Abstract
The hot and arid lowlands of southwestern Saudi Arabia are home to two common lianas, Cocculus pendulus and Leptadenia arborea. This paper attempts to relate the adaptation of these two climbing woody perennials to such a harsh environment to the anatomy and hydraulic characteristics of their wood. The stems of these lianas have wood with wide xylem vessels and high hydraulic conductivity which should enhance water flow to the upper canopy despite their severe twisting. Hydraulic conductivity is further helped by the simple perforation plates of xylem vessels. The circular thickening of xylem walls gives them strength and reduces the risk of their collapse and the ensuing embolism in the advent of high tension created by severe water deficit and high evapo-transpiration demand. Wide vessels, on the other hand, are more susceptible to embolism. This problem may be overcome by reducing the solute potential of xylem sap by hydrolysis of starch grains which were found to be abundant in the vicinity of the vessels. This should help absorb water by the deep roots from the capillary fringes of the typically shallow water table in this particular habitat. Furthermore, the abundance of ray parenchyma cells between xylem groups of both lianas provides great flexibility with minimum damage to water conduits in the stem during climbing and twisted growth. It was concluded that these wood features in both lianas are crucial for survival under the harsh conditions of arid Tihama plains of southwestern Saudi Arabia.
Keywords: Cocculus pendulus, Leptadenia arborea, Lianas, Wood anatomy, Ecology, Saudi Arabia
1. Introduction
Lianas are woody plant climbers that begin life as terrestrial seedlings, but need the physical support of nearby trees (or any other supports) for their weak stems and branches to lean on and ascend to get better exposure to sunlight (Gentry, 1991; Maheshwari et al., 2009). Climbing can also put their canopies beyond the reach of most herbivorous animals. However, the twisting that their stems undergo while climbing can cause physical damage and deformation to their tissues; as a result, water flow to the upper canopy can be constrained, especially if these species grow in dry habitats.
The low lands of southwestern Saudi Arabia (Tihama plains of Jazan province) represent a harsh habitat with prolonged periods of drought. The mean annual precipitation is 150 mm while the mean annual temperature is 30–31 °C, making this region among the hottest and driest in Saudi Arabia (Abdel-Rahman and Balegh, 1974; Masrahi, 2012). Moreover, the rainy season coincides with summer when evaporation is highest (Masrahi, 2012). According to Walter’s classification of climatic zonobiomes, Tihama plain of Jazan represents a hot desert (Breckle, 2002). The eastern part of the plain and the adjacent rocky hills (100–700 m above sea level) have some relatively “mesic” habitats in dry wadi (ravines) beds and around villages. The dominant vegetation consists of scattered xerophytes like Acacia spp., Panicum turgidum and Salvadora persica as well as halophytic vegetation along the coast (Masrahi, 2012).
Despite its harsh dry climate, Tihama plain is home to two common lianas, Cocculus pendulus (J.R. & G. Forst.) Diels (Menispermaceae) and Leptadenia arborea (Forssk.) Schweinf. (Apocynaceae-Asclepiadoideae) (Masrahi, 2012). The former is a white-stemmed liana, grows in the dry sandy plain of Tihama, while the latter has light brown stems with thick corky bark in the older stems and grows in the eastern part of the plain and adjacent rocky hills, as well as around villages. These species climb by their twining stems over shrubs and trees, especially Acacia spp. The wide distribution of these two lianas in such harsh habitats is peculiar since most lianas are only found in humid forests or along river banks (Gentry, 1991); this implies a high degree of adaptation to unfavorable climatic conditions especially drought.
The objective of the present study was to investigate stem wood anatomy of two lianas, C. pendulus and L. arborea, and its ecological significance in the context of the hot desert of southwestern Saudi Arabia.
2. Materials and methods
Stems of C. pendulus and L. arborea plants were collected during March and April, 2012 from Tihama coastal plain and low rocky habitats east of Tihama in Jazan province, southwestern Saudi Arabia (Fig. 1). The stem were cut and stored in 70% ethanol until examined. Transversal sections of the stems were cut with a sharp razor. The sections were then stained with neutral red (Foster, 1965) and examined under an optical microscope (Zeiss Scope A1 with an AxioCam camera, Germany) to estimate the average xylem vessel diameter and frequency. The vessel diameter values presented are the means of more than 25 determinations as recommended by the IAWA Committee (1989). Vessel frequency (VF) represents the number of vessels per mm2 of stem’s cross-sectional area. The vessels with a diameter between 25 and 100 μm were considered narrow; those with a diameter larger than 100 μm were considered wide while the vessels with a diameter less than 25 μm were not considered because of their limited contribution to water conductivity (Ewers et al., 1997; Gutiérrez et al., 2009). Relative hydraulic conductivity (HC) was estimated using the modified Hagen–Poisseuille equation (Carlquist, 2001), while vulnerability to cavitation (vulnerability index, VI) was calculated using the equation proposed by Carlquist (1977), as follows:
where r is vessel radius in μm, VF is vessel frequency (N/mm2) and VD is the vessel diameter in μm.
Figure 1.
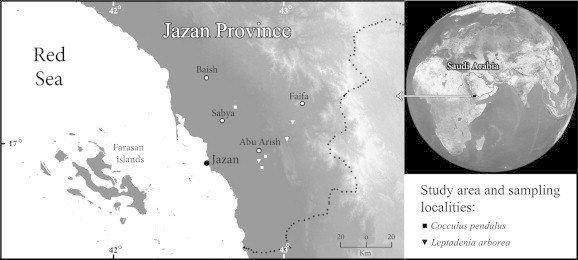
Study area and sampling localities of tow lianas.
Another set of stem samples were examined with a scanning electron microscope (SEM). The samples were placed on the double side carbon tape on an aluminum stub. The specimens were examined without coating by a field emission SEM (QUANT FEG 450, Amsterdam, Netherlands).
Leaf stomatal density (N/mm2) was estimated microscopically, while leaf surface to volume ratio (S/V) was determined according to Mauseth (2000). These leaf anatomical features greatly affect water flow in the xylem.
Data were statistically analyzed using student t-test.
3. Results
The growth habit of the stems of C. pendulus and L. arborea is illustrated in Figs. 2 and 4, respectively. The transverse-section (TS) of mature stems of the two species reveals cambial variants represented by anomalous secondary growth (Figs. 3 and 5, respectively). TS of mature stems of C. pendulus showed successive rings of cambia (successive rings of xylem alternating with phloem) (Figs. 2b, 3a and b). Ray parenchyma cells revealed a dense accumulation of starch grains (Fig. 3d). TS of mature stems of L. arborea (Figs. 4b, 5a and b) showed segmented groups of xylem with inter-xylary phloem separated by relatively large rays. The segmented groups of xylem are sheathed with fibers. Ray parenchyma cells of this species also showed a dense accumulation of starch grains (Fig. 5d). Stem xylem parenchyma cells were more abundant in L. arborea compared to C. pendulus.
Figure 2.
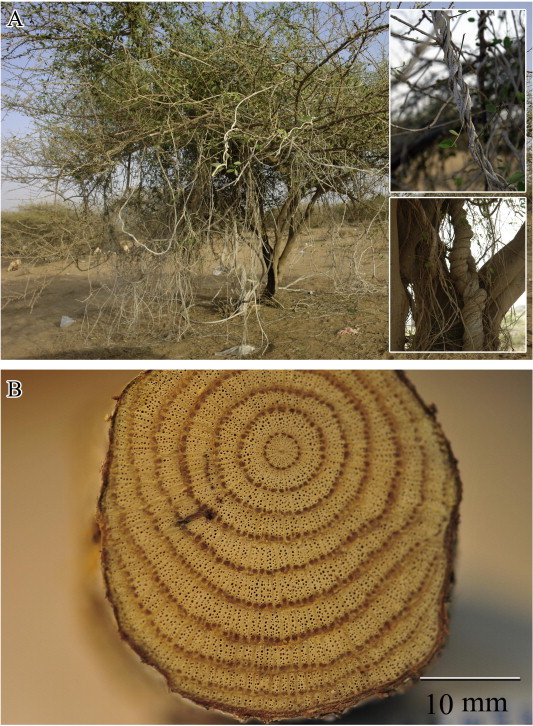
Cocculus pendulus, (A) Stems growing on Acacia tortilis with the nature of twining (in the frames). (B) Cross-section of the stem reveals successive rings of cambia.
Figure 4.

Leptadenia arborea, (A) Stems growing on Acacia ehrenbergiana. Nature of twining on fence (in the frame). (B) Cross-section of the stem.
Figure 3.
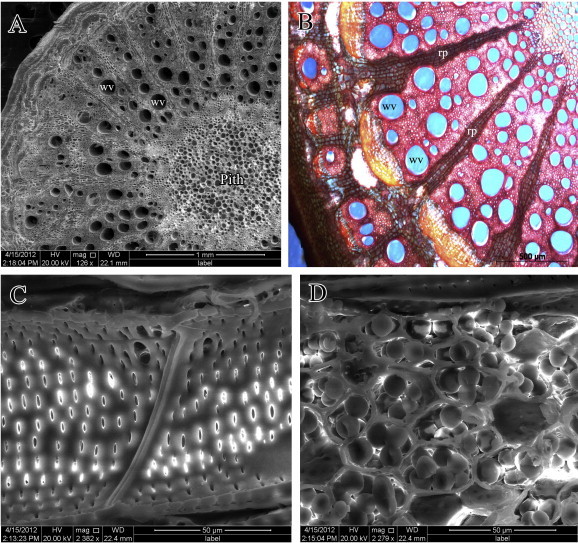
Cocculus pendulus, (A and B) Scanning electron photomicrograph and photomicrograph of stem cross-section reveal wide vessels (wv) and ray parenchyma (rp). (C) Scanning electron photomicrograph of a vessel shows simple perforation plate and circular thickening of wall. (D) Scanning electron photomicrograph of Xylem parenchyma reveals a dense accumulation of starch grains.
Figure 5.
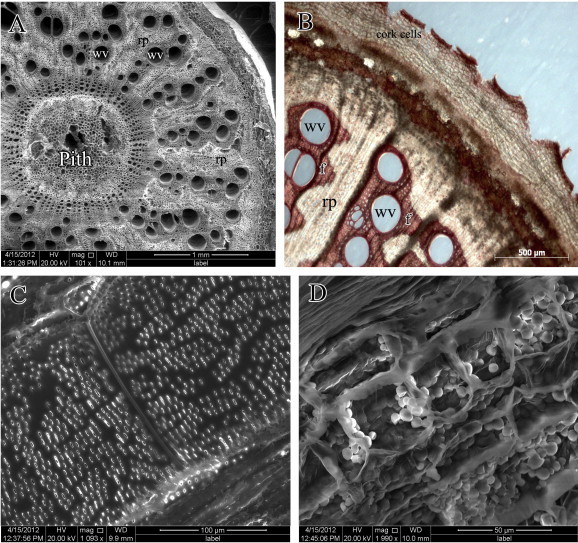
Leptadenia arborea, (A and B) Scanning electron photomicrograph and photomicrograph of stem cross-section reveal wide vessels (wv) and ray parenchyma (rp). Note that xylem groups are sheathed by fibers (f), and stem covered by thick corky bark. (C) Scanning electron photomicrograph of a vessel shows simple perforation plate and circular thickening of wall. (D) Scanning electron photomicrograph of Xylem parenchyma reveals a dense accumulation of starch grains.
Table 1 shows the quantitative anatomical features of stem wood and leaves of the two species. Vessel diameter of both species was large but L. arborea had wider vessels than C. pendulus (P < 0.001).
Table 1.
Anatomical characters of C. pendulus and L. arborea.
| Species | VD (μm) | VF (N/mm2) | HC | PP | TWT | VI | SF of leaf (N/mm2) |
S/V ratio of leaf | |
|---|---|---|---|---|---|---|---|---|---|
| Upper | Lower | ||||||||
| Cocculus pendulus | 128.4 ± 44.4⁎ | 32 ± 14 | 530872.4 | Simple | Circular | 4 | 25.6 ± 4.6 | 59 ± 8.2⁎ | 9 ± 1.3⁎⁎ |
| Leptadenia arborea | 168.6 ± 48.8⁎ | 16 ± 3 | 3156387.5 | Simple | Circular | 10.5 | 29.3 ± 2.3 | 30 ± 8 | 7.5 ± 0.8⁎⁎ |
VD = Vessel diameter, VF = Vessel frequency, HC = Hydraulic conductivity, PP = Perforation plates, TWT = Type of wall thickening, VI = Vulnerability index, SF of leaf = Stomatal frequency of leaf, S/V ratio of leaf = surface to volume ratio of leaf.
Significance at P < 0.001.
Significance at P < 0.05.
Vessel frequency was low in the stems of both species, with no significant difference between them (P > 0.05). Calculated hydraulic conductivity was high in both species but was higher in the stems of L. arborea than in the stems of C. pendulus by about 6 fold. Perforation plates of xylem vessels of both species were simple. The type of wall thickening of both species was circular (Figs. 3c and 5c). Vulnerability index was high (more than 1) for both species but L. arborea showed higher vulnerability index values than C. pendulus by about 2.5 fold.
The lower surface of C. pendulus leaves had a higher mean stomatal density than the upper surface (P < 0.001). In contrast, L. arborea had similar stomatal densities on the upper and lower sides of its leaves (P > 0.05). The Upper surfaces of the leaves of the two species did not differ in stomatal density, whereas the lower surfaces of C. pendulus leaves showed higher stomatal densities than L. arborea. Leaf surface to volume ratio was higher in C. pendulus than in L. arborea (P < 0.05).
4. Discussion
The occurrence and survival of any plant species depend on the degree of its adaptation to its habitat. In xeric habitats, like the Tihama plains of Jazan, plants differ widely in their capacity to cope with drought, the most limiting factor to growth in such context. Adaptations exist to explain these differences, which can be mainly attributed to a plant’s capacity to maintain a favorable water status (Gutterman, 1993; Kozlowski and Pallardy, 2002). Many adaptations of woody plants have been identified in their stems, chief among them is the low resistance to water flow in vascular tissues (Kozlowski and Pallardy, 2002).
The two studied lianas had wide xylem vessels in their stem’s wood with high calculated hydraulic conductivity. Typically, liana stems tend to have unusually wide (large diameter) xylem vessels (Carlquist, 1985, 1991; Rowe and Speck, 2005; Angyalossy et al., 2012). In fact, the wide vessels appear to compensate also for the limited cross-sectional area of the stems which can achieve great lengths while remaining proportionately narrow in diameter; they appear to do so by spending greater amounts of energy on elongation than on radial growth. In doing so, the cylinder of tissue is modified in which more effective water-conducting cells (vessel elements) are produced at the expense of mechanical cells (fibers) in contrast to the situation in self-supporting woody plants (trees and shrubs) (Carlquist, 1985). Wide vessels also offer low friction and deliver larger volumes of water per unit time (Zimmermann, 1983; Mauseth, 1988; Ewers et al., 1991; Sperry, 1995).
The two lianas have high water conducting capacity. Simple perforation plates of xylem vessels of the two lianas should enhance the conductivity of water and reduce the resistance to water flow while the circular thickening of xylem walls gives them strength against collapse under increased tension (Mauseth, 1988; Baas et al., 2004). Strength of xylem is also enhanced by sheathing with fibers as in L. arborea. This species also had wider vessels and higher hydraulic conductivity than C. pendulus; this may be related to the relatively “mesic” nature of L. arborea’s habitat.
However, whereas increased vessel diameter greatly increases water conduction efficiency, it also decreases safety and renders vessels more vulnerable to cavitation, that is, the formation of air bubbles within the conduits resulting in breakage of water columns. Conduit diameter is directly related to cavitation frequency (Sperry, 1995). Cavitation can precipitate an air embolism; the embolism spreads from element to element through the perforations on xylem walls, and the entire vessel becomes useless (Zimmermann, 1983; Mauseth, 1988; Evert, 2006). The resulting reduction in water supply to the leaves can lead to water stress and then eventually death of the plant (Zimmermann, 1983). Vulnerability index of both species showed high values, but L. arborea was 2.5 times more vulnerable than C. pendulus. This vulnerability due to wood structure may explain why most lianas grow in very moist areas, such as rain forests or along riverbanks, where water stress never occurs. Lianas make up about one-fifth of all plant types in these habitats (Gentry, 1991). The lack of safety mechanisms against drought-induced embolism in such lianas would not be surprising (Carlquist, 1985). Lianas tend to avoid areas prone to extreme drought. Generally, in most habitats with limited water resources, lianas represent only a small proportion of the flora. For instance, in southwestern Saudi Arabia, liana species represent only about 0.8% of the total flora (Masrahi, 2012). Furthermore, the advantage of the climbing habit diminishes when arboreal canopies are infrequent as is the case in this area (Carlquist and Hoekman, 1985). Nevertheless, some of them do occur in places where drought is occasional and moderate, but even in this case, they generally lose their leaves during the dry season (Gartner et al., 1990; Angyalossy et al., 2012).
In both species, ray parenchyma cells near the vessel groups showed a dense accumulation of starch grains, which, besides supporting growth, may enhance water flow and reduce the risk of embolism by hydrolysis into soluble sugars which enter the vessels and reduce the solute potential of the sap as shown to be the case in other lianas (Carlquist, 1985, 2001).
As phreatophytes, lianas have deep root systems (commonly grow 5–10 m deep) that proliferate in the capillary fringe (phreatic zone) just above the water table (Nilsen and Orcutt, 1996; Andrade et al., 2005; Johnson et al., 2013). Thus, these two lianas seem to have a relatively stable supply of ground water. This idea may be supported by the fact that the depth of ground water table in Tihama plains generally varies between 5 and 20 m depending on proximity to wadi courses (Müller et al., 1984). The two lianas, especially C. pendulus, frequently grow on Acacia species, mainly A. ehrenbergiana and A. tortilis, which grow mainly along wadi courses (Masrahi, 2012).
Ray parenchyma in the stems of both species separates the groups of xylem into sectors. L. arborea had a greater abundance of parenchyma compared to C. pendulus. Parenchyma between wood segments provides great flexibility, permitting lianas to adapt to shifts induced by growth around supporting trees. This permits torsion of the stems with minimum damage to vessels and sieve tubes (Sieber and Kučera, 1980; Carlquist, 2001; Rowe and Speck, 2005). Parenchyma is also responsible for the repair of the vascular system by formation of new cambia which can replace deactivated conducting cells with new functional ones. Redundancy of conducting tissues, when it is scattered throughout a stem, provides a degree of safety that permits non injured xylem and phloem to continue conduction even though some parts of a stem are injured (Angyalossy et al., 2012). This was evident in L. arborea whose stems tend to twist severely around its support (Fig. 4a – in the frame).
In Tihama plains, C. pendulus and L. arborea behave as evergreens with dense canopies overwhelming their hosts (Y.S. Masrahi, unpublished observations). Therefore, water supply to their leaves does not seem to be jeopardized even during the long dry season. Factors influencing water supply play a central role in the adaptation of plants to their environment (Nobel, 2009). The flow of water from the soil to the leaves requires regulation of plant water potential by stomatal control and leaf area adjustment which may be necessary to maximize water uptake on the one hand, while avoiding loss of hydraulic contact with the soil water (due to vessel embolism) on the other. It is well known that hydraulic limits are the cause of partial or complete foliar dieback in response to drought (Sperry et al., 2002), which was not observed in the dense canopies of C. pendulus and L. arborea.
The process of moving water to the site of evaporation with minimum energy investment is a major factor driving the architecture and physiology of plants, including the function of stomatal regulation (sperry et al., 2002). In general, liana stems (like the two studied here) are quite narrow in relation to the amount of foliage they supply with water, but wide vessels are thought to hydraulically compensate for their narrow stem diameters (Ewers et al., 1990). In the case under study, wood anatomical features favor high hydraulic conductivity which is necessary to maintain the high midday transpiration and photosynthesis rates observed in these C3 plants (Y.S. Masrahi, unpublished data). Stomatal frequency of both species was low and lie within the range of xerophytes (15–80 stomata/mm2) (Gibson, 1982; Nobel, 2009). However, surface to volume ratio (S/V) of leaves was high as for xerophytic plants. S/V ratio affects transpiration, photosynthesis and water storage capacity. High S/V values (>3) suit mesic habitats (Mauseth, 2000). A high S/V means great surface area (for photosynthesis and transpiration) and small internal volume (for water storage). Great surface area of leaves compensate for low stomatal frequency in both species. Compared with L. arborea, C. pendulus had a larger S/V ratio and a higher stomatal frequency essentially on the lower surface which reduces transpiration.
To conclude, it appears that the main adaptations of both lianas to the harsh conditions in lowlands of southwestern Saudi Arabia are related to stem wood-structure. This wood had large xylem vessels and high hydraulic conductivity. The stem is divided into sectors of xylem groups with ray parenchyma where starch grains accumulate. These reserves can be hydrolyzed into soluble sugars which can improve the upward flow of water and repair air filled vessels. Furthermore, these wood features minimize damage to the hydrosystem in the climbing stems and aid the healing process when injury occurs. Frequently growing on Acacia spp. along wadi courses may also support survival by having deep roots to reach the capillary fringes above the relatively shallow ground water table.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Rahman A.A., Balegh S.E. Analysis of the climatic elements in Saudi Arabia. Bull. Fac. Sci. Riyadh Univ. 1974;6:98–123. [Google Scholar]
- Andrade J.L., Meinzer F.C., Goldstein G., Schnitzer S.A. Water uptake and transport in lianas and co-occurring trees of a seasonally dry tropical forest. Trees. 2005;19:282–289. [Google Scholar]
- Angyalossy V., Angeles G., Pace M.R., Lima A.C., Dias-Leme C.L., Lohmann L.G., Madero-Vega C. An overview on the anatomy, development and evolution of the vascular system of lianas. Plant Ecol. Divers. 2012;5(2):167–182. [Google Scholar]
- Baas P., Ewers F.W., Davis S., Wheeler E.A. Evolution of xylem physiology. In: Hemsley A.R., Poole I., editors. The Evolution of Plant Physiology. Elsevier Academic Press; London: 2004. pp. 273–295. [Google Scholar]
- Breckle S. fourth ed. Springer; Berlin: 2002. Walter’s Vegetation of the Earth. [Google Scholar]
- Carlquist S. Ecological factors in wood evolution: a floristic approach. Am. J. Bot. 1977;64(7):887–896. [Google Scholar]
- Carlquist S. Observations on functional wood histology of vines and lianas: vessel dimorphism, tracheids, vasicentric tracheids, narrow vessels, and parenchyma. Aliso. 1985;11(2):139–157. [Google Scholar]
- Carlquist S. Anatomy of the vine and liana stems: a review and synthesis. In: Putz F.E., Mooney H.A., editors. The Biology of Vines. Cambridge University Press; Cambridge: 1991. pp. 53–71. [Google Scholar]
- Carlquist S. second ed. Springer-Verlag; Berlin: 2001. Comparative Wood Anatomy. [Google Scholar]
- Carlquist S., Hoekman D.A. Ecological wood anatomy of the woody southern Californian Flora. IAWA Bull. 1985;6(4):319–347. [Google Scholar]
- Evert R.F. third ed. Wiley & Sons; New Jersey: 2006. Esau’s Plant Anatomy. [Google Scholar]
- Ewers F.W., Fisher J.B., Chiu S. A survey of vessel dimensions in stems of tropical lianas and other growth forms. Oecologia. 1990;84:544–552. doi: 10.1007/BF00328172. [DOI] [PubMed] [Google Scholar]
- Ewers F.W., Fisher J.B., Fichtner K. Water flux and xylem structure in vines. In: Putz F.E., Mooney H.A., editors. The Biology of Vines. Cambridge University Press; Cambridge: 1991. pp. 127–160. [Google Scholar]
- Ewers F.W., Carlton M.R., Fisher J.B., Kolb K.J., Tyree M.T. Vessel diameters in roots versus stems of tropical lianas and other growth forms. IAWA J. 1997;18(3):261–279. [Google Scholar]
- Foster A.S. D. Van Nostrand Company; New Jersey: 1965. Practical Plant Anatomy. [Google Scholar]
- Gartner B.L., Bullock S.H., Mooney H.A., Brown V.B., Whitbeck J.L. Water transport properties of vine and tree stems in a tropical deciduous forest. Am. J. Bot. 1990;77(6):742–749. [Google Scholar]
- Gentry A.H. The distribution and evolution of climbing plants. In: Putz F.E., Mooney H.A., editors. The Biology of Vines. Cambridge University Press; Cambridge: 1991. pp. 3–49. [Google Scholar]
- Gibson A.C. The anatomy of succulence. In: Ting I.P., Gibbs M., editors. Crassulacean Acid Metabolism. University of California; Riverside: 1982. pp. 1–17. [Google Scholar]
- Gutiérrez M., Miguel-Chávez R.S., Terrazas T. Xylem conductivity and anatomical traits in diverse lianas and small tree species from a tropical forest of southwest Mexico. Int. J. Bot. 2009;5(4):279–286. [Google Scholar]
- Gutterman Y. Springer-Verlag; Berlin: 1993. Seed Germination in Desert Plants. [Google Scholar]
- Committee I.A.W.A. IAWA list of microscopic features for hardwood identification. IAWA Bull. 1989;10(3):219–332. [Google Scholar]
- Johnson D.M., Domec J.-C., Woodruff D.R., McCulloh K.A., Meinzer F.C. Contrasting hydraulic strategies in two tropical lianas and their host trees. Am. J. Bot. 2013;100(2):374–383. doi: 10.3732/ajb.1200590. [DOI] [PubMed] [Google Scholar]
- Kozlowski T.T., Pallardy S.G. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002;68(2):270–334. [Google Scholar]
- Maheshwari R., Rao K.S., Ramachandra T.V. Structural characteristics of a giant tropical liana and its mode of canopy spread in an alien environment. Curr. Sci. 2009;96(1):58–64. [Google Scholar]
- Masrahi Y.S. Published by Author; Jeddah: 2012. The Illustrated Guide to the Wild Plants of Jazan Region. [Google Scholar]
- Mauseth J.D. The Benjamin/Cummings Pub. Comp., Inc.; California: 1988. Plant Anatomy. [Google Scholar]
- Mauseth J.D. Theoretical aspects of surface-to-volume ratios and water-storage capacities of succulent shoots. Am. J. Bot. 2000;87(8):1107–1115. [PubMed] [Google Scholar]
- Müller E., Fredrich K., Kling H. Hydrology of Tihamat Asir. In: Jado A., Zötl J.G., editors. vol. 2. Springer-Verlag; Wien: 1984. pp. 174–194. (Quaternary Period in Saudi Arabia). [Google Scholar]
- Nilsen E.T., Orcutt D.M. John Wiley & Sons, Inc.; New York: 1996. Physiology of Plants Under Stress. Abiotic Factors. [Google Scholar]
- Nobel P.S. fourth ed. Academic Press; Oxford: 2009. Physicochemical and Environmental Plant Physiology. [Google Scholar]
- Rowe N., Speck T. Plant growth forms: an ecological and evolutionary perspective. New Phytol. 2005;166:61–72. doi: 10.1111/j.1469-8137.2004.01309.x. [DOI] [PubMed] [Google Scholar]
- Sieber M., Kučera L.J. On the stem anatomy of Clematis vitalba L. IAWA Bull. 1980;1(1–2):49–54. [Google Scholar]
- Sperry J.S. Limitations on stem water transport and their consequences. In: Gartner B.L., editor. Plant Stems: Physiology and Functional Morphology. Academic Press; San Diego: 1995. pp. 105–124. [Google Scholar]
- Sperry J.S., Hacke U.G., Oren R., Comstock J.P. Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ. 2002;25:251–263. doi: 10.1046/j.0016-8025.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann M.H. Springer-Verlag; Berlin: 1983. Xylem Structure and the Ascent of Sap. [DOI] [PubMed] [Google Scholar]


