Abstract
Chronic kidney disease (CKD) is a progressive pathological condition marked by deteriorating renal function over time. Diagnostic of kidney disease depend on serum creatinine level and glomerular filtration rate which is detectable when kidney function become half. The detection of kidney damage in an early stage needs robust biomarkers. Biomarkers allow monitoring the disease progression at initial stages of disease. On the onset of impairment in cellular organization there is perturbation in signaling molecules which are either up-regulated or down-regulated and act as an indicator or biomarker of diseased stage. This review compiled the cell signaling of different kidney biomarkers associated with the onset of chronic kidney diseases. Delay in diagnosis of CKD will cause deterioration of nephron function which leads to End stage renal disease and at that point patients require dialysis or kidney transplant. Detailed information on the complex network in signaling pathway leading to a coordinated pattern of gene expression and regulation in CKD will undoubtedly provide important clues to develop novel prognostic and therapeutic strategies for CKD.
Keywords: Cell signaling, Kidney, Biomarker, NGAL, Clusterin
1. Introduction
Cells use a large number of clearly defined signaling pathways to regulate their activity. (Mendoza et al., 2011; Schneider et al., 2012). Phenotypic modification and chaotic of signaling pathways lead to critical diseases in human like mental illness, hypertension, heart disease, diabetes, kidney disease etc. On the onset of impairment in cellular organization there is perturbation in signaling molecules which are either up-regulated or down-regulated and act as an indicator or biomarkers of diseased stage (Rifai et al., 2006).
Chronic kidney disease (CKD) is characterized by progressive destruction of the renal parenchyma and the loss of functional nephrons, which finally lead to chronic renal failure (Amandine et al., 2010). CKD progresses slowly and their mechanism is not well understood. CKD leads to the reduction of functional nephrons and for the compensation nephrons triggers molecular and cellular events promoting compensatory growth of the remaining ones (Hostetter, 1995). In some cases, this compensatory process becomes pathological, with the development of renal lesions and End stage renal disease (Kliem et al., 1996; Pillebout et al., 2003; Terzi et al., 1995). In CKD clinical diagnosis is dependent on levels of blood urea, nitrogen and serum creatinine. Serum creatinine, however, has shortcomings because of its low predictive value. Therefore, next generation that is candidate biomarkers are being identified for early diagnosis of CKD (Charles, 2008). Biomarkers are protein and act as signaling molecule in diseased state. In this review, attention is focused on intracellular signaling pathway of biomarkers associated with the onset of kidney impairment. During the process of development of disease, specific protein selects their signaling systems that are suitable to control their particular functions. This review is all about cell-specific biomarker function to regulate different signaling pathways in response to chronic kidney damage.
This review updates the reader on the current status of biomarker signaling associated with kidney impairment. At present, the main contenders include Asymmetric dimethylarginine, clusterin, Neutrophil gelatinase-associated lipocalin, and Hepatocyte growth factor. Characteristics of these biomarkers are presented in Table 1.
Table 1.
Characteristics of biomarkers.
| Biomarkers | Discovery | Nature | Signaling pathways |
|---|---|---|---|
| Asymmetric dimethylarginine | Vallance et al., 1992 first reported the role of asymmetric dimethylarginine | It is a metabolic by-product chemical found in blood plasma | It involves in transforming growth factor-beta, nuclear factor-κB and Mitogen-activated protein kinase signaling pathways |
| Clusterin | Fritz et al., 1983 was first identified in ram rete testis fluid. | 75–80 kDa disulfide-linked heterodimeric protein | It involves in transforming growth factor-beta, signaling pathway |
| Neutrophil gelatinase-associated lipocalin | Kjeldsen et al., 2000 first identified this protein as a component of neutrophil granules | 25 kDa protein and expressed in neutrophils | It involves in Epidermal growth factor receptor and Mitogen-activated protein kinase signaling pathways |
| Hepatocyte growth factor | Nakamura et al., 1984 first identified and purified Hepatocyte growth factor in rat | It is secreted as a single inactive polypeptide and consists of 69-kDa alpha-chain and 34-kDa beta-chain | It involves in phosphatidylinositol 3-kinase-Akt, growth factor-beta, nuclear factor-κB and the Stat3 signaling pathways |
1.1. Signaling pathways associated with Asymmetric dimethylarginine and CKD
Asymmetric dimethylarginine (ADMA), an analog of l-arginine, is a naturally occurring product of metabolism found in human circulation. Elevated levels of ADMA inhibit nitric oxide (NO) synthesis and therefore impair endothelial function and thus promote kidney impairment (Vallance et al., 1992). Nitric oxide is a major player in the regulation of renal function. Increased levels of ADMA have been shown to be the strongest risk predictor (Hasdan et al., 2002; Kielstein et al., 2002; Passauer et al., 2005; Zoccali et al., 2001) and are involved in the evolution of progressive nephropathies suggested by two studies. Both studies concluded high ADMA constantly predicted a faster rate of renal function loss and lend support to the hypothesis that ADMA may engender renal damage as it triggers glomerular hypertension, endothelial damage, salt accumulation, and cell senescence (Fliser et al., 2005; Ravani et al., 2005).
Matsuguma et al. (2006) explored the molecular mechanism for increase in ADMA and its role for hypertension in CKD using a rat remnant kidney model. Production of ADMA in cell is through an enzyme protein methyltransferase (PRMT) (Boger et al., 2000) and metabolized mainly by NG, NG-dimethylarginine dimethylaminohydrolase (DDAH) (Leiper and Vallance, 1999; Ogawa et al., 1998; Ueda et al., 2003). Results of semiquantitative reverse transcription-PCR and Western blot showed that DDAH protein levels as well as increased PRMT gene expression may cause the elevation of plasma ADMA levels in kidney by inhibiting the production of endothelial nitric oxide, thereby bring out hypertension in CKD. The mechanism is represented in Fig. 1.
Figure 1.
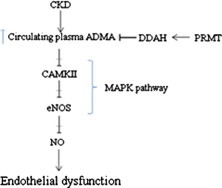
Plasma ADMA level is controlled by DDAH. In CKD, ADMA level is elevated which stops the production of CAMKII (via MAPK pathway), eNOS and NO. Elevated levels of ADMA inhibit Nitric oxide (NO) synthesis and therefore impair endothelial function and thus promote kidney impairment. MAPK (Mitogen activated protein kinase); DDAH (NG, NG-dimethylarginine dimethylaminohydrolase); PRMT (protein methyltransferase); eNOS (endothelial nitric oxide synthase).
Another signaling involved in ADMA associated kidney injury is through transforming growth factor (TGF-β) and nuclear factor-κB (NF-κB) pathways. Wang and co-worker (2012) explored the mechanism by treating the human renal glomerular endothelial cells (HRGECs) with long term low dose of ADMA. Exogenous ADMA notably increased stress fiber formation in HRGECs and upregulated nuclear factor-κB (NF-κB) and TGF-β expression. Further to inhibit the stress fiber formation, HRGECs were treated with actin depolymerizing and stabilizing agent, p38 MAPK inhibitor and NADPH oxidase to elucidate that actin cytoskeleton may be involved in the modulation of ADMA-induced nuclear factor-κBNF-κB activation and the following TGF-β expression in human renal glomerular endothelial cells. The same research was reported by Feng et al., 2013 in endothelial cells.
Kajimoto et al. (2012) had explored the novel mechanism of ADMA signaling link with impaired endothelial function in CKD. Fig. 1 explains the ADMA signaling. Study divulged that ADMA reduces arterial endothelial nitric oxide synthase (eNOS) phosphorylation by inhibiting Ca/Calmodulin-Dependent Protein Kinase CaMKII (one of the upstream kinases for eNOS activation)-mediated signaling showing that ADMA may also reduce nitric oxide production via decreased eNOS phosphorylation; this effect is mediated by the mitogen-activated protein kinases (MAPK) pathway and can be reversed in vivo by increased catabolism of ADMA through dimethylarginine dimethylaminohydrolase-1 over expression.
1.2. Signaling pathways associated with clusterin and CKD
Clusterin also termed as complement cytolysis inhibitor, testosterone repressed prostate message-2 (TRPM-2), dimenic acidic glycoprotein (DAG), SP-40, gp8O, apobipoprotein J and NA 1/N A 2. It is disulfide-linked heterodimeric glycoprotein of 75 kD, consisting of α- and β-subunits (Jones and Jomary, 2002; Kujiraoka et al., 2004). Clusterin is coupled with different biologic functions comprised of reproduction, cell regression, cell aggregation, and regulation of the cytolytic activity of the membrane attack complex of complement (Caccamo et al., 2004; Redondo et al., 2002). Up-regulation of clusterin suggests the occurrence of renal injury and proves to be a potential biomarker of nephrotoxicity (Girton et al., 2002). Clusterin is a component of glomerular immune deposits in the kidney and clusterin protein is translated in the renal tubular epithelium after de novo expression of clusterin mRNA following renal injury, and by post translation processing it is immediately converted into 2 subtypes. The α subtype was excreted in the urine and the β subtype was accumulated in the cytoplasm of the renal tubular epithelial cells (Saunders et al., 1994).
Different pathways were reported to be associated with clusterin in response to kidney damage. Studies reported that expression of clusterin is associated with the signaling pathway of TGF β. unilateral ureteral obstruction (UUO) kidney is the condition of marked interstitial fibrosis. Clusterin is over expressed in UUO kidney and confirmed by the up-regulation of fibronectin.
Liu, 2006 reported that TGF β 1 plays an important role in the process of fibrosis. Expression of clusterin is induced by TGF β 1 via the activation of AP-1 transcriptor protein and protein kinase C (Jin and Howe, 1997). Diagrammatic representation is shown in Fig. 2.
Figure 2.
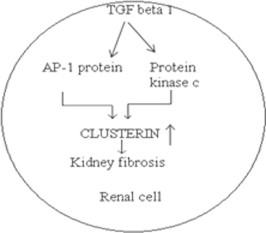
Clusterin is over expressed in kidney fibrosis. AP 1 protein and protein kinase C enzyme in TGF β 1 pathway trigger the expression of clusterin.
Clusterin is expressed on the dedifferentiated tubular cells after injury in polycystic kidney disease and renal cell carcinoma (Correa-Rotter et al., 1998; Harding et al., 1991). Kidney cancer cells express nuclear form and secretory form of clusterin (sCLU) which are upregulated after molecular stress and found to be antiapoptotic and prosurvival in nature. Shannan et al. (2006) revealed the role of clusterin to cure kidney and other cancer which expresses secretory form of clusterin. Finding suggests that by using antisense oligonucleotides or short interfering double-stranded RNA with the help of some drugs targeting sCLU may become novel strategy for cancer therapy, especially in the treatment of kidney, prostate, colon, breast, and lung tumors that over express sCLU.
1.3. Signaling pathway associated with neutrophil gelatinase-associated lipocalin and CKD
Neutrophil gelatinase-associated lipocalin (NGAL) also called Lnc2 is a member of a family of over 20 proteins called lipocalins which function in intracellular chemical signaling (Xu et al., 1994). NGAL is one of the first molecules to trigger kidney development, expressed by the ureteric bud. It has been shown to convert embryonic mesenchymal cells into epithelial cells which form tubules and then complete nephrons (Yang et al., 2002a,b). NGAL is present in the kidney proximal tubule in a patchy distribution and has recently been found to be expressed in patients with CKD (Bolignano et al., 2009). The level of NGAL expression appears to be related to the degree of kidney dysfunction and may even be able to predict which patients are going to have a faster decline in their kidney function. Viau et al. (2010) used genomic approach to delineate a novel mechanism of progression of CKD through cross talk between NGAL and EGFR. NGAL acts as a growth regulator by mediating the mitogenic effect of epidermal growth factor receptor (EGFR) signaling. Activation of the EGFR stimulates hypoxia-inducible factor (HIF-1α) and ensuing expression of LCN2 resulting in increased cell proliferation, cytogenesis, renal damage and CKD progression. Fig. 3 presents the signaling of NGAL, EGFR and HIF-1 α in renal tubular cells leading to CKD.
Figure 3.
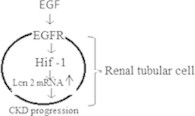
Renal tubular cells treated with epidermal growth factor activates EGF receptor protein which further activates HIF-1 α protein and promotes the formation of NGAL mRNA thus increases the process of CKD.
Mao et al. (2011) explored the signaling of NGAL receptor named NGALR (NGAL receptor) in injured glomeruli and investigated the possible mechanism of the NGALR involvement in inflammation in human mesangial cells (HMC). The findings showed that NGALR is differentially expressed in human glomerular disease and is significantly up-regulated by interleukin Il-1β in HMC via MAPK/ERK activation.
1.4. Signaling pathway associated with hepatocyte growth factor and CKD
Hepatocyte growth factor (HGF) is a heterodimer, mesenchymally derived, pleiotropic, and multifunctional cytokine consisting alpha-chain and a beta-chain of 60-kDa and 34-kDa, respectively (Nakamura et al., 1995). HGF is also known as a renotrophic factor, and highly expressed in kidney cells (Ishibashi et al., 1992; Matsumoto and Nakamura, 2001).
HGF is highly involved in the signaling of CKD. HGF binds to its tyrosine kinase receptor, c-Met, and activates various signaling pathways (Furge et al., 2000; Stuart et al., 2000), including cell growth via phosphatidylinositol 3-kinase-Akt, cell proliferation via Ras-Mek-Erk, cell inflammation via NF-κB, TGF beta-1 pathway and the Stat3 pathways.
HGF slows down the progression of chronic obstructive nephropathy reported by Gao et al. (2002). Researchers worked on apoptotic signaling pathway and found that after liposome mediated HGF gene transfer in unilateral ureteral obstruction (UUO) rats, HGF and c-Met were up-regulated in UUO kidneys and apoptotic pathway molecule Bcl-2 protein was found to be up regulated while no changes were found in Bcl-xl and Bax protein. Fig. 4 explains the increased HGF protein level in unilateral ureteral obstruction kidney with the activation of apoptotic pathway proteins.
Figure 4.
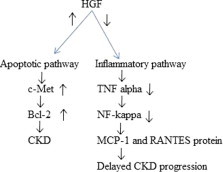
Anti-inflammatory and apoptotic activities of HGF. Bcl-2, c-Met and HGF levels are increased in UUO CKD while decreased in MCP-1 and RANTES protein level helps in delayed CKD progression.
Gong et al. (2006) explained anti-inflammatory activity of HGF in inflamed renal interstitial kidney of rat. Researchers worked on the expression of the chemokines macrophage chemoattractant protein-1 (MCP-1) and RANTES (regulated upon expression normal T cell expressed and secreted) which are down regulated in inflamed condition of glomeruli and tubulointerstitium. In-vitro study revealed that HGF down regulated TNF-α-induced expression of MCP-1 and RANTES at both the mRNA and protein levels in proximal tubular epithelial cells. TNF-α stimulates nuclear translocation and activation of NF-κB, a key transcription factor that regulates chemokine expression is also halted by HGF. Diagrammatic representation of antiinflammatory activity of HGF is explained in Fig. 4.
2. Conclusion
Increasing knowledge in the science of biology and medicine has accelerated the discovery of novel biomarkers and elucidated their roles in molecular pathways triggered by physiological and/or pathological conditions. The detection of kidney disease genes holds great promise for detecting novel pathways that initiate renal fibrosis and lead to progressive loss of renal function. Detailed information on the complex network in signaling pathway leading to a coordinated pattern of gene expression and regulation in CKD will undoubtedly provide important clues to develop novel prognostic and therapeutic strategies for CKD. Above mentioned pathways allied with kidney impairment are likely to offer new therapies that may slow or halt development of chronic kidney failure.
Acknowledgements
I would like to thank my colleague Hemant Pandey for guiding me and also like to thank my Institute for providing me internet facility to complete my review. I am also grateful to Dr. Manoj Pandey for his valuable guidance.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Zeba Khan, Email: zebakhan_04@yahoo.co.in.
Manoj Pandey, Email: manojpandey66@gmail.com.
References
- Amandine V., Khalil E.K., Denise L. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J. Clin. Invest. 2010;120(11):4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger R.H., Sydow K., Borlak J. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells involvement of S-adenosylmethionine-dependent methyltransferases. Circ. Res. 2000;87:99–105. doi: 10.1161/01.res.87.2.99. [DOI] [PubMed] [Google Scholar]
- Bolignano D., Lacquaniti A., Coppolino G. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. CJASN. 2009;4(2):337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A.E., Scaltriti M., Caporali A. Cell detachment and apoptosis induction of immortalized human prostate epithelial cells are associated with early accumulation of a 45 kDa nuclear isoform of clusterin. Biochem. J. 2004;382:157–168. doi: 10.1042/BJ20040158. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Charles L.E. Biomarkers of acute kidney injury. Adv. Chronic Kidney Dis. 2008;15(3):222–234. doi: 10.1053/j.ackd.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Rotter R., Ibarra-Rubio M.E., Schwochau G. Induction of clusterin in tubules of nephrotic rats. J. Am. Soc. Nephrol. 1998;9:33–37. doi: 10.1681/ASN.V9133. [DOI] [PubMed] [Google Scholar]
- Feng Y., Zhang D., Zhang Y. The mechanism of long-term low-dose asymmetric dimethylarginine inducing transforming growth factor-β expression in endothelial cells. Int. J. Mol. Med. 2013;31:67–74. doi: 10.3892/ijmm.2012.1190. [DOI] [PubMed] [Google Scholar]
- Fliser D., Kronenberg F., Kielstein J.T. Asymmetric dimethylarginine and progression of chronic kidney disease: the mild to moderate kidney disease study. J. Am. Soc. Nephrol. 2005;16:2456–2461. doi: 10.1681/ASN.2005020179. [DOI] [PubMed] [Google Scholar]
- Fritz I.B., Burdzy K., Setchell B. Ram rete testis fluid contains a protein (clusterin) which influences cell-cell interactions in vitro. Biol. Reprod. 1983;28:1173–1188. doi: 10.1095/biolreprod28.5.1173. [DOI] [PubMed] [Google Scholar]
- Furge K.A., Zhang Y.W., Vande Woude G.F. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene. 2000;19:5582–5589. doi: 10.1038/sj.onc.1203859. [DOI] [PubMed] [Google Scholar]
- Gao X., Mae H., Ayabe N. Hepatocyte growth factor gene therapy retards the progression of chronic obstructive nephropathy. Kidney Int. 2002;62(4):1238–1248. doi: 10.1111/j.1523-1755.2002.kid579.x. [DOI] [PubMed] [Google Scholar]
- Girton R.A., Sundin D.P., Rosenberg M.E. Clusterin protects renal tubular epithelial cells from gentamicin-mediated cytotoxicity. Am. J. Physiol. Renal Physiol. 2002;282:F703–709. doi: 10.1152/ajprenal.00060.2001. [DOI] [PubMed] [Google Scholar]
- Gong R., Rifai A., Dworkin L.D. Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: targeting the inflamed vascular endothelium. JASN. 2006;17(9):2464–2473. doi: 10.1681/ASN.2006020185. [DOI] [PubMed] [Google Scholar]
- Harding M.A., Chadwickm L.J., Gattone V.H. The SGP-2 gene is developmentally regulated in the mouse kidney and abnormally expressed in collecting duct cysts in polycystic kidney disease. Dev. Biol. 1991;146:483–490. doi: 10.1016/0012-1606(91)90249-3. [DOI] [PubMed] [Google Scholar]
- Hasdan G., Benchetrit S., Rashid G. Endothelial dysfunction and hypertension in 5/6 nephrectomized rats are mediated by vascular superoxide. Kidney Int. 2002;61:586–590. doi: 10.1046/j.1523-1755.2002.00166.x. [DOI] [PubMed] [Google Scholar]
- Hostetter T.H. Progression of renal disease and renal hypertrophy. Annu. Rev. Physiol. 1995;57:263–278. doi: 10.1146/annurev.ph.57.030195.001403. [DOI] [PubMed] [Google Scholar]
- Ishibashi K., Sasaki S., Sakamoto H. Hepatocyte growth factor is a paracrine factor for renal epithelial cells: stimulation of DNA synthesis and NA, K-ATPase activity. Biochem. Biophys. Res. Commun. 1992;182:960–965. doi: 10.1016/0006-291x(92)91825-b. [DOI] [PubMed] [Google Scholar]
- Jin G., Howe P.H. Regulation of clusterin gene expression by transforming growth factor beta. J. Biol. Chem. 1997;272:26620–26626. doi: 10.1074/jbc.272.42.26620. [DOI] [PubMed] [Google Scholar]
- Jones S.E., Jomary C. Clusterin. Int. J. Biochem. Cell Biol. 2002;34:427–431. doi: 10.1016/s1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- Kajimoto H., Kai H., Aoki H. Inhibition of eNOS phosphorylation mediates endothelial dysfunction in renal failure: new effect of asymmetric dimethylarginine. Kidney Int. 2012;81(8):762–768. doi: 10.1038/ki.2011.476. [DOI] [PubMed] [Google Scholar]
- Kielstein J.T., Boger R.H., Bode-Boger S.M. Marked increase of asymmetric dimethylarginine in patients with incipient primary chronic renal disease. J. Am. Soc. Nephrol. 2002;13:170–176. doi: 10.1681/ASN.V131170. [DOI] [PubMed] [Google Scholar]
- Kjeldsen L., Cowland J.B., Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim. Biophys. Acta. 2000;1482:272–283. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- Kliem V., Johnson R.J., Alpers C.E. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 1996;49(3):666–678. doi: 10.1038/ki.1996.95. [DOI] [PubMed] [Google Scholar]
- Kujiraoka T., Takano M., Hattori H. Apolipoprotein J (apo J) Nippon Rinsho. 2004;62:117–120. [PubMed] [Google Scholar]
- Leiper J., Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc. Res. 1999;43:542–548. doi: 10.1016/s0008-6363(99)00162-5. [DOI] [PubMed] [Google Scholar]
- Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- Mao S., Jiang T., Shang G. Increased expression of neutrophil gelatinase-associated lipocalin receptor by interleukin-1β in human mesangial cells via MAPK/ERK activation. Int. J. Mol. Med. 2011;27(4):555–560. doi: 10.3892/ijmm.2011.613. [DOI] [PubMed] [Google Scholar]
- Matsuguma K., Ueda S., Yamagishi S. Molecular mechanism for elevation of asymmetric dimethylarginine and its role for hypertension in chronic kidney disease. JASN. 2006;17(8):2176–2183. doi: 10.1681/ASN.2005121379. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001;59:2023–2038. doi: 10.1046/j.1523-1755.2001.00717.x. [DOI] [PubMed] [Google Scholar]
- Mendoza M.C., Er E.E., Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem. Sci. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Morishita R., Higaki J. Expression of local hepatocyte growth factor system in vascular tissues. Biochem. Biophys. Res. Commun. 1995;215:483–488. doi: 10.1006/bbrc.1995.2490. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nawa K., Ichihara A. A purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem. Biophys. Res. Commun. 1984;122:1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Kimoto M., Sasaoka K. Purification and properties of a new enzyme, NG, NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J. Biol. Chem. 1998;264:10205–10209. [PubMed] [Google Scholar]
- Passauer J., Pistrosch F., Bussemaker E. Reduced agonist-induced endothelium-dependent vasodilation in uremia is attributable to an impairment of vascular nitric oxide. J. Am. Soc. Nephrol. 2005;16:959–965. doi: 10.1681/ASN.2004070582. [DOI] [PubMed] [Google Scholar]
- Pillebout E., Weitzman J.B., Burtin M. JunD protects against chronic kidney disease by regulating paracrine mitogens. J. Clin. Invest. 2003;112(6):843–852. doi: 10.1172/JCI17647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravani P., Tripepi G., Malberti F. Asymmetrical dimethylarginine predicts progression to dialysis and death in patients with chronic kidney disease: a competing risks modeling approach. J. Am. Soc. Nephrol. 2005;16:2449–2455. doi: 10.1681/ASN.2005010076. [DOI] [PubMed] [Google Scholar]
- Redondo M., Villar E., Torres-Munoz J. Overexpression of clusterin in human breast carcinoma. Am. J. Pathol. 2002;157:393–399. doi: 10.1016/S0002-9440(10)64552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifai N., Gillette M.A., Carr S.A. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat. Biotechnol. 2006;24:971–983. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- Saunders J.R., Aminian A., McRae J.L. Clusterin depletion enhances immune glomerular injury in the isolated perfused kidney. Kidney Int. 1994;45:817–827. doi: 10.1038/ki.1994.108. [DOI] [PubMed] [Google Scholar]
- Schneider A., Klingmüller U., Schilling M. Short-term information processing, long-term responses: insights by mathematical modeling of signal transduction. BioEssays. 2012;34(7):542–550. doi: 10.1002/bies.201100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannan B., Seifert M., Leskov K. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 2006;13:12–19. doi: 10.1038/sj.cdd.4401779. [DOI] [PubMed] [Google Scholar]
- Stuart K.A., Riordan S.M., Lidder S. Hepatocyte growth factor/scatter factor-induced intracellular signalling. Int. J. Exp. Pathol. 2000;81:17–30. doi: 10.1046/j.1365-2613.2000.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi F., Ticozzi C., Burtin M. Subtotal but not unilateral nephrectomy induces hyperplasia and protooncogene expression. Am. J. Physiol. 1995;268(5 pt 2):F793–F801. doi: 10.1152/ajprenal.1995.268.5.F793. [DOI] [PubMed] [Google Scholar]
- Ueda S., Kato S., Matsuoka H. Regulation of cytokine-induced nitric oxide synthesis by asymmetric dimethylarginine: role of dimethylarginine dimethylaminohydrolase. Circ. Res. 2003;92:226–233. doi: 10.1161/01.res.0000052990.68216.ef. [DOI] [PubMed] [Google Scholar]
- Vallance P., Leone A., Calver A. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- Viau A., Karoui K.E., Laouari D. Lipocalin 2 is essential for chronic kidney disease in mice and human. J. Clin. Invest. 2010;120(11):4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang D., Zheng J. Actin cytoskeleton-dependent pathways for ADMA-induced NF-κB activation and TGF-β high expression in human renal glomerular endothelial cells. Acta Biochim. Biophys. Sin. 2012;44(11):918–923. doi: 10.1093/abbs/gms077. [DOI] [PubMed] [Google Scholar]
- Xu S.Y., Carlson M., Engstrom A. Purification and characterization of a human neutrophil lipocalin (HNL) from the secondary granules of human neutrophils. Scand. J. Clin. Lab. Invest. 1994;54(5):365–376. doi: 10.3109/00365519409088436. [DOI] [PubMed] [Google Scholar]
- Yang J., Blum A., Novak T. An epithelial precursor is regulated by the ureteric bud and by the renal stroma. Dev Biol. 2002;246:296–310. doi: 10.1006/dbio.2002.0646. [DOI] [PubMed] [Google Scholar]
- Yang J., Goetz D., Li J.Y. An iron delivery pathway mediated by a lipocalin. Mol. Cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- Zoccali C., Bode-Boger S., Mallamaci F. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: a prospective study. Lancet. 2001;358:2113–2117. doi: 10.1016/s0140-6736(01)07217-8. [DOI] [PubMed] [Google Scholar]


