Abstract
The present study was investigated for soil bioremediation through sababul plant biomass (Leucaena leucocephala). The soil contaminated with textile effluent was collected from Erode (chithode) area. Various physico-chemical characterizations like N, P, and K and electrical conductivity were assessed on both control and dye contaminated soils before and after remediation. Sababul (L. leucocephala) powder used as plant biomass for remediation was a tool for textile dye removal using basic synthetic dyes by column packing and eluting. The concentration of the dye eluted was compared with its original concentration of dye and were analyzed by using UV–vis spectrophotometer. Sababul plant biomass was analyzed for its physico-chemical properties and active compounds were detected by GC–MS, HPTLC and FTIR. Plant growth was assessed with green gram on the textile contaminated soil and sababul had the potential of adsorbing the dye as the contaminated soil and also check the growth of green gram.
Keywords: Biodegradation, Adsorption, Green gram, Leucaena leucocephala, Vigna radiate, Phytoremediation
1. Introduction
Soil health plays an important role in the growth of plants and trees, maintaining the ecosystem with its natural fauna, flora and indirectly sustains the environment to its natural conditions. Soil health is defined as the continued capacity of the soil to function as a vital living system, by recognizing that it contains biological elements that are key to ecosystem function within land use boundaries. These functions are able to sustain biological productivity of soil, maintain the quality of surrounding air and water environments, as well as promote plant, animal, and human health (Nathan, 2009). Soil pollution is caused by anthropogenic activities of man and due to rapid industrialization or due to other alteration the natural soil environment. This type of contamination typically arises from the rupture of underground storage links, application of pesticides, and percolation of contaminated surface water to subsurface strata, oil and fuel dumping, leaching of wastes from landfills or direct discharge of industrial wastes to the soil (Shaylor et al., 2009).
Textile finishing also requires the input of a wide range of chemicals which, if not contained in the final product, become waste treatment and disposal problems. The textile dyes and dye intermediates with high aromaticity and low biodegradability have emerged as major environmental pollutants (Arslan et al., 2000) and nearly 10–15% of the dye is lost in the dyeing process and is released in the wastewater which is an important source of environmental contamination. Considerable amount of water is used for dyeing and finishing of fabrics in the textile industries.
Dye-synthesizing wastewater and textile wastewater are two types of poorly treated wastewater that contain organic dyes. These wastewaters are characterized by strong color, highly fluctuating pH, high COD and bio-toxicity (Shen and Wang, 2001). Dye pollutants produced from the textile industries are becoming a major source of environmental contaminations. It is estimated that about 15% of the total world production of dyes is lost during the dyeing and finishing operations and is released in the textile effluents. Azo dyes, which contain one or more nitrogen to nitrogen double bonds (–N N–) and constitute a significant portion of dye colorants, are widely used in the dyeing industry today, and are also resistant to aerobic degradation and under anaerobic conditions they can be reduced to potentially carcinogenic aromatic amines.
Mccutecheon and Schnoor (2003) reported by the phytoremediation is an emerging green technology that uses plants to degrade some toxic chemicals in soils, sediments, groundwater, surface water, and air. It can be used as a remediate or as part of a broader site management alternative comprising a number of remediation technologies. Plants have grown naturally at contaminated waste sites and have been planted for esthetic value or land stabilization. Currently, it is used for treating many classes of contaminants, including petroleum hydrocarbons, pesticides, explosives, heavy metals and radionuclides, as well as CVOCs. Leucaena leucocephala was known as the ‘miracle tree’ because of its worldwide success as long-lived. Recently it has been recommended for contour planting in small scale tropical farming systems as a means of soil conservation and fertility maintenance (Nyambati et al., 2006).
L. leucocephala is a colonizing plant which has spread to a very wide range of sites which are more or less frost-free and has naturalized itself in many areas, some far outside the tropics. L. leucocephala requires warm temperatures (25–30 °C) for optimum growth. A large amount of pods and seeds are fallen which is of no use. The study was carried out to investigate the potential of L. leucocephala pods in the form of dry powder for phytoremediation of soil contaminant with textile dye (Brewbaker and Sorensson, 1990). The present study was done to understand the phytoremediating potential of L. leucocephala (subabul) for dye contaminated soil and it was assessed for germination and growth potential of Vigna radiata (green gram).
2. Materials and methods
2.1. Soil sample
Chithode area is located with many dyeing industries and textile dyeing process involves large volumes of water mixed with dyes. The untreated textile waste water is being discharged which contaminates the soil around the dyeing units. Plant growth is sparse and thus this soil was identified for the study. Five different locations were selected and soil was taken (100 kg/ha) at a depth of 0–25 cm. Composite replicates were prepared, air-dried, sieved and stored for further analysis. In the same area unpolluted soil was used as control.
2.2. Collection of plant test material
The test material (V. radiate: Green gram) was obtained from the Centre for Plant Genetics and Breeding, Tamil Nadu Agricultural University (TNAU), Coimbatore and it was stored for further studying the growth pattern.
2.3. Plant biomass used as remediation
The seed pods were dried and then powdered. Fine powder was used as phytoremediator for further analysis. No binding agent was used so as to study the exact nature of L. leucocephala biomass and it was treated with 20 sterile seeds individually. The seeds of each group were transferred into individual pots for the respective treatment and growth promotion effect was observed.
2.4. Soil characterization
The soil collected was cleaned of debris, shade dried, sieved to uniform size and used for further studies. Initial soil profile was done and the soil characteristics like N, P, K, pH, electrical conductivity, lime, and texture were assessed before and after remediation.
2.5. Spectral studies with soil and remediating plant material
The soil used in the study was milled until fine powder and was filtrated with sieves 0.071 and 0.500 mm mesh size. Potassium bromide of spectroscopy grade was also filtered with sieves 0.071 mm mesh size. 2 mg sample was taken and mixed uniformly with 100 mg KBr (2% w/w) homogenized by using stir vortex and was used for the analysis.
2.6. Characterization of L. leucocephala dye adsorption study
Column experiment was carried out with immobilized L. leucocephala powder and a varying concentration of reactive red dye was eluted down the column. Dye adsorption study was carried out with red reactive dye since most dye effluent is contaminated with this dye. Different concentrations of the dye ranging from 25% as minimum followed by 50%, 75% and 100% as maximum were eluted down a column packed with L. leucocephala seed pod biomass powder.
2.7. Spectral study for L. Leucocephala by FTIR
To 100 g of dye contaminated soil was added 3 g of L. leucocephala (Pods) powder and mixed well and placed on a vertical shaker for 24 h. After 24 h the corresponding solutions were equilibrated and then filtered. About 20 ml of the clear filtrate was digested with triple acid was used for Fourier Transform Infrared Spectroscopy (FTIR) analysis to compare the dyeing binding capacity with L. leucocephala and soil using Thermo Nicolet FTIR Nexus spectrometer coupled with DTGS (deuterated tri-glycine sulphate) detector.
2.8. Preliminary phytochemical screening
This was done qualitatively (various phytochemical constituents) and quantitatively (phenols, flavonoids and tannins).
2.8.1. Identification of active principle of L. Leucocephala seed pod biomass using HPTLC
A CAGMAG LINOMAT 5 HPTLC instrument and CAGMAG REPROSTRAR 3 photo- documentation chamber were used for HPTLC analysis. 10% methanolic extract of L. leucocephala was prepared by keeping in boiling water bath, filtered and used for HPTLC. 5 μl of the plant extract was loaded as 8 mm band length in the 5 × 10 silica gel 60F254 TLC plate using Hamilton syringe. The sample loaded plate was kept in a TLC twin trough developing chamber with respective mobile phases up to 90 mm.
2.8.2. Identification of active principle of L. leucocephala using GC–MS
GC–MS analysis was carried out on a GC clarus 500 Perkin Elmer comprising a AOC −20i autosampler and gas chromatograph interfaced to a mass spectrometer (GC–MS).
2.8.3. Radical scavenging assays
This test evaluated the DPPH (1,1,diphenyl 2-picryl hydrazyl) and Superoxide radical evaluated the L. leucocephala as a phytoremidiator on contaminated soil and on the growth of green gram. To evaluate the germination of green gram (%) from the treated and untreated plant biomass the root and shoot lengths of germinated green gram before and after remediation with L. Leucocephala were checked.
3. Results and discussion
3.1. Soil characterization
Dye contaminated soil characteristics should be thoroughly investigated before it is subjected to remediation and only then be recommended for use in agriculture and irrigation (Rajeswari et al., 2005). The soil is a complex living system and the success of its decontamination and the choice of remediation depend on its characteristics (Table 1).
Table 1.
Physico-chemical characterization of dye contaminated soil and control soil (The reactive dyes are containing salt, alkali, surfactants, defoamer and diluents).
| S. No. | Particulars | Dye contaminated soil (100 kg/ha) | Control soil (100 g/ha) |
|---|---|---|---|
| 1 | pH | 8.84 | 8.4 |
| 2 | Electrical conductivity (dS m−1) | 0.28 | 0.26 |
| 3 | Texture | CL | CL |
| 4 | Lime | C | SC |
| 5 | Available N (kg ha−1) | 199 | 200 |
| 6 | Available P (kg ha−1) | 25 | 51.6 |
| 7 | Available K (kg ha−1) | 140 | 983 |
| 8 | Copper (ppm) | 2.86 | 1.07 |
| 9 | Manganese (ppm) | 3.77 | 2.03 |
| 10 | Iron (ppm) | 207 | 439.4 |
| 11 | Zinc (ppm) | 1.95 | 2.2 |
3.2. Physico chemical characterization of dye contaminated soil and control soil
Soil analysis done with the polluted and unpolluted soils revealed that there was no change in the physical properties of soil like pH, electrical conductivity and texture. The chemical constituents changed with polluted and unpolluted soils. The lime content of soils increased in the dye polluted soil when compared to unpolluted soil. The macronutrients such as phosphorus and potassium were drastically affected. The phosphorus content was lowered from 51.6 to 25 kg ha−1. Similarly the potassium content was reduced to 140 from 983 kg ha−1. The micronutrient such as copper and manganese increased from 1.07 to 2.86 ppm and 2.03to 3.77 ppm, whereas iron and zinc decreased from 439.4 to 20.77 ppm and 2.2 to 1.95 ppm. Thus soil characteristic analysis showed that dye contamination in soils can affect the macro and micro nutrient contents of soils (Garg and Kowshik, 2007).
3.3. Spectral studies with soil and remediating plant material
FTIR was carried out in control soil, polluted soil and remediated soil with plant material-L. leucocephala biomass. A comparison done between the spectral reflectance of the control soil, dye contaminated soil, and remediated soil showed the following results. A peak was observed at 3394–3363 cm−1 corresponding to amines (N–H) in the control soil and remediated soil but absent in polluted soil. Similarly the presence of carboxylic acid at a peak of 2515–2430 cm−1 was missing in the polluted soil and present in the control and remediated soils. Phenyl and methyl groups are known functional groups of all materials and were present in the control soil and remediated soil but absent in polluted soil. A shift in the band at 1442–1380 cm−1 of the remediated soil which was absent in the control soil and dye contaminated soil indicated the presence of alkanes which could also play a role in the binding property of the dye to the plant material. The presence of amines, carboxylic acid, phenyl group and alkanes of the plant material could be responsible for the binding property of the dye. The same pattern of research by Moran et al. (1997) reported that low-cost biosorbents are able to bind to dye molecules and easily be regenerated.
3.4. Dye adsorption study
Dye adsorption study was carried out with a red reactive dye since most dye effluent is contaminated with this dye. Different concentrations of the dye ranging from 25% as minimum followed by 50%, 75% and 100% as maximum were eluted down a column packed with L. leucocephala seed pod biomass powder. It was observed that L. Leucocephala biomass powder was able to absorb the dye upto 50% and after which the rate of adsorption decreased. Upto 50% a colorless effluent was collected above which the dye was seen in the effluent. Absorbance at 540 nm confirmed the gradation of the color of the dye.
3.5. Spectral study for L. leucocephala biomass by FTIR
FTIR was carried out (Fig. 1a) with L. Leucocephala seed pod biomass powder which revealed the presence of amines (3363 cm−1), carboxyl groups, (2430 cm−1), phenyl groups (1890 cm−1), alkanes and alkenes (1643–1380 cm−1). These groups could be responsible for the binding capacity of the dye to plant material on phytoremediation.
Figure 1.
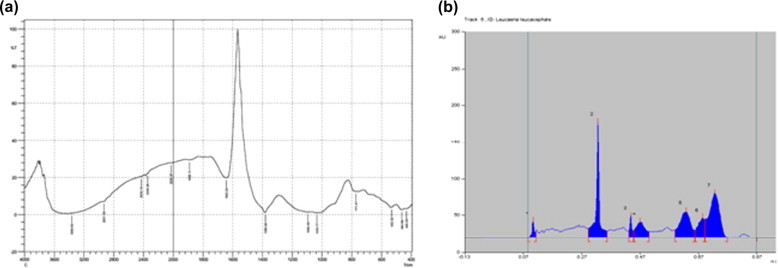
Spectra (a) and identification of active principal (b) study of Leucaena leucocephala.
3.6. Phytochemical screening
3.6.1. Qualitative phytochemical screening
The qualitative phytochemical analysis of L. leucocephala biomass revealed the presence of phytochemical in 50% ethanolic extracts as shown in Table 2. Presence of phytochemical was indicated by the + sign.
Table 2.
Preliminary phytochemical screening of ethanolic extract of Leucaena leucocephala biomass.
| S. No. | Phytochemical test | Result |
|---|---|---|
| 1 | Alkaloids Dragendroff’s test Wagners test Mayers test |
++ ++ ++ |
| 2 | Flavonoids | ++ |
| 3 | Tannins Ferric chloride Lead acetate test |
+ + |
| 4 | Phenols Ferric chloride Lead acetate test Liebermann’s test |
++ ++ ++ |
| 5 | Saponins | +++ |
| 6 | Carbohydrates Fehlings test Benedict’s test Molisch’s test |
++ ++ ++ |
| 7 | Proteins–Amino acids Millon’s test Biuret reagent Ninhydrin reagent |
++ ++ ++ |
3.6.2. Identification of active principle of L. leucocephala using HPTLC and GC–MS HPTLC profiling
In the last two decades HPTLC has emerged as an important tool for the qualitative, semi-quantitative phytochemical analysis of herbal drugs and formulations (Man Mohan Srivastava, 2011). HPTLC is a powerful analytical technique due its merits of reliability, simplicity, reproducibility and speed (Agarwal et al., 2004). A solvent system that would give dense and compact spots with significant Rf values was desired for quantification of L. leucocephala biomass. The desired resolutions of compounds in HPTLC analysis were displayed as both chromatogram and the peak densitogram (Fig. 1b).
3.6.3. Identification of the active constituents by GC–MS
GC–MS is extensively used for the analysis of compounds which include esters, fatty acids, alcohols, aldehydes, terpenes etc. and has been widely heralded as a “gold standard for the detection of chemical constituents of biological materials. A prominent peak at 17.95 RT reveals the presence of tetradecyne having properties like antioxidant, and cancer preventive. A major peak at 14.97 RT reveals the presence of palmitic acid which has nematicidal activity acts as an antioxidant, and as a lubricant. Presence of pelargonic acid at peak 18.47 shows that L. leucocephala has anti-inflammatory and antimicrobial properties. A minor peak at 34.36 RT reveals the presence of pyridine which exhibits the antioxidant, and nematicide activity. Yet another minor peak at 35.59 RT reveals the presence of myristic acid which has properties like antitumor, and cancer preventive. Another peak at 36.42 RT reveals the presence of dioxolane but it has no activity. In conclusion based on the RT the plant biomass contains pyridine, myristic acid and dioxolone.
3.7. Radical scavenging assays of L. leucocephala biomass
3.7.1. DPPH scavenging activity by spectrophotometry
DPPH is a free radical compound and has been widely used to test the free radical scavenging activity of various samples. DPPH scavenging activity of plant extract and ascorbate is illustrated in Fig. 2. The plant extract shows the DPPH scavenging activity of 59.68% at 1000 μg/ml where as for ascorbate it was found to be 61.58% at 1000 μg/ml. The Ic50 of the plant extract and ascorbate was found to be 499 and 478 μg/ml, respectively. The model of scavenging the stable DPPH radical is a widely used method to evaluate the free radical scavenging ability of various samples (Ebrahimzadeh et al., 2008). DPPH is a stable nitrogen-centered free radical the color of which changes from violet to yellow upon reduction by either the process of hydrogen or electron-donation. Substances which are able to perform this reaction can be considered as antioxidants and therefore radical scavengers (Dehpour et al., 2009).
Figure 2.

Identification of DPPH scavenging activity of Leucaena leucocephala.
3.7.2. Superoxide scavenging activity
Superoxide scavenging activity of the plant extract at various concentrations (200, 400, 600, 800 and 1000 μg/ml) was found. The maximum scavenging activity of plant extract and ascorbate at 1000 μg/ml was found to be 53.3% and 56.6%, respectively. The IC50 value of plant extract and ascorbate was found to be 478 and 438 μg/ml, respectively. From IC50 value and percentage scavenging capacity, it was found that plant extract is more effective in scavenging superoxide radical than that of ascorbate. Superoxide anion which is reduced from molecular oxygen has been implicated in initiating oxidation reactions associated with aging. It plays an important role in the formation of other reactive oxygen species such as H2O2, hydroxyl radical and singlet oxygen, which induced oxidative damage, proteins and DNA (Moran et al., 1997).
3.8. Evaluating the growth potential of green gram before and after remediation of dye contaminated soil
The third phase was carried out to establish plant growth. It was carried out before and after remediation in the dye contaminated soil. It was done by studying the germinating and growth potential of green gram (V. radiata CO-6). The study involved the assessment of biometric levels of plant growth followed by biochemical and stress parameters as inducers of growth of green gram. The optimum ratio of biomass to soil was 3 g/100 g of soil. This ratio was found to be optimum for remediation and for growth of green gram.
3.9. Percentage germination of green gram before and after remediation with L. leucocephala biomass
A study on the percentage germination of green gram grown on dye contaminated soil and remediated with L. leucocephala biomass powder was done and compared with reference control. Percentage germination was calculated upto the third day after sowing when maximum activity occurs and plant biomass was assessed on the 30th day after germination in the soil. Fig. 3b shows the germination results. Seedling growth is sensitive to dye contaminated soil than remediated and control. L. leucocephala biomass treatment overcame the effect of dye contaminated soil and improved the percentage of germination in green gram. Excessive accumulation of dye in cotyledons compromises the seeds’ germination through a negative interference of pollutants with mineral and organic reserve mobilization. This influences the seedling growth and alters physiological and biochemical processes of the growing plant. Sahai et al. (1983) investigated the impact of various concentrations (0, 25, 50, 75 and 100% v/w) of the effluent textile mill on seed germination, seedling growth and pigment content of the Arachis hypogea. The effluent caused toxicity to the seedlings at higher concentrations. Undiluted effluents had an inhibitory effect (28.9%), whereas 25% effluent had a growth promoting effect (4.7%) which was significantly better than control.
Figure 3.
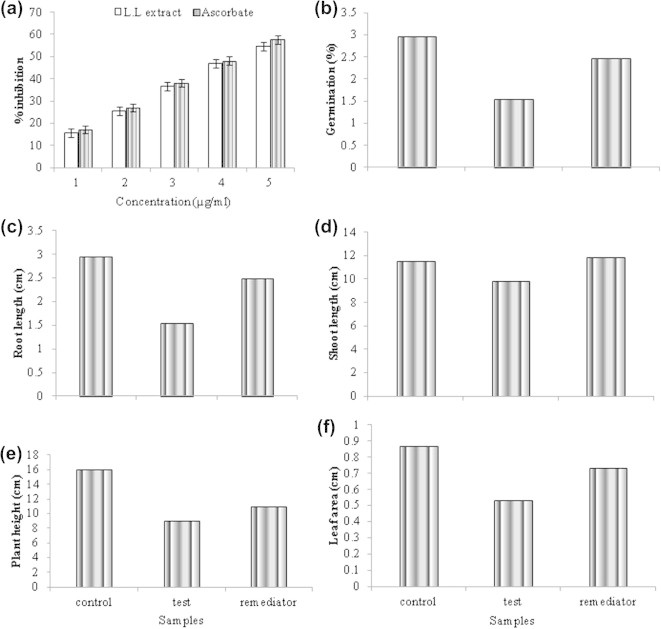
Study of Root length (a), percentage germination (b), root length (c), shoot length (d), height (e) and leaf area (f) with Leucaena leucocephala biomass.
3.10. Biometric study
Root length, shoot length, plant height and leaf area were assessed in plants grown before and after remediation of the dye contaminated soil.
3.10.1. Root length of green gram
The data corresponding to the root growth of the green gram plant grown on dye contaminated soil vs remediated soil are seen in Fig. 3c. Use of L. leucocephala boomass as phytoremediator enhanced the germination rate and promoted root induction in green gram.
3.10.2. Shoot length of green gram
As seen in Fig. 3d the shoot length of green gram with dye contaminated soil did not change significantly when compared with reference control. Thus dye contaminated soil does not inhibit shoot growth in green gram.
3.10.3. Plant height in green gram
As observed in Fig. 3e there was a change in the plant height on remediation. Delayed germination and slow growth rate were predominant in the dye contaminated soil in contrast to remediated soil.
3.10.4. Leaf area in green gram
As observed in Fig. 3f the leaf area decreased in plants with dye contaminated soil when compared with control and remediated plants. Initha et al. (2013) reported that germination, shoot and root percentages were reduced as concentration of dye increased which is similar to our results.
3.11. Biochemical analysis of green gram root and leaf
3.11.1. Protein and total free amino acid
The roots and leaves of green gram were sacrificed from the plant on the 15th day after germination and were used for the estimation. The protein content changed significantly in leaves before and after remediation as shown in Fig. 4a. As seen in Fig. 4b the free amino acid content was decreased in the leaves significantly before and after remediation. When comparison between protein and free amino acid was seen, free amino acid changed remarkably in leaves of plants grown in the dye contaminated red soil than protein. The protein content and free amino acid content of roots had a significant change before and after remediation as seen in Fig. 4a and b. A comparison was made between protein and amino acid levels, and there was no change in their levels in the root. L. leucocephala plant biomass remediates the soil for the growth of green gram in the dye contaminated soil and enhanced the levels of protein and amino acid. This is in accordance with the results of Ameta et al. (2003) who showed that carbohydrate, protein, chlorophyll contents may be altered due to the destabilization of the chloroplast and reduced photosynthesis. Protein, carbohydrate and chlorophyll contents are related to plant growth, a decrease in their content is a clear indication of the toxic nature of the dye present in the industry effluent.
Figure 4.
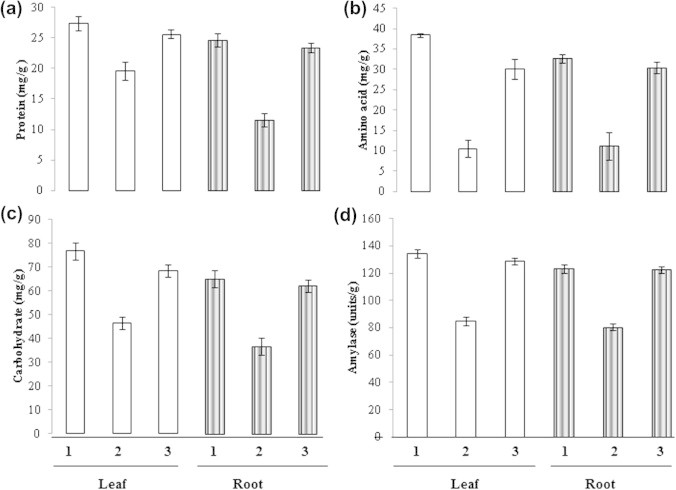
Study of protein (a), total free amino acid (b), carbohydrate (c) and amylase (d) with Leucaena leucocephala biomass. Note: (1) Control, (2) Test, (3) Remediator.
3.11.2. Carbohydrate and amylase
As shown in Fig. 4c and d the carbohydrate content was low when compared with amylase activity in the leaves. A comparison made for carbohydrate level and amylase activity before and after remediation reveals carbohydrate content decreased in the leaves before remediation and increased after remediation and amylase activity was also similar to carbohydrate before and after remediation. The carbohydrate content and amylase activity in the root are similar to the leaves when compared before and after remediation. Thus L. leucocephala biomass remediates the level of carbohydrate and amylase in plants grown in the dye contaminated soil. Amylase is the first enzyme of germination and it mobilizes the carbohydrate reserve for germination and growth of the plant. Based on the fact our values stand good for root and leaves of green gram plan used in this study (Beck and Ziegler, 1989).
3.11.3. Chlorophyll
Fig. 5 shows the Chlorophyll content of plants decreasing in response to industrial effluent could be associated with higher concentration of dissolved solids or increase in chlorophyllase, an enzyme responsible for chlorophyll degradation or decrease in the endogenous cytokinins involved in stimulating chlorophyll synthesis (Gadallah, 1996). The total chlorophyll content of leaves of plants grown in the dye contaminated soil was comparatively less than the control and L. leucocephala biomass remediated contaminated soil. There was a significant change in the level of chlorophyll content of leaves of plants before and after remediation of soil.
Figure 5.

Study of chlorophyll with Leucaena leucocephala biomass.
3.12. Stress markers
3.12.1. Phenol
Fig. 6a shows the phenol content of leaves and root of green gram. The phenol content was low in leaves under stress condition when compared to roots. A comparison done for roots and leaves before and after remediation reveals the phenol content decreased in the leaves before remediation and increased after remediation of soil. There was no significant change in the root system. Phenols are generally thought to prevent oxidative damage by scavenging active oxygen species and by grating the radical chain reaction during lipid peroxidation (Sakihama et al., 2002). The stress caused by the dye on plant growth was remediated by L. leucocephala biomass and was able to accelerate the effect of phenol in abiotic stress in green gram roots and leaves.
Figure 6.

Study of phenol (a), salicylic acid (b), ascorbic acid (c), hydrogen peroxide (d), total glutathione (e) and glutathione peroxidase (d) with Leucaena leucocephala biomass. Note: (1) Control, (2) Test, (3) Remediator.
3.12.2. Salicylic acid
The level of salicylic acid decreased significantly before remediation and enhanced after remediation when compared with control in both roots and leaves. Salicylic acids are important plant signaling molecules (Fig. 6b). A comparison between the dye contaminated soil and remediated soil reveals the salicylic acid decreased in the leaves in the dye contaminated soil than in remediated soil. Several studies also support the major role of salicylic acid in modulating the plant response to several abiotic stresses (Yalpani et al., 1994, Senarthana et al., 2000).
3.12.3. Ascorbic acid
Fig. 6c shows the level of ascorbic acid in leaves and root. The level of ascorbic acid decreased significantly before remediation and enhanced after remediation when compared with control in both roots and leaves. Ascorbic acid is considered as the most powerful reactive oxygen species due to its ability to donate the electrons in a number of enzymatic and non-enzymatic reactions. Thus it proves to be a good detoxicant. Ascorbic acid reduces the 2,6-dichlorophenol indophenols dye to colorless. The ascorbic acid gets oxidized to dehydroascorbic acid.
3.12.4. Hydrogen peroxide
Hydrogen peroxide is a product of superoxide dismutase reaction. From Fig. 6d it is observed that the level of hydrogen peroxide increased in the leaves and root under stress condition. L. leucocephala biomass remediated the level of hydrogen peroxide in plants grown in dye contaminated soil (Choudhury and Panda, 2004).
3.12.5. Total glutathione and glutathione peroxidase
Glutathione is the substrate of glutathione peroxidase reactions and reduced glutathione they serve in the removal of ROS and their reaction products. Reduced glutathione and glutathione peroxidase are known to be responsive to biotic and abiotic stress. Fig. 6e and f shows the level of total glutathione and glutathione peroxidase in the test. A comparison made for total glutathione and glutathione peroxidase in the leaves before and after remediation reveals total glutathione content increased in the leaves before remediation and decreased after remediation and glutathione peroxidase activity decreased before and increased after remediation. The same pattern of change occurred in roots also. Thus L. leucocephala biomass remediates the level of total glutathione and glutathione peroxidase in plants grown in the dye contaminated soil.
3.12.6. Lipid peroxidation
The suitability of the lipid peroxidation level, as biomarker for environmental monitoring was evaluated (Mohan and Hosetti, 1999). Fig. 7a shows the level of LPO. Lipid peroxidation level was high in leaves and roots compared to the reference control and remediated soil with L. leucocephala biomass in plants. The increased accumulation of lipidperoxide is indicative of enhanced production of toxic oxygen species (Hendry, 2000).
Figure 7.

Study of Lipid peroxidation (a), Proline (b) and Methyl glyoxylate (c) with Leucaena leucocephala biomass. Note: (1) Control, (2) Test, (3) Remediator.
3.12.7. Proline
As shown in Fig. 7b the proline content of leaves and roots were high in plants grown in the dye contaminated soil. Remediating soil with L. leucocephala biomass changed the level. Proline accumulates in several plants under stress, providing plants against damage by ROS (Prasad and Strzalka, 1995).
3.12.8. Methyl glyoxylate
Under stress conditions cells become metabolically active, which is reflected by up regulation of enzymes involved in glycolysis and TCA cycle and as a result triose phosphate level increases which, instead of giving only pyruvate could be converted to methyl glyoxal. As observed from Fig. 7c there was a significant increase in methyl glyoxal content of roots and leaves of green gram grown in the contaminated soil than in control and remediated soil. L. leucocephala biomass has a potential remediating effect for dye contamination in soil. The contamination of soil by dye enhances plant uptake causing accumulation in different organs. The results of the three phases show that soil contamination by dye will alter the chemical constituents of the soil and inhibit plant growth (Hendry, 2000). Characterization of L. leucocephala seed pod biomass reveals the potentiality of the phytoremediator to bind to the biomass and phytostabilize the soil for plant growth. The use of L. leucocephala as phytoremediator enhanced the macromolecular level of leaves and root after remediation. The stress induced by the dye reduced by the phytoremediation was seen by the level of stress markers before and after remediation in leaves and roots.
4. Conclusion
The present study was done to understand the phytoremediating potential of L. leucocephala (subabul) for the dye contaminated soil. This was assessed on the germination and growth potential of V. radiata (green gram). A pilot study was carried out with a varying mass of seed pod biomass of L. leucocephala (subabul) and the optimum ratio for dye contaminated soil was observed to be 3 g of plant biomass per 100 g of soil. It was found that stress markers like proline, methyl glyoxal content of leaves and roots were increased significantly in plants grown in the dye contaminated soil than in remediated soil using L. leucocephala biomass. The above observation indicates the protective effect of L. leucocephala biomass against abiotic stress caused by dye contamination. It can act as a biodegradable organic phytostabiliser for dyes such as reactive dye of different hues. It can remediate contaminated soils upto 50% an upper limit found in the study and the remediated soil can be used for the cultivation of green gram.
Acknowledgement
The authors should thank the Department of Biochemistry and Biotechnology for their support and also thank the Principal of PSG College of Arts and Science, Coimbatore.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agarwal A.A., Conner J.K., Stinchcombe J.R. Evolution of plant resistance and tolerance to frost damage. Ecol. Lett. 2004;7:1199–1208. [Google Scholar]
- Ameta C., Punjabi P.B., Kothari S., Sancheti A. Effect of untreated and photo catalytically treated dyeing industry effluent on growth and biochemical parameters of Allium cepa (Onion) Pollut. Res. 2003;22:389–392. [Google Scholar]
- Arslan I.A., Balcioglu D.W., Bahnemann Phytoremediation studies at Milan army ammunition plant, (Environmental Executive Notes, 1996) Dyes Pigm. 2000;47 [Google Scholar]
- Beck E., Ziegler P. Biosynthesis and degradation of starch in higher plants. Annu. Rev. Plant Phys. 1989;40:95–117. [Google Scholar]
- Brewbaker J.L., Sorensson C.T. In: Advances in New Crops. Janick J., Simon J.E., editors. Timber Press; Portland: 1990. New tree crops from interspecific Leucaena hybrids; pp. 283–289. [Google Scholar]
- Choudhury S., Panda S.K. Induction of oxidative stress and ultrastuctural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under lead and arsenic phytotoxicity. Curr. Sci. 2004;87:342–348. [Google Scholar]
- Dehpour A.A., Ebrahimzadeh M.A., Nabavi S.F., Nabavi S.M. Antioxidant activity of methanol extract of Ferula assafoetida and its essential oil composition. Grasas Y Aceites. 2009;60(4):405–412. [Google Scholar]
- Initha C.Lebanon Ebency, Rajan S, Murugesan A.G., Rajesh R., Elayarajah B. Biodegradation of textile azo dyes and its bioremediation potential using seed germination efficiency. Int. J. Curr. Microbiol. App. Sci. 2013;2(10):496–505. [Google Scholar]
- Ebrahimzadeh M.A., Navabi S.M., Navabi S.F., Bahramian F., Bekhradnia A.R. Antioxidant and free radical scavenging activity of H. Officinalis l. var Angustifolius V. Odorata, B. Hyrcana and C. Speciosum. Pak. J. Pharm. Sci. 2008;23:29–34. [PubMed] [Google Scholar]
- Gadallah M.A.A. Phytotoxix effects of industrial sewage waste waters on growth, chlorophyll content, transcription rate and relative water content of potted sunflower plants. Water Air Soil Poll. 1996;89:33–47. [Google Scholar]
- Garg V.K., Kowshik P. Influence of textile mill waste water irrigation on the growth of sorghum cultivars. Appl. Eco. Environ. Res. 2007;6(1):165–176. [Google Scholar]
- Hendry J.R. D.C.U.S. Environmental Protection Agency; Washington: 2000. An overview of phytoremediation of lead and mercury. National Network of Environmental Management Studies (NNEMS) p. 51. [Google Scholar]
- Man Mohan Srivastava . Springer; Heidelberg, Dordrecht, London, New York: 2011. High-Performance Thin-Layer Chromatography (HPTLC) p. 380. [Google Scholar]
- McCutecheon S.C., Schnoor J.L. Wiley-Inter Science Inc; Hoboken, New Jersey: 2003. Phytoremediation: Transformation and Control of Contaminants. [Google Scholar]
- Mohan B.S., Hosetti B.B. Aquatic plants for toxicity assessment. Environ. Res. 1999;81:259–274. doi: 10.1006/enrs.1999.3960. [DOI] [PubMed] [Google Scholar]
- Moran C., Hall M.E., Howell R.C. Effects of sewage treatment on textile effluent. J. Soc. Dyes Color. 1997;113:272–274. [Google Scholar]
- Nathan, M., 2009. Soil testing for lead for garden and landscape soils. Missouri environment and garden Columbia, MO: University of Missouri, College of Agriculture, Food and Natural Resources, Division of Plant Sciences, Plant Protection Programs. 15 (4), 23–32.
- Nyambati E.M., Sollenberger L.E., Karue C.N., Musimba N.K.R. The value of Acacia brevispica and Leucaena leucocephala seedpods as dry season supplements for calves in dry areas for Kenya. Afr. J. Agric. Res. 2006;1(4):118–124. [Google Scholar]
- Prasad M.N.V., Strzalka K. Kluwer Academic Publishers; Dordrecht: 1995. Physiology and Biochemistry of Heavy Metal Toxicity and Tolerance in Plants. pp. 303–324. [Google Scholar]
- Rajeswari S., Sunita S., Rasheed V.S., Andrew J.H. The soil sciences in India: policy lessons for agricultural innovation. Res. Policy. 2005;35(5):643–654. [Google Scholar]
- Sahai R., Agarwal N., Khosala N. Effect of fertilizer factory effluent on seed germination, seedling growth and chlorophyll content of Phaseolous radiates. Trop. Ecol. 1983;20:135. [Google Scholar]
- Sakihama Y., Cohen M.F., Grace S., Hideo C., Yamasaki H. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicology. 2002;17:67–80. doi: 10.1016/s0300-483x(02)00196-8. [DOI] [PubMed] [Google Scholar]
- Senarthana T., Touchell D., Bunns E.K. Acetyl salicylic acid and salicylic acid induce multiple stresses to tolerance in bean and tomato plants. Plant Growth Regul. 2000;30:157–161. [Google Scholar]
- Shaylor H., McBride M., Harrison E. Cornell University, Department of Crop and Soil Sciences, Waste Management Institute; Ithaca, NY: 2009. Soil Contamination and Best Practices for Healthy Gardens. pp. 1–4. [Google Scholar]
- Shen Y.S., Wang D.K. Development of photo reactor design equation for the treatment of dye wastewater by UV/H2O2 process. J. Hazard. Mater. 2001;89:267–277. doi: 10.1016/s0304-3894(01)00317-x. [DOI] [PubMed] [Google Scholar]
- Yalpani N., Encydi A.J., Leon J., Raskin I. UV light and ozonestimulate accumulation of salicylic acid and pathogenedsis related proteins and virus resistance in tobacco. Planta. 1994;193:373–376. [Google Scholar]


