Abstract
Basella alba is a soft green vegetable, survives in adverse environmental circumstances, for example, very cold temperature although the mechanism and the temperature sensitivity in this species are not clarified. Pot experiment for cultivation of B. alba was carried out to examine the effects of low temperature on the synthesis of two enzymes, polyphenol oxidase (PPO) and peroxidase (POD) in leaf of this plant. They were exposed to 8 °C for 24 h, 48 h and 72 h periods and the respective controls were kept in ambient room temperature for the above mentioned time. Low temperature causes the higher activity of PPO and the threshold level was found after 48 h period when compared to the respective controls. The activity was higher at 10 mM catechol, substrate for this enzyme, than 100 mM and 200 mM concentration, however, the three doses yielded the gradual increase in activity. Similar stimulatory effects on peroxidase (POD) activity in leaf were observed whenever the plants were exposed to cold for 24 h, 48 h and 72 h periods and maximal after 48 h period. Our findings demonstrate that the higher activity of these enzymes in leaf might be an index for the regulatory mechanism of the survival of these species in such adverse environment.
Keywords: Temperature stress, Metabolic effects, Basella alba, Adaptive response
1. Introduction
Basic stresses such as drought, salinity, temperature and chemical pollutants are simultaneously acting on the plants causing cell injury and producing secondary stresses such as osmotic and oxidative ones (Wang et al., 2003; Abu-Khadejeh et al., 2012). Plants could not change their sites to avoid such stresses but have different ways and morphological adaptations to tolerate these stresses. Environmental stress can disrupt cellular structures and impair key physiological functions of plants. Drought, salinity and low temperature stress impose an osmotic stress that can lead to turgor loss. Membranes may become disorganized, proteins may undergo loss of activity or be denatured and often excess levels of reactive oxygen species (ROS) are produced leading to oxidative damage. Recent investigations reveal that chilling induced injury is associated with the formation of reactive oxygen species (ROS) such as superoxide , hydrogen peroxide (H2O2), hydroxyl radical (OH−) and singlet oxygen (1O2) (Basra, 2001; Lee and Lee, 2000). To prevent the oxidative damage caused by such abiotic stress, plants generate the different mechanism by which they survive in such critical environment. Anti oxidative enzymes like superoxide dismutase (SOD), catalase (CAT) and peroxidase (PRX) are the most important components in the scavenging system of ROS. Several lines of evidences reveal that anti oxidative enzymes and anti oxidant molecules can neutralize ROS (Oidaira et al., 2000; Lee and Lee, 2000). Polyphenol oxidase (PPO) and peroxidase (POD) have been widely recognized to be an anti oxidative causing the biosynthesis of diverse metabolites essential for diagnosis and other purposes and have been found to be involved in the scavenging system of reactive oxygen species synthesized in the biological system. Polyphenol oxidases are enzymes with molecular weight of 60 kDa located in the chloroplast bound to thylakoid membranes, belonging to a group of copper containing metalloproteins and are members of oxido-reductases that catalyze the oxidation of a wide range of phenolic compounds by utilizing molecular oxygen (Queiroz et al., 2008). In the presence of atmospheric oxygen and PPO, monophenol is hydroxylated to o-diphenol and diphenol can be oxidized to o-quinones which then undergo polymerization to yield dark brown polymers (Chisari et al., 2007). Peroxidases are a single-polypeptide chain, heme containing enzymes with molecular weight between 28 and 60 kDa and have been involved to oxidize a wide variety of organic and inorganic substrates by reducing H2O2 and peroxides. They are mainly located in the cell wall (Chen et al., 2002) and are one of the key enzymes controlling plant growth and development. During the cold environment, these two enzymes might be involved in the prevention of oxidative damage in plant and therefore could be an essential index for the adaptive mechanism in adverse circumstances.
Basella alba (Pui) is a very soft leafy common vegetable available in Bangladesh and grows both in summer and winter and therefore, both seasons were believed to be involved in regulating metabolic alterations in this species of vegetable. The diverse clinical importance of this plant was demonstrated by recent investigations (Roshan et al., 2012; Premalatha and Rajgopal, 2005). In response to low temperature, these species of plant have been found to survive in the atmosphere although the physiological mechanism of survival is not clarified. It has been revealed that temperature variation is a common environmental phenomenon causing diverse metabolic alterations in plants and other organisms (Janska et al., 2010). Changes in environmental temperature affect the plant kingdom either by suppression of their total growth and development or by augmenting diverse physiological, metabolic and superficial changes. Moreover, low temperature has been recognized to be a major stimulatory effector involved in metabolic regulation and has been shown to cause the synthesis of ROS in plants (Mahajan and Tuteja, 2005). Therefore, it is assumed that variation of temperature may affect both metabolic activities as well as its biological importance of this species of plant. The aim of this study is to examine the interrelationship between anti oxidative status and preventive mechanism against temperature stress causing cell injury and physiological alterations in this vegetable and both PPO and POD might be involved in playing the critical role in this respect. Therefore, the current investigation has been undertaken to find the role of cold acclimation on the regulation of metabolic functions regarding the alteration and synthesis of PPO and POD in leaf of B. alba and may assist in the clarification of such stress induced mechanisms.
2. Materials and methods
2.1. Plant materials and low temperature treatment
For this experiment, two plastic pots were used; each pot size was 70 cm in diameter and 24 cm in height. An adequate amount of soil was taken in each plastic pot and the plastic pots were seeded with B. alba. For the germination of seeds, the following points were carried out: (i) the strong seeds were selected; the seeds were added to normal water and the floating seeds were discarded; (ii) the seeds were kept in normal water with temperature below 37 °C overnight; (iii) the seeds which were swollen by water absorption, were expected to be effective for germination; (iv) the seeds were seeded in the pots prepared with soil and the efficiency of seed germination was 65–75%. After 30 days of germination, the two different pots were described as control and low temperature induced plants. Control pot was used for 24 h, 48 h and 72 h treatments in the room temperature without cold acclimation. The second pot was used for 24 h, 48 h and 72 h duration in the temperature controlled cooling chamber and given cold exposure (8 °C) with full aeration. After the treatments, leaves were collected consecutively from each pot for 24 h, 48 h and 72 h duration and kept in −80 °C.
2.2. Assay of polyphenol oxidase (PPO) activity
The leaves of the different treatments (24 h, 48 h and 72 h) and their respective controls were homogenized with 22 mL of distilled water in a mortar kept on ice. Approximately, 1.5 g of low temperature induced and their respective control leaves were used for homogenization. The homogenates were centrifuged at 9000 rpm for 15 min and the supernatants were used as crude extract for assay of PPO activity spectrophotometrically as described by Mahadevan and Sridhar (1982) based on an initial rate of increase in absorbance at 495 nm where, catechol was used as substrate. One unit of enzyme activity is defined as a change in absorbance of 0.001 min−1 mL−1 of enzyme extract. For determination of PPO activity in leaf, 3 mL of 0.1 M phosphate buffer (pH 6.0) and 2 mL of crude enzyme extract were taken in the test tube and kept on ice. The contents were mixed, placed in a spectrophotometer using a cuvette and the absorbance was adjusted to zero at 495 nm. The cuvette was removed, 1 mL of catechol (10 mM, 100 mM and 200 mM) was added, quickly mixed by inversion and the changes in absorbance at 495 nm were recorded for up to 3 min (1, 2, 3 min). In all experiments, three replicates were performed for each sample. The following calculation was used to assay PPO activity in a sample: change in A495 = Af − Ai, where, Ai = initial absorbance reading and Af = final absorbance reading. Change in A495 for each sample was used to calculate the units of PPO activity and the activity is expressed as Unit g−1 of leaf weight.
2.3. Assay of peroxidase (POD) activity
The leaves of the different treatments (24 h, 48 h and 72 h) and their respective controls were homogenized with 15 mL of 0.5 M phosphate buffer (pH 7.0) in a mortar kept on ice. Approximately, 1.5 g of low temperature induced and their respective control leaves were used for homogenization. The homogenates were centrifuged at 9000 rpm for 15 min and the supernatants were used as crude extract for assay of POD activity as described by Daz et al. (2001). Peroxidase activity was determined spectrophotometrically at 470 nm using guaiacol as a phenolic substrate with hydrogen peroxide. The enzymatic oxidation of guaiacol changed the substrate into orange–pink product which was measured by spectrophotometer as a change in absorbance of 0.001 min−1 and the absorbance was recorded for up to 4 min. For determination of POD activity in leaf, 2.5 mL of 0.1 M phosphate buffer (pH 7.0), 2 mL of crude enzyme extract and 0.6 mL of 1% (v/v) H2O2 were taken in a test tube and kept on ice. The spectrophotometer was adjusted to zero initially and the content of test tube was transferred into a cuvette and the absorbance was taken as initial reading. 0.6 mL of 4% (v/v) guaiacol was added to the cuvette, quickly mixed by inversion and the change in absorbance at 470 nm was measured for 1, 2, 3 and 4 min. In all experiments, three replicates were performed for each sample. The following calculation was used to assay peroxidase activity in a sample: change in A470 = Af − Ai, where, Ai = initial absorbance reading and Af = final absorbance reading. Change in A470 for each sample was used to calculate the units of POD activity and the activity was expressed as Unit g−1 of leaf weight.
2.4. Statistical analysis
Results of the experiments were expressed as mean and standard error of different groups based on three independent determinations. The differences between the mean values were evaluated by ANOVA followed by paired t-test using SPSS software.
3. Results
3.1. Effect of 10 mM substrate concentration on PPO activity in leaf induced by low temperature
To properly identify physiological responses to environmental stress such as low temperature, plants were exposed to 8 °C in the temperature controlled cooling chamber for 24 h, 48 h and 72 h periods and the respective controls were kept in ambient room temperature. Polyphenol oxidase activities in leaf exposed to the cold temperature for the above mentioned periods were examined at 10 mM catechol, substrate for the enzyme. As shown in Table 1, the average PPO activity in leaf of vegetable in response to low temperature for 24 h period was 395.42 ± 57.77 Unit g−1 of leaf whereas for control leaf kept in ambient temperature, the PPO activity was 251.68 ± 75.97 Unit. A significant 57.11% (p < 0.05) increased PPO activity was observed after 24 h when compared to the control plant (Fig. 1A). The results appeared to indicate that the PPO activities were affected by cold acclimation. Therefore, it is reasonable that an adaptive response by the species of plant was created and the higher synthesis of PPO was observed to serve as the factor in adverse environmental situation and might be sensitive to the temperature variation. Leaves of B. alba were exposed to low temperature for 48 h period and the average PPO activity was 2122.33 ± 205.70 Unit while for the respective control plant, the enzyme activity was 1198.71 ± 187.98 Unit g−1 of leaf. The results indicated that 77.05% (p < 0.01) increased PPO had been found after 48 h in response to low temperature compared to the control plant as illustrated in Fig. 1A. The increased synthesis of PPO in leaf in response to low temperature might be involved in the regulation of metabolic functions of this species of plant. The alteration of PPO level in leaf is an index for characterization of the sensitivity to the environmental temperature. To find the optimum effect of cold acclimation on PPO level in leaf, the extended time was 72 h. As shown in Table 1, the low temperature induced leaf had PPO level 3168.25 ± 180.81 Unit while for the respective control leaf, the average PPO level was 2615.21 ± 281.47 Unit g−1 of leaf. The results showed that the PPO level in leaf had been enhanced significantly (p < 0.05) by 21.14% when the plants were exposed to cold temperature for 72 h when compared to control (Fig. 1A). The results appeared to indicate that the PPO levels were severely affected by cold acclimation for prolonged exposure, however, seems to be lower than 48 h period, therefore reasonably assumed to be optimally enhanced after 48 h of low temperature exposure. The results suggest that the increased PPO induced by low temperature might be caused by such abiotic stress and could be considered as the survival factor for this species of plant in critical environment.
Table 1.
Effect of low temperature on the regulation of PPO activity in leaf of Pui vegetable. The plants were exposed to 8 °C for 24 h, 48 h and 72 h in the cold chamber. After the treatments, the plants were immediately removed from the chamber and sampling of leaf was performed. For assay of PPO activity, 10 mM concentration of catechol was used as a substrate of the enzyme. Control plants were similarly used except giving low temperature exposure.
| Treatments | Polyphenol oxidase (PPO) activity (Unit g−1 of leaf) |
|---|---|
| Control | 251.68 ± 75.97 |
| 24 h | 395.42 ± 57.77A |
| Control | 1198.71 ± 187.98 |
| 48 h | 2122.33 ± 205.70B |
| Control | 2615.21 ± 281.47 |
| 72 h | 3168.25 ± 180.81A |
The results are means of ± SE for three values in each group.
p < 0.05 versus respective control.
p < 0.01 versus respective control.
Figure 1.
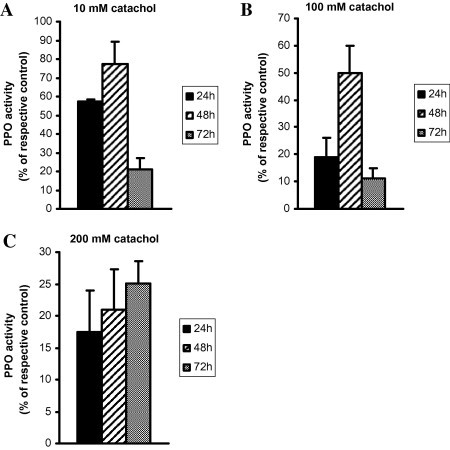
Alteration of PPO activity in response to 10 mM (A), 100 mM (B) and 200 mM (C) catechol in leaves of Basella alba during cold acclimation. The plants were exposed to 8 °C for 24 h, 48 h and 72 h in cold chamber, however, the respective controls were used without any cold acclimation. After the treatments, the leaves of plants and their respective controls were used for the assay of PPO activity. The results are expressed as percentage of the respective controls.
3.2. Effect of 100 mM substrate concentration on PPO activity in leaf induced by low temperature
The activity of PPO is related to the concentration of substrate and therefore, to get the maximal response of enzyme, 100 mM of catechol was used. As shown in Table 2, the PPO activity in response to 100 mM catechol in leaf of B. alba was recorded to determine the effect of low temperature on the regulation of this enzyme activity. After 24 h of treatment, the leaf enzyme level was calculated as 2837.02 ± 123.85 Unit for control and for low temperature induced plant, the value was 3377.29 ± 107.54 Unit g−1 of leaf. Low temperature causes a significant (p < 0.01) increase (19.04%) in PPO level when compared to the respective control, however, the response was found to be lower than 10 mM substrate concentration for the respective period of exposure (Fig. 1A and B). The increase in PPO activity determines the higher anti oxidative status for the prevention of chilling induced physiological stress of the plant. It is important to note that higher the oxidative stress, higher the synthesis of the enzyme thereby the plants survive in the adverse environment. To find the maximal response, plants were exposed to cold for 48 h period and the enzyme activity in leaf was 2199.33 ± 80.62 Unit, while for the respective control plant for the above mentioned time, the activity was recorded as 1465.54 ± 95.57 Unit g−1 of leaf. The results indicated that PPO activity in leaf had been increased by 50.06% significantly (p < 0.01) when compared to control; however, the activity was higher than that of 24 h period. Compared to 10 mm substrate concentration for the similar time, the PPO activity had been demonstrated to be lower (Fig. 1A and B). The increased activity in response to low temperature determines the higher conversion of phenolic compounds to the desirable products as this enzyme has higher specificity for the oxidation of phenolic compounds. Higher activity of PPO is an essential parameter for producing the colored pigment substantial for industrial and other purposes. Although the higher activity of PPO in response to the increased concentration of substrate was observed, the percentage of increase in activity in response to low temperature was found to be lower (Tables 1 and 2 and Fig. 1A and B). In response to low temperature for 72 h, the PPO activity in leaf was 3603.65 ± 115.46 Unit while the respective control leaf had 3238.99 ± 59.99 Unit enzyme activity for the above mentioned time. The results (shown in Table 2 and Fig. 1B) demonstrated that prolonged exposure of cold temperature had been involved in the synthesis of enzyme in leaf of this species of plant and the activity was increased by 11.25% significantly (p < 0.05) when compared to the respective control, however the efficiency of cold temperature on synthesis of enzyme was found to be lower than that of 24 h or 48 h period (Fig. 1B).
Table 2.
Effect of low temperature on the regulation of PPO activity in leaf of Pui vegetable. The plants were exposed to 8 °C for 24 h, 48 h and 72 h in the cold chamber. After the treatments, the plants were immediately removed from the chamber and sampling of leaf was performed. For assay of PPO activity, 100 mM concentration of catechol was used as a substrate of the enzyme. Control plants were similarly used except giving low temperature exposure.
| Treatments | Polyphenol oxidase (PPO) activity (Unit g−1 of leaf) |
|---|---|
| Control | 2837.02 ± 123.85 |
| 24 h | 3377.29 ± 107.54A |
| Control | 1465.54 ± 95.57 |
| 48 h | 2199.33 ± 80.62A |
| Control | 3238.99 ± 59.99 |
| 72 h | 3603.65 ± 115.46B |
The results are means of ± SE for three values in each group.
p < 0.01 versus respective control.
p < 0.05 versus respective control.
3.3. Effect of 200 mM substrate concentration on PPO activity in leaf induced by low temperature
To find the physiological role of low temperature on PPO activity in leaf of B. alba, we further determined PPO activity after 24 h, 48 h and 72 h periods of cold exposure in response to the higher dose of substrate. Table 3 shows the effect of cold temperature on PPO activity after 24 h of treatment. Plants exposed to low temperature had PPO level 5273.96 ± 240.33 Unit where as for control, the PPO activity 4490.94 ± 294.41 Unit g−1 of leaf was observed. The results indicated that the enzyme activity of Basella leaf had been increased by 17.43% significantly (p < 0.1) for low temperature treatment when compared to the respective control. Acclimation to cold similarly causes the enhanced PPO synthesis in prolonged time (48 h) and the plants may survive in such critical environment either by synthesis of PPO in their tissues or by other phenomenon. Fig. 1C also shows that the PPO level was increased significantly (p < 0.05) by 20.96% when they were exposed to 8 °C compared to the respective control where the values were 4232.43 ± 111.46 Unit and 5119.86 ± 146.11 Unit g−1 of leaf respectively for control and cold acclimation. After 72 h of treatment, low temperature causes the synthesis of PPO enzyme and the activity was 5548.87 ± 181.12 Unit while for the control leaf the value was recorded to be 4433.96 ± 56.60 Unit g−1 of leaf showing the higher synthesis of enzyme after 72 h period and enhanced significantly (p < 0.05) by 25.14% when compared to the respective control however the activity was not declined after 72 h of exposure rather increased than the 24 h or 48 h of exposure of low temperature. The results are also illustrated in Fig. 1C when expressed as percentage of control. Although the higher PPO level in leaf in response to higher dose of substrate was demonstrated during the experiment (Tables 1–3), the efficiency of low temperature on enhancing increased activity of PPO was assumed to be optimal and higher at 10 mM substrate concentration for 48 h of exposure. Therefore, the faster increase in enzyme activity in leaf in response to cold acclimation might be dependent on the substrate concentration as well as the period of exposure and might be involved in regulation of metabolism of phenolic substrates by which the plants survive in such a critical environment and gives a new insight for the prevention of the oxidative stress and might be involved to give a signal to the physiological level.
Table 3.
Effect of low temperature on the regulation of PPO activity in leaf of Pui vegetable. The plants were exposed to 8 °C for 24 h, 48 h and 72 h in the cold chamber. After the treatments, the plants were immediately removed from the chamber and sampling of leaf was performed. For assay of PPO activity, 200 mM concentration of catechol was used as a substrate of the enzyme. Control plants were similarly used except giving low temperature exposure.
| Treatments | Polyphenol oxidase (PPO) activity (Unit g−1 of leaf) |
|---|---|
| Control | 4490.94 ± 294.41 |
| 24 h | 5273.96 ± 240.33A |
| Control | 4232.43 ± 111.46 |
| 48 h | 5119.86 ± 146.11B |
| Control | 4433.96 ± 56.60 |
| 72 h | 5548.87 ± 181.12B |
The results are means of ± SE for three values in each group.
p < 0.1 versus respective control.
p < 0.05 versus respective control.
3.4. Time course effect of low temperature on POD activity in leaf of B. alba
Anti oxidative enzymes involved in scavenging system of reactive oxygen species (ROS) include peroxidase. They are a family of isoenzymes found in almost all plants; they are heme-containing monomeric glycoproteins that utilize either H2O2 or O2 to oxidize a wide variety of molecules. Peroxidase is an oxidoreductase that is directly involved in many plant functions such as hormone regulation, defense mechanisms, indolacetic degradation and lignin biosynthesis. Therefore, during cold acclimation, peroxidase might be involved in enzymatic defense of plant cells. To examine the role of low temperature on the regulation of POD activity in leaf of B. alba, plants in the pot were exposed to 8 °C in temperature controlled chamber for 24 h period and the respective control was kept in ambient room temperature. The average POD activity in response to low temperature was 3324.18 ± 185.05 Unit g−1 of leaf whereas for control leaves, the POD activity was 2144.82 ± 51.93 Unit. The results demonstrated that POD activity in leaf had been significantly enhanced and stimulated (54.98%, p < 0.05) by low temperature compared to the respective control (shown in Table 4 and Fig. 2). The increase in activity in response to low temperature might be the regulatory mechanism of enhancing the synthesis of compounds responsible for browning color since the enzyme has higher specificity for the phenolic substrate guaiacol. The higher the activity of this enzyme, higher the conversion of the phenolic substrate to colored o-quinones.
Table 4.
Changes of POD activity in leaf of Pui vegetable exposed to low temperature. The plants were exposed to 8 °C for 24 h, 48 h and 72 h in the cold chamber. After the treatments, the plants were immediately removed and sampling of leaf was performed. Control plants were similarly used except giving low temperature exposure.
| Treatments | Peroxidase (POD) activity (Unit g−1 of leaf) |
|---|---|
| Control | 2144.82 ± 51.93 |
| 24 h | 3324.18 ± 185.05A |
| Control | 2942.72 ± 68.01 |
| 48 h | 4961.71 ± 4.68B |
| Control | 2979.76 ± 118.22 |
| 72 h | 4530.46 ± 211.92B |
The results are means of ± SE for three values in each group.
p < 0.05 versus respective control.
p < 0.01 versus respective control.
Figure 2.
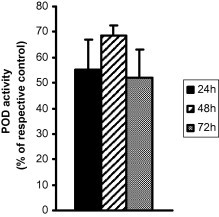
Alteration of POD activity in leaves of Basella alba during cold acclimation. The plants were exposed to 8 °C for 24 h, 48 h and 72 h in cold chamber, however, the respective controls were used without any cold acclimation. After the treatments, the leaves of plants and their respective controls were used for determination of POD activity. The results are expressed as percentage of the respective controls.
As shown in Table 4, the POD activity in leaves of plant was recorded to determine the effect of low temperature on POD synthesis for prolonged exposure. After 48 h of treatment, the leaf POD level was estimated as 2942.72 ± 68.01 Unit for control and for low temperature induced plant, the value was 4961.71 ± 4.68 Unit g−1 of leaf. Low temperature causes a significant and more pronounced increase in POD activity in leaf by 68.60% (p < 0.01) (Fig. 2) when compared to the respective control. The increase of POD activity in leaf was found to be higher than the previous 24 h of exposure as demonstrated in Fig. 2. Therefore, the activity of this enzyme in leaf is assumed to be regulated by the variation of temperature and be strictly followed by the extension of time.
Table 4 also shows the effect of low temperature on POD activity in leaf of plant after 72 h of exposure. Plants acclimated to low temperature had leaf POD level 4530.46 ± 211.92 Unit, whereas 2979.76 ± 118.22 Unit g−1 of leaf for control plant was observed during the experiment. As the time extended, the average POD activity in leaf had been enhanced in response to 8 °C and was increased both for control and low temperature induced plants. Low temperature for prolonged exposure causes increased synthesis of POD significantly (p < 0.01) by 52.04% compared to the control (Fig. 2), however, the effect was lower than the previous 24 h or 48 h of exposure. Although the optimum activity of POD in leaf was observed after 48 h of exposure in response to low temperature, the gradual increase in activity for both the control and cold acclimated plants was noted as the time extended. Therefore, the synthesis of POD in leaf was found to be augmented in response to low temperature time dependently. The results suggest that the increased POD activity in leaf might be due to the higher sensitivity of temperature and caused by temperature stress in the environment where they survive and could be considered as the survival factor as well as index for characterization of physiology of leaf of this species of plant.
4. Discussion
To understand the mechanism of plant species responses to low temperature regarding the physiological and adaptive responses, assay of PPO and POD activity in leaf of B. alba was performed. In this respect, plants were grown in pot and exposed to 8 °C for 24 h, 48 h and 72 h periods. In the present study, low temperature has been found to be involved in causing higher PPO and POD activities in leaf however the effects were more pronounced after 48 h of the exposures. The mechanism of formation of these enzymes in response to the temperature stress is not known in this species of plant, however, several lines of evidences might be involved to clarify and recognize the formation of these molecules in such adverse situation. It has been shown that low temperature causes the higher oxidative stress inducing the synthesis of active oxygen species (AOS) (Lee and Lee, 2000) and increases tolerance to AOS in cereals and with an increase in anti oxidative enzymes (Anderson et al., 1995; Seebba et al., 1999). Anti oxidative enzymes can neutralize AOS (Oidaira et al., 2000) and thereby prevents the cellular membranes and organelles from the damaging effects of AOS (Foyer et al., 1991). It is reasonable that fluctuation of temperature can cause stress to the normal physiological functions of plants, and hence alteration of metabolic activities in leaf of the plant might be observed. Previous studies revealed that low temperature had been associated with pronounced modifications in the ultrastructure of leaf cells, disorganization of cellular compartments (Stefanowska et al., 2002) and therefore, may induce the synthesis of new enzymes and proteins. B. alba is a common green vegetable grown in Bangladesh and other countries during both winter and summer seasons. Therefore, the plants have the higher sensitivity to these temperatures; however, the plants survive in very cold environment although the mechanism is not clarified. Since low temperature causes the significant alteration in metabolic functions of plant and has been revealed to cause reactive oxygen species (ROS), they therefore might be involved in causing the synthesis of diverse metabolites essential for various purposes. The reactive oxygen species (ROS) have been shown to cause the injury in plants during the critical circumstance and therefore, to survive in this environment, plants generate different mechanisms and synthesize the compounds.
To find the optimum effect of catechol, dose response characteristics of substrate for the enzyme PPO have been adopted in this study. Among the different concentration of catechol, 10 mM concentration has been found to cause the higher response although the three different concentrations of catechol (10 mM, 100 mM and 200 mM) produced the gradual increase in PPO activity for both the control and low temperature induced plants showing the validity of the substrate effect. Accordingly, the higher dose of catechol enhanced the PPO activity maximally in leaf extract therefore, higher colored quinones were synthesized which might be an effective approach for producing the essential pigments. Polyphenol oxidase catalyzes the oxidation of phenol to quinone which can covalently modify and crosslink various cellular nucleophiles, undergo melanin-forming auto oxidation reactions, or participate in other reactions. The enzymes are found in higher plants and responsible for the enzymatic browning of raw fruits and vegetables. Such reactions are generally considered to be undesirable in food preservation and processing because of the unpleasant appearance and the concomitant development of a substandard flavor. Several lines of evidences revealed that PPO had been considered to be an important reagent for clinical diagnosis and micro-analytical immunoassays because of its high sensitivity (Siers, 1991) while many fruits and vegetables contain POD in amounts that contribute to browning-like reactions (Vamos-Vigyazo, 1981). Although PPO is involved primarily in the degradation of phenolics, they can also be degraded by POD (Thypyapong et al., 1995) and the activities of both enzymes have been found to increase in response to biotic and abiotic stresses (Kwak et al., 1996). Both PPO and POD have been considered in defensive mechanisms for plants against stress (Vamos-Vigyazo, 1981). Oxidative stress can arise from an imbalance between the generation and elimination of reactive oxygen species (ROS), leading to excess ROS levels that causes indiscriminate damage to virtually all biomolecules, leading, in turn, to various diseases and cell death (Scandalios, 2005). Reactive species can be eliminated by a number of enzymatic and non-enzymatic antioxidant defense mechanisms (Boullier et al., 2001) therefore, the higher activity of PPO and POD in response to low temperature in species of Pui vegetable might be linked to the defense mechanisms and the results are consistent with their findings.
Abiotic stress leads to a series of molecular, biochemical, physiological and morphological changes that adversely affect plant growth and productivity. Low temperature is a major factor limiting the productivity and geographical distribution of many species, including important agricultural crops. Higher plants manifest a unique capability of the synthesis of a large amount of diverse molecules so-called secondary metabolites, such as phenolic compounds and the synthesis and release of phenolics are induced by various biotic and abiotic factors (Makoi and Ndakidemi, 2007). It has been demonstrated in the previous study that these enzyme activities increased in response to different types of stress, both biotic and abiotic (Yadegari et al., 2007). More specifically, both enzymes have been related to the appearance of physiological injuries caused by thermal stress. During the experiment, it was observed that both low temperature induced and their respective controls caused the color pigmentation quickly, however low temperature induced leaves had higher pigmentation than the control, therefore, it is reasonable that cold acclimation causes the higher oxidation of phenolic compounds and might be an effective approach for producing the colored pigment essential for the several purposes. Of course, the phenomenon is a substantial mechanical and physiological way by which the plants survive in the adverse environment. The oxidation of phenolic compounds might be related to the temperature variation in the environment. It has been observed that the optimum PPO activity was found in the range of 25–30 °C and then declined at temperature above 40 °C (Doğan and Doğan, 2004).
Metabolic adjustments in response to unfavorable conditions are dynamic and not only depend on the type and strength of the stress, but also on the cultivar and the plant species. Some metabolic changes are common to salt, drought, and temperature stress, whereas others are specific. Enzymatic browning is a significant problem in a number of fruits and vegetables such as strawberry (Chisari et al., 2007), grape (Muñoz et al., 2004), potato (Lee and Park, 2007) and lettuce (Gawlik-Dziki et al., 2008). The discoloration in fruits and vegetables by enzymatic browning, resulting from conversion of phenolic compounds to o-quinones which subsequently polymerize to be a brown or dark pigment and the enzymes involved in these processes are PPO and POD (Jiang et al., 2004). Because PPO and POD are the main enzymes involved in phenolic oxidation of many fruits and vegetables, their activities have attracted much attention. The relationship between the degree of browning and PPO activity was studied in processing apple varieties to provide reference for raw material selection (Ye et al., 2007). Recent studies reveal that cold acclimation adversely affects physiological and morphological structures of plants (Stefanowska et al., 1999) and the nutritional deficiency has been observed in response to cold. Therefore, it is reasonable that adverse oxidative effects caused by cold acclimation might be correlated to the alteration of physiology of leaf of B. alba and also to the nutritional deficiencies particularly the uptake of essential nutrients from the soil and also from the environment. Further studies are needed to clarify the mechanisms linked to the above approaches. Measurement of PPO and POD in leaf of B. alba might be an essential approach and will give a new insight to clarify the mechanism of diverse metabolic functions of plant as well as help in analysis of physiology of B. alba. Moreover, regulation of these enzymes is not only mediated by cold environment but also might be by other chemical mediators in the environment.
5. Conclusion
It is obvious from the current investigation that low temperature induces a severe metabolic alterations regarding the enhancement of anti oxidative enzyme activity in leaf therefore, assumes to be involved in the alteration of physiology of plants B. alba. The adverse environment caused by low temperature produced severe effect on the plants and thereby plants face the stress to physiological and molecular level and therefore, different regulatory metabolic alterations were observed in the circumstances. Chilling induced oxidative stress and injury is frequently observed in the critical environment and to overcome these effects, some enzymes are over expressed where PPO and POD might be involved. Species adapted by natural selection to cold environments have evolved a number of physiological and morphological means to improve survival in the face of extended cold periods. During low temperature acclimation, plants possess nutritional or energy deficiency as they survive in such critical circumstances however, the complications might be due to the higher oxidative effects and the enzymes may play the critical role in this respect.
Acknowledgements
This study was carried out in the Department of Biochemistry and Molecular Biology, Rajshahi University and was supported by the University Grant Commission (UGC), Bangladesh.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abu-Khadejeh A., Shibli R., Makhadmeh I., Mohammad M. Influence of increased salinity on physiological responses of hydroponic grown tomato (Lycopersicon esculentum Mill.) Jordan J. Agric. Sci. 2012;8(3):321–331. [Google Scholar]
- Anderson M.D., Prasad T.K., Stewart C.R. Changes in isozyme profiles of catalase, peroxidase and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol. 1995;109(4):1247–1257. doi: 10.1104/pp.109.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basra A.S. Crop responses and adaptations to temperature stress. In: Prasad T.K., editor. Mechanisms of Chilling Injury and Tolerance. Haworth Press Inc; New York: 2001. pp. 1–34. [Google Scholar]
- Boullier A., Bird D.A., Chang M.K., Dennis E.A., Friedman P., Gillotre-Taylor K., Horrko S., Palinski W., Quehenberger O., Shaw P., Steinberg D., Terpstra V., Witztum J.L. Scavenger receptors, oxidized LDL, and atherosclerosis. Ann. NY. Acad. Sci. 2001;947:214–222. doi: 10.1111/j.1749-6632.2001.tb03943.x. [DOI] [PubMed] [Google Scholar]
- Chen E.L., Chen Y.A., Chen L.M., Liu Z.H. Effect of copper on peroxidase activity and lignin content in Raphanus sativus. Plant Physiol. Biochem. 2002;40(5):439–444. [Google Scholar]
- Chisari M., Barbagallo R.N., Spagna G. Characterization of polyphenol oxidase and peroxidase and influence on browning of cold stored strawberry fruit. J. Agric. Food Chem. 2007;55(9):3469–3476. doi: 10.1021/jf063402k. [DOI] [PubMed] [Google Scholar]
- Daz J., Bernal A., Pomar F., Merino F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci. 2001;161(1):179–188. [Google Scholar]
- Doğan S., Doğan M. Determination of kinetic properties of polyphenol oxidase from Thymus (Thymus logicaulis subsp. chaubardii var. chaubardii) Food Chem. 2004;88(1):69–77. [Google Scholar]
- Foyer C., Lelandais M., Galap C., Kunert K.J. Effect of elevated cytosolic glutathione reductase activity on the cellular glutathione pool and photosynthesis in leaves under normal and stress conditions. Plant Physiol. 1991;97(3):863–872. doi: 10.1104/pp.97.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawlik-Dziki U., Zlotek U., Swieca M. Characterization of polyphenol oxidase from butter lettuce (Lactuca sativa var. capitata L.) Food Chem. 2008;107(1):129–135. [Google Scholar]
- Janska A., Marsik P., Zelenkova S., Ovesna J. Cold stress and acclimation-what is important for metabolic adjustment? Plant Biol. 2010;12(3):395–405. doi: 10.1111/j.1438-8677.2009.00299.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y.M., Duan X.W., Joyce D., Zang Z.Q., Li J.R. Advances in understanding of enzymatic browning in harvested litchi fruit. Food Chem. 2004;88(3):443–446. [Google Scholar]
- Kwak S.S., Kim S.K., Park I.H., Liu J.R. Enhancement of peroxidase activity by stressed-related chemicals in sweet potato. Phytochemistry. 1996;43(3):565–568. [Google Scholar]
- Lee D.H., Lee C.B. Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Sci. 2000;159(1):75–85. doi: 10.1016/s0168-9452(00)00326-5. [DOI] [PubMed] [Google Scholar]
- Lee M.K., Park I. Studies on inhibition of enzymatic browning in some foods by Du-Zhong (Eucommia uimoides Oliver) leaf extract. Food Chem. 2007;114:154–163. [Google Scholar]
- Mahadevan A., Sridhar R. second ed. Sivakami Publications; Madras, India: 1982. Methods in Physiological Plant Pathology. p. 316. [Google Scholar]
- Mahajan S., Tuteja N. Cold, salinity and drought stresses: an overview. Arch. Biochem. Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Makoi J.H.J.R., Ndakidemi P.A. Biological, ecological and agronomic significance of plant phenolic compounds in rhizosphere of the symbiotic legumes. Afr. J. Biotechnol. 2007;6(12):1358–1368. [Google Scholar]
- Muñoz O., Sepúlveda M., Schwartz M. Effects of enzymatic treatment on anthocyanin pigments from grapes skin from Chilean wine. Food Chem. 2004;87(4):487–490. [Google Scholar]
- Oidaira H., Satoshi S., Tomokazu K., Takashi U. Enhancement of antioxidant enzyme activities in chilled rice seedlings. Plant Physiol. 2000;156:811–813. [Google Scholar]
- Premalatha B., Rajgopal G. Cancer–an ayurvedic perspective. Pharmacol. Res. 2005;51:19–30. doi: 10.1016/j.phrs.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Queiroz C., Lopes M.L.M., Fialho E., Valente-Mesquita V.L. Polyphenol oxidase: characteristics and mechanisms of browning control. Food Rev. Int. 2008;24(4):361–375. [Google Scholar]
- Roshan A., Naveen K.H.N., Shruthi S.D. A review on medicinal importance of Basella alba L. Int. J. Pharm. Sci. Drug Res. 2012;4(2):110–114. [Google Scholar]
- Scandalios J.G. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005;38(7):995–1014. doi: 10.1590/s0100-879x2005000700003. [DOI] [PubMed] [Google Scholar]
- Seebba F., Sebustiani L., Vitagliano C. Protective enzymes against activated oxygen species in wheat (Triticum aestivum L.) seedlings: responses to cold acclimation. J. Plant Physiol. 1999;155:762–768. [Google Scholar]
- Siers H. Oxidative stress: from basic research to clinical application. Am. J. Med. 1991;91:31–38. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- Stefanowska M., Kuras M., Kubacka-Zebalska M., Kacperska A. Low temperature affects of growth and structure of cell walls in winter oilseed rape (Brassica napus L. var. oleifera L.) plants. Ann. Bot. 1999;84:313–319. [Google Scholar]
- Stefanowska M., Kuras M., Kacperska A. Low temperature-induced modifications in cell ultrastructure and localization of phenolics in winter oilseed rape (Brassica napus L. var. oleifera L.) leaves. Ann. Bot. 2002;90:637–645. doi: 10.1093/aob/mcf241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thypyapong P., Hunt M.D., Steffens J.C. Systemic wound induction of potato (Solanum tuberosum) polyphenol oxidase. Phytochemistry. 1995;40(3):673–676. [Google Scholar]
- Vamos-Vigyazo L. Polyphenoloxidase and peroxidase in fruits and vegetables. CRC Crit. Rev. Food Sci. Nutr. 1981;15:49–126. doi: 10.1080/10408398109527312. [DOI] [PubMed] [Google Scholar]
- Wang W., Vinocur B., Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218(1):1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- Yadegari L.Z., Heidari R., Carapetian J. The influence of cold acclimation on proline, malondialdehyde (MDA), total protein and pigments contents in soybean (Glycine max) seedlings. J. Biol. Sci. 2007;7(8):1436–1441. [Google Scholar]
- Ye S., Yo-Xin Y., Heng Z., Yuan-Peng D., Feng C., Shu-Wei W. Polyphenolic compound and the degree of browning in processing apple varieties. Agric. Sci. China. 2007;6(5):607–612. [Google Scholar]


