Abstract
Ziziphora tenuior L. (Lamiaceae) is an aromatic herb used for its medicinal values against fungi, bacteria. Micropropagation can be used for large-scale multiplication of essential oil producing plants thus avoiding an overexploitation of natural resources. This work aims to develop a reliable protocol for the in vitro propagation of Z. tenuior, and to compare the antioxidant activity between in vitro propagated and wild plants.
The explants were sterilized and cultured on MS medium containing different concentrations of growth regulators naphthalene acetic acid (NAA) or indole-3-butyric acid (IBA) with 0.5 mg/L of kinetin (Kin) callus formation was 70.2% after 45 days of incubation in dark on medium supplemented with 1.5 mg/L of NAA. After one month of callus culture on medium supplemented with 2 mg/L BA the shoot number was 5.12 and for the multiplication stage. The shoot number was 4.21 and length was 6.17 cm on medium supplemented with 1 mg/L Kin + 0.1 mg/L NAA.
DPPH• reagent was used to test the antioxidant activity. The aqueous and methanol extracts of in vitro plants which were treated with 1.5 and 1 mg/L of kin plus 0.1 mg/L of NAA showed a strong DPPH• scavenging activity where IC50 was 0.307 and 0.369 mg/ml, respectively, while the IC50 of aqueous and methanol extracts of wild plants was 0.516 and 9.229 mg/ml, respectively. Our results suggested that plant growth regulators and in vitro culture conditions increased the antioxidant activity.
Keywords: In vitro; Micropropagation; Auxin; Cytokinin; Radical scavenging activity (RSA); Reactive oxygen species (ROS); DPPH• (2,2-diphenyl-1-picrylhydrazyl)
1. Introduction
Reactive oxygen species (ROS) are a byproduct of normal metabolism. ROS are additionally produced in cells as a response to several factors, including oxidative and thermal stresses, ultraviolet light, chemical agents, and ionizing radiation. Oxidative stress can cause DNA damage, cell functions inhibition (Yoo et al., 2008), lipid and protein peroxidation, and disturbance of glutathione levels. In addition, ROS contribute to the development of cancer, diabetes, atherosclerosis, inflammatory diseases, and ageing (Harman, 1956, 1992; Beal, 1995; Maxwell, 1995; Lee et al., 2005). ROS are divided into free radical species, such as superoxide anion radical, hydroxyl radical, and non-free radical species that are involved in oxidative reactions, such as singlet oxygen. ROS free radicals contain unpaired electrons (Halliwell and Gutteridge, 1999), which usually show a high degree of reactivity with biological macromolecules such as proteins, lipids, and DNA. Free radicals can oxidize DNA bases (especially guanine), leading to mutations (Harman, 1956, 1992).
Oxidative damages can be reduced through enzymatic mechanisms such as superoxide dismutase (SOD) and catalase (CAT) (Niki et al., 1994) or by antioxidants presented as natural products (Rice-Evans et al., 1997). Many synthetic drugs protect against oxidative damage, but some have adverse side effects. An alternative solution to overcome ROS is to consume natural antioxidants from food, beverages, and traditional medicines containing natural antioxidants such as l-ascorbic acid, tocopherol, and polyphenols (Van Wyk and Wink, 2004; Yazdanparast and Ardestani, 2007; Wink and Van Wyk, 2008; Wink and Abbas, 2013).
Medicinal plants played an important role in the treatment of diseases and health disorders for thousands of years and are still important in traditional medicine systems around the world (Van Wyk and Wink, 2004). Experiments with animal models have shown that the green tea polyphenol epigallocatechin gallate (EGCG) decreased the oxidative damages of superoxide anion radicals and increased the lifespan in the model organism Caenorhabditis elegans (Maupas) (Rhabditidae) (Abbas and Wink, 2009). In addition, EGCG reduced the formation of β-amyloid oligomers involved in Alzheimer’s disease (Abbas and Wink, 2010).
A sufficient supply of the plant raw material contains a consistent quality of valuable natural products becomes difficult with increasing the need to consume such natural products. Therefore, laboratories worldwide are trying to produce secondary metabolites from plant tissue cultures for commercial applications (Wink et al., 2005; Alfermann, 2009) as an alternative or addition to plants produced in fields or greenhouses. Lamiaceae include several important species that contain secondary metabolites such as phenolic compounds, flavonoids, iridoids, and terpenoids (Richardson, 1992; Zegorka and Glowniak, 2001; Lu and Yeap-foo, 2002), which have antioxidant, antiinflammatory, antibacterial, and antiviral properties (Van Wyk and Wink, 2004; Wink, 2008). Ziziphora tenuior L. (Lamiaceae) is an annual and aromatic herb of 5–15 cm height (Fig. 1A), and the stem is simple or branched from the base. The leaves are simple and lanceolate, the flowers are hermaphrodite and zygomorphic with a narrow and tubular calyx (size: 1–2 × 6–9 mm). The corolla is pink and contains five petals. The androecium consists of two fertile stamens and the gynoecium with two carpels (ACSAD, 2008; Mouterde, 1966). Z. tenuior volatile oil contains pulegone as a main constituent. Extracts of Z. tenuior exhibited antifungal and antibacterial effects (Naeini et al., 2010; Ghasemi Pirbalouti et al., 2012; Mahboubi et al., 2012). In addition, Z. tenuior has been used to treat fever, dysentery (Talebi et al., 2012), diarrhea, gut inflammation, cough (Safa et al., 2012), bladder stones, and painful menstruation (Naghibi et al., 2005).
Figure 1.
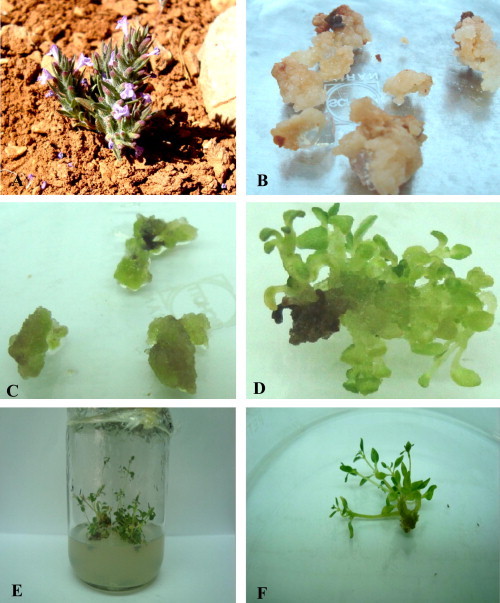
Micropropagation of Z. tenuior. (A) Z. tenuior from the field. (B) Callus induction from leaf explants after 45 days in the dark on a medium supplemented with 1.5 mg/L NAA + 0.5 mg/L Kin. (C) Calli after incubation for 7 days in light. (D) Shoot formation from calli after incubation for 4 weeks on a medium supplemented with 2 mg/L BA. (E) Micropropagated shoots after incubation for 4 weeks on a medium supplemented with 1 mg/L Kin + 0.1 mg/L NAA. (F) Number of shoots on medium supplemented with 1 mg/L Kin + 0.1 mg/L NAA.
Plant tissue culture is the process whereby small pieces of living tissue are isolated from an organism and grown aseptically for indefinite periods on a nutrient medium under controlled conditions (Ali et al., 2007). In vitro cultivation of plants is a necessary step in many experiments like micropropagation, creation of virus-free plants and genetic transformation. (Georgieva et al., 1996).
For increased human needs of medicines, plant tissue culture is used widely for micropropagation of medicinal plants to produce enough amounts of drugs and secondary metabolites, using this technology, the natural products can be provided at any time of the year without waiting for the suitable season to collect the plant and controlling the environmental conditions of plant growth (Sidhu, 2010). In addition to that, it could be obtained of the plants in a short time and a small place (Prakash and Van staden, 2007).
Z. tenuior is an important medicinal plant. Therefore, the current study aimed to explore possibilities to propagate the plant in vitro employing tissue culture via callus and shoot induction using different growth regulators. In addition, the antioxidant activities of aqueous and methanol extracts of the in vitro propagated and wild plants were studied, using the reagent DPPH• (2,2-diphenyl-1-picrylhydrazyl).
2. Materials and methods
This research was carried out in the Plant Tissue Culture and Molecular Biology Laboratory in Damascus University, Faculty of Science, Department of Plant Biology.
2.1. Chemicals and reagents
Indole-3-butyric acid (IBA), benzyladenine (BA), kinetin (Kin), naphthalene acetic acid (NAA), and 2,2-diphenyl-1-picrylhydrazyl (DPPH•) were purchased from Sigma–Aldrich GmbH (Munich, Germany).
2.2. Plant material
Wild plants were collected from Kalamoon Mountains, Assal Al-Ward (Syria), and authenticated by Dr. Imad Alkadi at the Department of Plant Biology, Damascus University, Syria and used as experimental material.
2.3. Establishment of plant tissue culture
Explants of wild plants were washed with water for 30 min. and treatment with 1% antifungal for 5 min, samples were washed three times with sterilized water. Final sterilization was done using 70% ethanol and 10% sodium hypochlorite for 5 min in the presence of few drops of Tween 20. Samples were washed three times with sterilized H2O and cultured on callus induction MS medium (Murashige and Skoog, 1962) supplemented with different concentrations of auxin (NAA or IBA) in the presence of kinetin. The basic nutrient medium (MS) contained mineral salts, vitamins, 3% sucrose, and 0.7% agar. The pH was adjusted to 5.8. The media were sterilized by autoclaving. 40 explants were cultured for each treatment and the experiments were repeated three times. Samples were incubated in the dark for 45 days at 23 ± 1 °C. The incidence of callus formation per leaf was evaluated.
2.4. Shoot induction
Calli were transferred to MS medium supplemented with different concentrations of BA. Samples were incubated in the light for 16 h (light 2000 lux, temperature 23 ± 1 °C, humidity 60–70%) followed with 8 h in darkness at 16 ± 1 °C. The number and length of the shoots produced from calli were established after 30 days of culture.
2.5. Shoot multiplication
The callus-derived shoots were transferred to MS medium supplemented with different concentrations of cytokines in combination with auxin (Kin + NAA). Using the conditions described for shoot induction, 40 shoots were cultured. The number and length of shoots were determined after 30 days of culture; the experiments were repeated three times.
2.6. Plant extract preparation
Aerial parts of wild and in vitro plants were dried and ground by pistil and mortar to a soft powder. For aqueous extract, 5 g of powder was immersed in 100 ml of distilled water; the solution was heated in a water bath for 30 min at 95 °C. For the methanol extract, 5 g of powder was immersed in 100 ml of 80% methanol and heated at 40 °C for 24 h. Then the samples were centrifuged and the supernatants were transferred to new tubes and stored at −20 °C.
2.7. Free radical scavenging activity test
The free radical scavenging activity of samples was measured using 2,2-diphenyl-1-picrylhydrazyl (DPPH•), following the method described by Blois (1958) with some modifications (Laouini et al., 2012). DPPH• is reduced to hydrazine when it reacts with hydrogen donors. Briefly, serial dilutions (10, 1, 0.1, 0.01, 0.001 mg/ml) of samples (aqueous and methanol extracts) were tested for radical scavenging activity. 0.2 mM DPPH• was prepared in methanol and 500 μl of this solution was added to 1000 μl of a sample at different concentrations. The samples were incubated in the dark at room temperature for 30 min. After that, the absorbance was measured at 517 nm using a spectrophotometer. The percentage of radical scavenging activity (RSA%) was calculated using the following equation:
in which A0 is the absorbance of the control reaction, and A1 is the absorbance in the presence of the sample. In addition, the inhibitory concentration that reduces 50% of free radicals (IC50) was determined. The experiments were repeated three times.
2.8. Statistical analysis
Statistical comparisons were done with the SPSS program using one-way analyses of variance (ANOVA) test. All figures indicate means and standard errors of the means. P < 0.05 was regarded as statistically significant.
3. Results
3.1. Effect of auxin on callus formation
Auxins are known to exhibit a significant effect on callus formation from leaf explants. 1.5 mg/L of NAA was the best concentration for callus induction with 70.2% ± 0.28 of leaf explants (Table 1) (Fig. 1B and C), whereas 2 mg/L of NAA reduced the callus formation to 60%. The treatment with 2 mg/L of IBA led to an increase in callus formation to 62.1%, while the growth regulator free MS medium did not show any callus formation and the leaf explants died after 10 days of culture (Table 1).
Table 1.
The effect of different concentrations of NAA and IBA with Kin on callus induction from leaf explants after 6 weeks of in vitro culture.
| Callus induction (in% ± SE of number of leaf explants) | Growth regulators (mg/L) |
|---|---|
| 10.2% ± 0.17 f | 1 IBA + 0.5 Kin |
| 40.1% ± 0.12 e | 1.5 IBA + 0.5 Kin |
| 62.1% ± 0.18 b | 2 IBA + 0.5 Kin |
| 50.5% ± 0.18 d | 1 NAA + 0.5 Kin |
| 70.2% ± 0.28 a | 1.5 NAA + 0.5 Kin |
| 60.2% ± 0.26 c | 2 NAA + 0.5 Kin |
| 0% g | No growth regulator |
3.2. Effect of BA on Shoot formation
Different concentrations of BA induced shoot formation from the callus without root formation, while MS medium without growth regulator did not show any shoot induction. Shoot numbers significantly increased by increasing the BA concentration; the shoot numbers were 5.12 ± 0.13 at 2 mg/L of BA and 2.35 ± 0.08 at 1 mg/L of BA (Table 2). Shoots treated with 1 mg/L of BA were the longest with 1.8 ± 0.04 cm and only 0.56 ± 0.02 cm at 2 mg/L BA (Table 2) (Fig. 1D).
Table 2.
The effect of different concentrations of BA on shoot induction from callus cultures. Number and length of shoots after 4 weeks from culture.
| Mean shoot length (cm ± SE) | Mean shoot numbers (no ± SE) | Growth regulators (mg/L) |
|---|---|---|
| 1.8 ± 0.04 a | 2.35 ± 0.08 c | 1 BA |
| 1.24 ± 0.04 b | 3.77 ± 0.15 b | 1.5 BA |
| 0.56 ± 0.02 c | 5.12 ± 0.13 a | 2 BA |
| 0 ± 0.0 d | 0 ± 0.0 d | 0 BA |
3.3. Shoot multiplication
The statistical analysis showed that the best of shoot multiplication (4.21 ± 0.093) and length (6.17 ± 0.072) were achieved with 1 mg/L of Kin and 0.1 mg/L of NAA. Increased concentration of Kin up to 1.5 mg/L led to a decrease in shoot number and length. (Table 3) (Fig. 1E and F).
Table 3.
The effect of Kin combination with NAA on the number and length of new shoots.
| Mean shoot length (cm ± SE) | Mean shoot numbers (no ± SE) | Growth regulators (mg/L) |
|---|---|---|
| 6.12 ± 0.031 b | 3.90 ± 0.052 b | 0.5 Kin + 0.1 NAA |
| 6.17 ± 0.072 a | 4.21 ± 0.093 a | 1 Kin + 0.1 NAA |
| 5.22 ± 0.081 d | 3.13 ± 0.051 c | 1.5 Kin + 0.1 NAA |
| 5.52 ± 0.082 c | 1.20 ± 0.044 d | 0 Kin + 0 NAA |
3.4. Free radical scavenging activity
The results of DPPH• assay (Table 4) indicate significant differences in the ability to scavenge free radicals between the in vitro propagated plants and wild plants. The aqueous and methanol extract of in vitro grown shoots on medium supplemented with 1.5 Kin + 0.1 NAA, exhibited a stronger antioxidant activity (IC50 = 0.307 ± 0.001 and 8.026 ± 0.013 mg/ml) than wild plants (IC50 = 0.516 ± 0.001 and 9.229 ± 0.144 mg/ml) respectively. As for the extract of in vitro propagated plants, increasing concentration of Kin led to increase IC50 but without significant difference for aqueous extract, while with significant differences for methanol extract.
Table 4.
Radical scavenging activity (IC50 ± SE) of in vitro propagated and wild plants using aqueous and methanol extracts.
| Extract type (growth regulators combination) | IC50 (mg/ml) |
|---|---|
| Methanol extract of wild plant | 9.229 ± 0.144 f |
| Aqueous extract of wild plant | 0.516 ± 0.001 b |
| Methanol extract of plant (0.5 Kin + 0.1 NAA) | 8.712 ± 0.016 e |
| Aqueous extract of plant (0.5 Kin + 0.1 NAA) | 0.399 ± 0.011 ab |
| Methanol extract of plant (1 Kin + 0.1 NAA) | 8.452 ± 0.016 d |
| Aqueous extract of plant (1 Kin + 0.1 NAA) | 0.369 ± 0.001 a |
| Methanol extract of plant (1.5 Kin + 0.1 NAA) | 8.026 ± 0.013 c |
| Aqueous extract of plant (1.5 Kin + 0.1 NAA) | 0.307 ± 0.001 a |
| Methanol extract of plant (0 Kin + 0 NAA) | 8.832 ± 0.035 e |
| Aqueous extract of plant (0 Kin + 0 NAA) | 0.411 ± 0.007 ab |
4. Discussion
Callus is a mass of undifferentiated cells, which are formed in vitro from an explant tissue cultured on nutrient medium supplemented with suitable plant growth regulators (PGRs) during a dedifferentiation process (Skoog and Armstrong, 1970; Letham, 1974; Akiyoshi et al., 1983; Fowler et al., 1993; Bhojwani and Razdan, 1996). In our experiments, callus occurred on the cut surfaces of leaves after 6 days on the media supplemented with 1.5 mg/L NAA + 0.5 mg/L Kin similar to the situation of callus formation in Salvia canariensis L. (Mederos-Molina, 2004) or Nicotiana tabacum L. (Ali et al., 2007). After 1 week of light subjecting, the calli became granular and green. Then the calli turned to a dark brown color and shrunk, indicating that light treatment induces the production of phenolics (Shabnum and Wagay, 2011).
The differentiation of callus to differentiated organs is a complex process controlled by many factors. PGRs play an important role in cell differentiation and organ formation, and the effective concentration of cytokinin and auxin can be different between tissues and species (Skoog and Miller, 1975). The presence of cytokinin into the nutrient media has special importance (Nordstrom and Eliasson, 1986). In the current study, the largest number of shoots (5.12) was obtained with 2 mg/L of BA, while the longest length was seen with 1 mg/L of BA. For Thymus vulgaris L., the optimal condition was 4 mg/L of benzylaminopurine (BAP) (Shabnum and Wagay, 2011) and for N. tabacum (K-399) was 2 mg/L of BAP combined with 0.2 mg/L NAA (Ali et al., 2007).
Cytokinin promotes the growth of axillary buds by reducing the apical dominance of buds during the micropropagation phase (Van Staden et al., 2008(. Furthermore low auxin concentrations are important for micropropagation)Christison and Warnick, 1988). In our experiments the combination of 1 mg/L Kin + 0.1 mg/L NAA was optimal for shoot multiplication, whereas in T. vulgaris L. 0.5 mg/L BA without auxin had the best results (Ozudogru et al., 2011). An increase of the concentration of Kin led to a decrease in the number of shoots and this corresponds with findings of Ozudogru and his colleagues (Ozudogru et al., 2011(. Steephen and his group showed that the increasing concentration of BA above 1 mg/L led to callus formation and decreased shoot development of Vitex negundo L. (Steephen et al., 2010).
Most of the antioxidant activity is due to particular secondary metabolites especially phenolic compounds and some terpenes (Marzouk et al., 2007; Awaad and AL-jaber, 2010). Our results showed that the IC50 value of aqueous and methanol extracts of wild Z. tenuior were 0.516 and 9.22 mg/ml, respectively. In another study, extracts of essential oil of Ziziphora clinopodioides Lam. and Ziziphora pamiroalacia Juz showed IC50 values of 8.1 and 5.3 mg/ml respectively (Si-lei et al., 2010). Water extract showed a strong antioxidant activity in compression with methanol extract, which could be due to a better solubility of antioxidant compounds in water. Wong and his colleagues found that water extracts of 23 from 30 medicinal plants have antioxidant activity higher than those of the methanol extracts (Wong et al., 2006). The results of the current study showed that the radical scavenging ability of in vitro propagated plant extracts of Z. tenuior was higher than aqueous and methanol extracts of wild plants. Stress conditions during in vitro cultivation may have stimulated polyphenol production, and (plant growth regulator cytokinin and auxin) treatment might have been responsible. The formation rate of some phenolic compounds depends on the growth rates of the cultured tissue (Barz, 1977), and on auxin/cytokinin levels into the medium (Sargent and Skoog, 1960; Skoog and Montaldi, 1961). The components in Z. tenuior responsible for the antioxidant activity are unknown. Further research is therefore needed for the identification and isolation of the corresponding antioxidant components.
5. Conclusions
In conclusion, our present investigation shows that micropropagation of Z. tenuior through in vitro is a reliable method for the rapid multiplication of this species. In the current study Z. tenuior has been cultured in vitro for the first time and it was possible to obtain more than 314 plants from one single explant after four subculture cycles of multiplication. This protocol will be helpful for rapid and large scale propagation. Also we can use plant tissue culture to increase the active substance, our results show that the water extracts of in vitro produced plants showed an increase in antioxidant activity as compared to the starting material.
Acknowledgements
The authors would like to thank Dr. Imad Alkadi for helping in the classification, and Prof. Dr. Gassan Ayash, Prof. Dr. Adnan Nizam and Dr. Bassam Alaarg for scientific support in Department of Plant Biology, Damascus University, Damascus.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas S., Wink M. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med. 2009;75:216–221. doi: 10.1055/s-0028-1088378. [DOI] [PubMed] [Google Scholar]
- Abbas S., Wink M. Epigallocatechin gallate inhibits beta-amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine. 2010;17:902–909. doi: 10.1016/j.phymed.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Akiyoshi D.E., Morris R.O., Hinz R., Mischke B.S., Kosuge T., Garfinkel D.J., Gordon M.P., Nester E.W. Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc. Natl. Acad. Sci. U.S.A. 1983;80:407–411. doi: 10.1073/pnas.80.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfermann, A.W., 2009. Production of natural products by plant cell and organ cultures. In: Wink, M. (Ed.), Annual plant reviews, vol. 39. Functions and biotechnology of plant secondary metabolites, 2nd ed. Wiley-Blackwell, Oxford, pp. 381–399.
- Ali G., Hadi F., Ali Z., Tariq M., Ali Khan M. Callus induction and in vitro complete plant regeneration of different cultivars of tobacco (Nicotiana tabacum L.) on media of different hormonal concentrations. Biotechnology. 2007;6:561–566. [Google Scholar]
- Arab Center For The Studies of Arid Zones and Dry Lands (ACSAD), 2008. Atlas of the syrian Badia Plants, Syria, pp. 327–28.
- Awaad S.A., Al-Jaber A.N. Antioxidant natural plant. In: Govil J.N., Singh V.K., editors. vol. 27. Studium Press Pvt. Ltd; India: 2010. pp. 1–35. (RPMP Ethnomedicine: Source and Mechanism). [Google Scholar]
- Barz W. Catabolism of endogenous and exogenous compounds by plant cell cultures. In: Barz W., Reinhard E., Zenk M.H., editors. Plant tissue culture and its biotechnological application Proceedings in Life Sciences. Springer-Verlag; Berlin: 1977. pp. 153–171. [Google Scholar]
- Beal M.F. Aging, energy and oxidative stress in neurodegenerative diseases. Ann. Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- Bhojwani S.S., Razdan M.K. Elsevier; Amsterdam, Netherlands: 1996. Plant tissue culture: theory and practice. [Google Scholar]
- Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–1200. [Google Scholar]
- Christison M.L., Warnick D.A. Organogenesis in vitro as a developmental process. Hort. Sci. 1988;23:115–119. [Google Scholar]
- Fowler M.R., Rayns F.W., Hunter C.F. The language and aims of plant cell and tissue culture. In: Hunter C.F., editor. In vitro cultivation of plant cells. Butterworth-Oxford; Heinemann Ltd.: 1993. pp. 1–18. [Google Scholar]
- Georgieva K., Yordanov I., Kroinova A. Photosynthetic characteristics of transformed tobacco plants grown in vitro after tgeir transplantation in natural conditions. Plant physiol. 1996;22:3–13. [Google Scholar]
- Ghasemi Pirbalouti G.A., Malekpoor F., Hamedi B. Ethnobotany and antimicrobial activity of medicinal plants of Bakhtiari Zagross mountains. Iran. J. Med. Plants Res. 2012;6:675–679. [Google Scholar]
- Halliwell B., Gutteridge J.M.C. 3rd ed. Oxford University Press, Oxford; UK: 1999. Free radicals in biology and medicine. [Google Scholar]
- Harman D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. A Biol. Sci. Med. Sci. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging. Mutat. Res. 1992;275:257–266. doi: 10.1016/0921-8734(92)90030-s. [DOI] [PubMed] [Google Scholar]
- Laouini E.S., Segni L., Ouahrani R.M., Gherraf N., Mokni S. Phytochemical analysis, antioxidant and antimicrobial activities of leaves extract of date palm grown in Algeria. J. Fund. App. Sci. 2012;4:48–58. [Google Scholar]
- Lee K.W., Hur H.J., Lee H.J., Lee Y.C. Antiproliferative effects of dietary phenolic substances and hydrogen peroxide. J. Agric. Food Chem. 2005;53:1990–1995. doi: 10.1021/jf0486040. [DOI] [PubMed] [Google Scholar]
- Letham D.S. Regulators of cell division in plant tissues. XX. The cytokinins of coconut milk. Plant physiol. 1974;32:66–70. [Google Scholar]
- Lu Y., Yeap-foo L. Polyphenolics of Salvia- a review. Phytochemistry. 2002;59:117–140. doi: 10.1016/s0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- Mahboubi M., Bokaee S., Dehdashti H., Feizabadi M.M. Antimicrobial activity of Mentha piperitae, Zhumeria majdae, Ziziphora tenuior oils on ESBLs producing isolates of Klebsiella pneumonia. Biharean Biol. 2012;6:5–9. [Google Scholar]
- Marzouk M., Moharram F., Mohamed M., Gamal-Eldeen A., Aboutabl E. Anticancer and antioxidant tannins from Pimenta dioica leaves. Z. Naturforsch. 2007;62:526–536. doi: 10.1515/znc-2007-7-811. [DOI] [PubMed] [Google Scholar]
- Maxwell S.R. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–361. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- Mederos-Molina S. In vitro callus induction and plants from stem and petiole explants of Salvia canariensis L. Plant tissue cult. 2004;14:167–172. [Google Scholar]
- Mouterde P. Editions de l’Imprimerie Catholique; Beirut, Lebanon: 1966. Nouvelle flore du liban et de la syrie 3. [Google Scholar]
- Murashige T., Skoog F. A revised medium for rapid growing and bioassay with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Naeini A., Khosravi A., Tadjbakhsh H., Ghazanfari T., Yaraee R., Shokri H. Evaluation of the immunostimulatory activity of Ziziphora tenuior extracts. Comp. Clin. Pathol. 2010;19:459–463. [Google Scholar]
- Naghibi F., Mosaddegh M., Motamed S.M., Ghorbani A. Labiatae family in folk medicine in Iran: from ethnobotany to pharmacology. Iran. J. Pharm. Res. 2005;2:63–79. [Google Scholar]
- Niki E., Shimaski H., Mino M. Gakkai Syuppn Center; Tokyo, Jaban: 1994. Antioxidantism-free radical and biological defense. pp. 3–16. [Google Scholar]
- Nordstrom A.C., Eliasson L. Uptake and translocation of C14-labeled benzylaminopurine in apple shoots grown in vitro in relation to shoot development. Physiol Plant. 1986;68:431–435. [Google Scholar]
- Ozudogru A.E., Kaya E., Kirdok E., Issever-Ozturk S. In vitro propagation from young and mature explants of thyme (Thymus vulgaris and T. longicaulis) resulting in genetically stable shoots. In Vitro Cell. Dev. Biol. Plant. 2011;47:309–320. [Google Scholar]
- Prakash S., Van Staden J. Micropropagation of Hoslundia opposita Vahl-a valuable medicinal plant. South Afr. J. Bot. 2007;73:60–63. [Google Scholar]
- Rice-Evans C.A., Miller J., Paganga G. Antioxidant properties of phenolic compounds. Trends in Plant Sci. 1997;2:152–159. [Google Scholar]
- Richardson, P., 1992. The chemistry of the Labiatae: An introduction and overview. In: Harley, R.M., Reynolds, T. (Ed.), Advances in Labiatae Science. Royal Botanical Garden, Kew, pp. 291–297.
- Safa O., Soltanipoor A.M., Rastegar S., Kazemi M., Dehkordi N.K., Ghannadi A. An ethnobotanical survey on Hormozgan province. Iran. Avicenna J. Phytomed. 2012;3:64–81. [PMC free article] [PubMed] [Google Scholar]
- Sargent J.A., Skoog F. Effects of indoleacetic acid and kinetin on scopoletin and scopolin levels in relation to growth of tobacco tissue in vitro. Plant Physiol. 1960;35:934–941. doi: 10.1104/pp.35.6.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabnum S., Wagay G.M. Micropropagation of different species of Thymus. J. Res. Dev. 2011;11:71–80. [Google Scholar]
- Sidhu Y. In vitro micropropagation of medicinal plants by tissue culture. Plymouth Student Sci. 2010;4:432–449. [Google Scholar]
- Si-lei X., Pi-hong Z., Qiao-ling J., Hong-li J., Xue-hua W. Essential oil compositions and antioxidant activities of two Ziziphora species in Xinjiang. Food Sci. 2010;31:154–159. [Google Scholar]
- Skoog F., Armstrong D.J. Cytokinin. Annu. Rev. J. Plant Physiol. 1970;21:359–384. [Google Scholar]
- Skoog F., Miller C.O. Chemical regulation of growth and organ information of buds in plant tissues. In: Skoog F., editor. Plant growth Substances. Wisconsin Press; Madison, Wisconsin Univ: 1975. pp. 263–285. [Google Scholar]
- Skoog F., Montaldi E. Auxin-kinetin interaction regulating the scopoletin and scopolin levels in tobacco tissue cultures. Proc. Natl. Acad. Sci. U.S.A. 1961;47:36–49. doi: 10.1073/pnas.47.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steephen M., Nagarajan S., Ganesh D. Phloroglucinol and silver nitrate enhances axillary shoot proliferation in nodal explants of Vitex negundo L. an aromatic plant. Iran. J. Biotech. 2010;8:82–89. [Google Scholar]
- Talebi M.S., Rezakhanlou A., Isfahani S.G. Trichomes plasticity in Ziziphora tenuior L. (Labiatae) in Iran: an ecological review. Ann. Biol. Res. 2012;3:668–672. [Google Scholar]
- Van Staden J., Zazimalova E., George E.F. Plant growth regulators II: Cytokinins, their analogues and antagonists. In: George E.F., Hall M., De Kleck G.J., editors. Plant propagation by tissue culture. Vol 1. Springer; Dordrecht, Netherlands: 2008. pp. 205–226. [Google Scholar]
- Van Wyk B.E., Wink M. Timber Press; Portland, OR, Cambridge: 2004. Medicinal Plants of the World. [Google Scholar]
- Wink M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008;9:996–1009. doi: 10.2174/138920008786927794. [DOI] [PubMed] [Google Scholar]
- Wink M., Abbas S. Epigallocatechin gallate (EGCG) from green tea (Camellia sinensis) and other natural products mediate stress resistance and slows down aging processes in Caenorhabditis elegans. In: Preedy V.R., editor. Tea in health and disease prevention. Elsevier; London: 2013. pp. 1105–1116. [Google Scholar]
- Wink M., Van Wyk B.E. Timber Press; Portland, OR, London: 2008. Mind-altering and poisonous plants of the world. [Google Scholar]
- Wink M., Alfermann A.W., Franke R., Wetterauer B., Distl M., Windhövel J., Krohn O., Fuss E., Garden H., Mohagheghzadeh A., Wildi E., Ripplinger P. Sustainable bioproduction of phytochemicals by plant in vitro cultures: anticancer agents. Plant Genet. Res. 2005;3:90–100. [Google Scholar]
- Wong C.C., Li B.H., Cheng W.K., Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006;97:705–711. [Google Scholar]
- Yazdanparast R., Ardestani A. In vitro antioxidant and free radical scavenging activity of Cyperus rotundus. J. Med. Food. 2007;10:667–674. doi: 10.1089/jmf.2006.090. [DOI] [PubMed] [Google Scholar]
- Yoo M.K., Lee H.C., Lee H., Moon B., Lee Y.C. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008;106:929–936. [Google Scholar]
- Zegorka G., Glowniak K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001;26:179–187. doi: 10.1016/s0731-7085(01)00354-5. [DOI] [PubMed] [Google Scholar]


