic, and embryonic development. The Journal of biological chemistry. 2003;278:1986–1990. doi: 10.1074/jbc.C200634200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.