Abstract
Essential for detection of relevant external stimuli and for fear processing, the amygdala is under modulatory influence of dopamine (DA). The DA transporter (DAT) is of fundamental importance for the regulation of DA transmission by mediating reuptake inactivation of extracellular DA. This study examined if a common functional variable number tandem repeat polymorphism in the 3′ untranslated region of the DAT gene (SLC6A3) influences amygdala function during the processing of aversive emotional stimuli. Amygdala reactivity was examined by comparing regional cerebral blood flow, measured with positron emission tomography and [15O]water, during exposure to angry and neutral faces, respectively, in a Swedish sample comprising 32 patients with social anxiety disorder and 17 healthy volunteers. In a separate US sample, comprising 85 healthy volunteers studied with blood oxygen level-dependent functional magnetic resonance imaging, amygdala reactivity was assessed by comparing the activity during exposure to threatening faces and neutral geometric shapes, respectively. In both the Swedish and the US sample, 9-repeat carriers displayed higher amygdala reactivity than 10-repeat homozygotes. The results suggest that this polymorphism contributes to individual variability in amygdala reactivity.
Introduction
Brain imaging studies demonstrate that the human amygdala is activated by external fear-inducing stimuli1, 2, 3 and that its reactivity is the subject of considerable interindividual variation. An influence of dopamine (DA) on amygdala function is well established on the basis of animal experiments,4, 5, 6 and gains support by recent functional neuroimaging studies in humans. The amygdala activation observed in healthy individuals during processing of angry and fearful faces is thus enhanced by the DA releaser amphetamine7 and reduced in patients with Parkinson's disease, but partially restored after DA repletion in the latter group.8 In line with these findings, acute treatment with DA D2 receptor antagonists resulted in a considerable attenuation of amygdala reactivity.9 Moreover, DA storage in the human amygdala, measured with 6-[18F]fluoro-L-DOPA positron emission tomography (PET), correlates with changes in amygdala blood oxygen levels during presentation of negative emotional visual stimuli.10
The DA transporter (DAT) mediates the active reuptake of extracellular DA into presynaptic terminals after release, and is hence a fundamental regulator of dopaminergic neurotransmission, determining the duration and amplitude of DA action. In addition, it is the molecular target for central stimulants such as amphetamine, which act by reversing the direction of transmitter transport.11 A variable number of tandem repeats (VNTR) polymorphism in the DAT gene (SLC6A3) 3′ untranslated region comprises 3–11 repeats of 40 bp each, with the 9- and 10-repeat variants being the most common in Caucasians.12 The results of studies exploring the influence of this polymorphism on DAT expression have not been unanimous, but the weight of evidence suggests the 9-repeat allele to be associated with higher DAT expression and hence with higher DA reuptake.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23
In the present study, we have explored the possible influence of SLC6A3 3′ untranslated region VNTR genotype on amygdala reactivity by using previously collected information regarding regional cerebral blood flow (rCBF) measured with PET during presentation of angry and neutral faces, respectively, in one Swedish sample comprising 32 subjects with social anxiety disorder (SAD) and 17 healthy volunteers. Subsequently, in an attempt to shed further light on the association between genotype and amygdala reactivity found in the Swedish sample, the possible association between SLC6A3 genotype and amygdala reactivity was assessed with another facial stimuli test using blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) in a cohort comprising 85 healthy volunteers from the US.
Materials and methods
Participants
PET study
The Swedish sample comprised 32 medication-free SAD patients (for sex and age: see Table 1; for details, see Furmark et al.24) and 17 healthy volunteers (8 men, 9 women, mean age 35.2 years); both subgroups had been enrolled through newspaper advertisements for studies that have been published previously.24 Originally 34 patients and 18 healthy volunteers assessed with PET were planned to be included in this study, but two patients and one healthy volunteer were excluded due to genotyping failure. According to self-report, all subjects in both samples were of Caucasian origin. Following a short telephone interview, the patients were asked to answer a battery of SAD questionnaires, and psychiatric status was evaluated by a clinical psychologist using face-to-face structured diagnostic interviews.25 A psychiatrist then conducted a MINI interview26 to exclude depressive disorder and other major psychiatric conditions except for comorbid anxiety disorders. To be included, the patients should meet DSM-IV27 criteria for SAD and display marked anxiety for public speaking. Major criteria for exclusion from the study were treatment for SAD during the past six months, chronic use of prescribed medication, substance abuse, pregnancy, menopause, left-handedness, previous PET examinations and any major somatic condition that could be expected to influence the outcome of the study (such as major neurological diseases). The same exclusion criteria, as well as any ongoing psychiatric disorder, were applied also to controls.
Table 1. SLC6A3 VNTR genotype frequencies, age and gender.
|
SLC6A3 VNTR genotype |
Age (mean±s.d.) | Sex (men/women) | |||
|---|---|---|---|---|---|
| 9/9 | 9/10 | 10/10 | |||
| Swedish sample | |||||
| SAD patients | 2 (6.3%) | 9 (28.1%) | 21 (65.6%) | 37.3±8.7 | 14 (43.8%)/18 (56.3%) |
| Healthy volunteers | 0 (0%) | 8 (47.1%) | 9 (52.9%) | 35.2±9.3 | 8 (47.1%)/9 (52.9%) |
| Total sample | 2 (4.1%) | 17 (34.7%) | 30 (61.2%) | 36.6±9.0 | 22 (44.9%)/27 (55.1%) |
| fMRI study | |||||
| Healthy volunteers | 10 (11.8%) | 33 (38.8%) | 42 (49.4%) | 45.2±6.6 | 41 (48.2%)/44 (51.8%) |
Abbreviations: fMRI, functional magnetic resonance imaging; SAD, social anxiety disorder; SLC6A3, dopamine transporter gene.
For the Swedish population, information both on the two subgroups (SAD patients and healthy volunteers) and on the total sample is provided.
The Uppsala University medical faculty ethical review board and the local radiation protection committee at Uppsala University Hospital approved the study. The participants gave informed consent in writing after having the procedure and its consequences explained to them.
fMRI study
After the removal of five multivariate outliers (see below), the US sample used in the analyses comprised 85 genotyped participants (for sex and age: see Table 1) that were recruited for the fMRI study from the Adult Health and Behaviour project, an archival database encompassing detailed measures of behavioral and biological traits among a community sample of 1379 nonpatient, middle-aged volunteers. According to self-reports, all subjects in the US sample were Caucasians. All participants were in good general health and free of the following study exclusions: (1) medical diagnoses of cancer, stroke, diabetes requiring insulin treatment, chronic kidney or liver disease or a lifetime history of psychotic symptoms; (2) use of psychotropic, glucocorticoid or cardiovascular (for example, antihypertensive, antiarrythmic) medication; (3) conditions affecting cerebral blood flow and metabolism (for example, hypertension); and (4) any current DSM-IV Axis I disorder as assessed by face-to-face structured diagnostic interview. Written informed consent according to the guidelines of the University of Pittsburgh Institutional Review Board was provided by all participants before their participation in the neuroimaging subcomponent of Adult Health and Behaviour.
Genotyping
PET study
Genomic DNA was extracted from plasma samples using QIAamp DNA kit (QIAGEN, Hilden, Germany). VNTR genotyping was achieved by amplifying part of the 3′ untranslated region with the polymerase chain reaction on a Perkin Elmer 9700 (PerkinElmer, Foster City, CA, USA) thermal cycler using 50 ng of genomic DNA, 0.15 μM of forward (5′-TGTGGTGTAGGGAACGGCCTGAG-3′) and reverse primer (5′-CTTCCTGGAGGTCACGGCTCAAGG-3′), 0.2 mM dNTP and 1.0 U HotStarTaq DNA polymerase (QIAGEN) in a buffered (QIAGEN HotStarTaq PCR Buffer) solution. The cycling conditions for amplification consisted of an initial denaturation period of 15 min at 95 °C, followed by 45 cycles of 25 s at 95 °C and 40 s at 72 °C, followed by 10 min at 72 °C. Analysis on a 3% agarose gel yielded distinct bands for the 9- and 10-repeat alleles. The two SLC6A3 VNTR genotype groups did not differ with respect to age.
fMRI study
In the US sample, high molecular weight DNA was isolated from EDTA anticoagulated whole blood samples obtained from all participants in the fMRI study using the Puregene kit (Gentra Systems, Minneapolis, MN, USA). Each sample was genotyped for the SLC6A3 3′ untranslated region VNTR using the protocol of Vandenbergh et al.28 All genotypes were scored by two independent readers by comparison with sequence verified standards and all call rates were >95%.
Brain imaging techniques
PET study
The Swedish sample was assessed by means of PET at Uppsala PET Centre using a 32-ring ECAT EXACT HR+ PET scanner (Siemens/CTI, Munich, Germany) that enables acquisition of 63 contiguous planes of data with a distance of 2.46 mm, resulting in a total axial field of view of 155 mm. Before recording, subjects were placed in the scanner, had their heads gently fixated and were inserted with a venous catheter for tracer injections. A 10-min transmission scan using three retractable 68Ge rotating line sources was performed. Subjects were then intravenously injected with the [15O] labeled water tracer (approximately 10 MBq kg−1 body weight). The emission scan started automatically in three-dimensional mode when the bolus reached the brain (50 ,000 counts per second), and consisted of three 30-s frames. The experimental task (see below) commenced immediately following tracer injection. Emission scans were reconstructed with a filter back projection using an 8 mm Hanning filter, resulting in a spatial resolution of about 5 mm in the field of view. Data were corrected for photon attenuation, decay, scattered radiation and random coincidences. After data reconstruction, a summation image of the three frames was made to obtain a better statistical reference for realignment and subsequent analyses. PET-images were realigned and normalized to the Montreal Neurological Institute's stereotactic template ICBM152 using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). Images were smoothed using a 12 mm Gaussian kernel and scaled to give all scans the same global value. Voxel size was 2 × 2 × 2 mm.
fMRI study
The US sample was assessed by means of fMRI using a Siemens 3 T MAGNETOM Allegra scanner developed specifically for advanced brain imaging applications and characterized by increased T2* sensitivity and fast gradients (slew rate=400 T m−1 s−1), which minimize echo-spacing thereby reducing EPI geometric distortions and improving image quality. BOLD functional images were acquired with a gradient echo EPI sequence (TR/TE=2000 per 25 ms, FOV=20 cm, matrix=64 × 64) that covered 34 interleaved axial slices (3 mm slice thickness) aligned with the AC-PC plane and encompassing the entire cerebrum and the majority of the cerebellum. All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, we acquired a reference EPI scan that we visually inspected for artifacts (for example, ghosting), as well as for good signal across the entire volume of acquisition, including the amygdala and ventral striatum. In addition, an autoshimming procedure was conducted before the acquisition of BOLD data in each subject to minimize field inhomogeneities. The fMRI data from all 85 participants included in this study met these quality standards. Whole brain image analysis was completed using SPM2. For each scan, images for each participant were realigned to the first volume in the time series to correct for head motion. Data sets were then selected for their high quality (scan stability) as demonstrated by small (<2 mm translational and <1 degree rotational) motion correction. On the basis of this criterion, data from all participants were included in subsequent analyses. Realigned images were spatially normalized into a standard Montreal Neurological Institute template stereotactic space using a 12-parameter affine model. These normalized images were then smoothed to minimize noise and residual difference in gyral anatomy with a Gaussian filter, set at 6 mm full-width at half-maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. The final voxel size in the fMRI analysis was 2 × 2 × 2 mm.
Affective face processing tasks
PET study
Simultaneously with the [15O]water tracer injection, a multislide presentation of 2.5 min was started. During the following emission scan, participants were shown black and white photographs of angry and neutral faces of different individuals taken from the set of Ekman and Friesen.29 Subjects were scanned twice and presented with 30 photographs of human faces (either angry or neutral) during each scan. The order of presentation was counterbalanced among participants. The procedure protocol was designed to be in line with those of previous PET studies,1 with each photograph being visible for 3 s, followed by a 2-s pause in which the computer screen was blank. To monitor the observance of the participants, they were instructed to press a mouse button when a new face was presented on the screen.
fMRI study
During fMRI scanning all 85 individuals in the US sample participated in an affective face processing task that was similar, but not identical, to that used in the PET experiments. The paradigm consisted of four blocks of the affective face processing task interleaved with five blocks of a sensorimotor control task. Subject performance (accuracy and reaction time) was monitored during all scans. During the affective face processing task, subjects viewed a trio of faces (expressing either anger or fear) and selected one of two faces (bottom) identical to a target face (top). Each affective face processing block consisted of six images, balanced for sex and target affect (angry or fearful) all derived from a standard set of pictures of facial affect.29 During the sensorimotor control blocks, subjects viewed a trio of simple geometric shapes (circles, vertical and horizontal ellipses) and selected one of two shapes (bottom) identical to a target shape (top). Each sensorimotor control block consisted of six different shape trios. All blocks were preceded by a brief instruction (‘Match Faces' or ‘Match Shapes') lasting 2 s. In the affective face processing blocks, each of the six face trios was presented for 4 s with a variable interstimulus interval of 2–6 s (mean=4 s) for a total block length of 48 s. In the sensorimotor control blocks, each of the six shape trios was presented for 4 s with a fixed interstimulus of 2 s for a total block length of 36 s. Total task time was 390 s.
Statistical analysis
Grouping of genotypes
To increase statistical power, and in line with previous studies on this polymorphism,30, 31, 32 genotypes were dichotomized so that subjects carrying at least one version of the relatively rare 9-repeat (9/9 and 9/10) were compared with those who were homozygous carriers of the 10-repeat (10/10). The genotype groups did not differ with respect to age (Swedish sample: 37±7 vs 36±10; US sample: 45±7 vs 45±7).
PET study
Mean rCBF data were extracted from the entire left and right amygdala, respectively, using the regions of interest based on the Talairach Daemon option of the Wake Forest University (WFU) PickAtlas tool.33 Global blood flow was scaled to 1. Extracted rCBF data were statistically evaluated in SPSS 18 (SPSS, Chicago, IL, USA) using repeated measures analysis of variance with facial affect (neutral, angry) as within group factor, genotype and diagnosis (SAD, control) as between group factors and sex as a covariate.
fMRI study
Because of the relatively high resolution when using fMRI (as compared with PET), the average of the cluster of activated voxels within the amygdala as defined in the Automated Anatomical Labeling atlas, rather than from the entire amygdalae, were used when relating amygdala activity to genotype in the fMRI study. To this end, following preprocessing, linear contrasts employing canonical hemodynamic response functions were used to estimate condition-specific (that is, threatening faces>shapes) BOLD activation for each individual. These individual contrast images (that is, weighted sum of the beta images) were then used in second-level random effects models to determine condition-specific amygdala responses. Bilateral amygdala regions of interest were based on the Automated Anatomical Labeling package of the WFU PickAtlas (v1.04). All fMRI data are reported using the coordinate system of the Montreal Neurological Institute. All SPM analyses were thresholded at P=0.05, FWE-corrected for multiple comparisons across the regions of interest.
Threat-related BOLD parameter estimates were extracted for each subject from the maximally activated voxels in the right and left amygdala. The effects of SLC6A3 genotype effects on these BOLD parameter estimates were analyzed in a regression analysis conducted in SPSS 18 (SPSS). Gender and age were entered as covariates. Multivariate outliers were identified by computing Cook's distance for each observation in accordance with the above model.34 Five data points with a Cook's distance exceeding the sample-specific cut-off of 0.045 were removed from the analyses yielding the final sample of 85 from a total sample of 90 subjects. The sample-specific threshold was calculated as previously described.34,35
Results
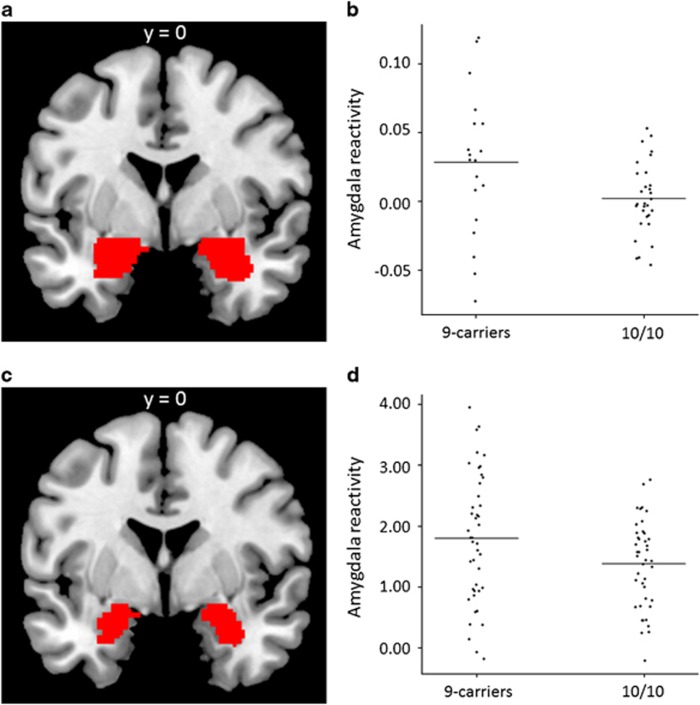
In neither the PET population nor the fMRI population did the distribution of observed SLC6A3 genotypes (Table 1) deviate from Hardy–Weinberg equilibrium. Left amygdala reactivity, that is, the mean rCBF during the processing of angry faces, as compared with that during the processing of neutral faces, was positively associated with the presence of one or two 9-repeats in the Swedish sample (Table 2, Figure 1), with no indication of an influence of diagnostic category (SAD: 9 repeats mean±s.d.=0.031±0.05, 10 repeats mean±s.d.=0.004±0.02; healthy volunteer: 9 repeats mean±s.d.=0.024±0.06, 10 repeats mean±s.d.=−0.003±.03) on this association. This observation is in line with the results of the US sample of healthy volunteers, in which amygdala reactivity, that is, regional BOLD parameter estimates from the comparison of threatening faces and geometric shapes, was also positively associated with carrying one or two 9-repeats (n=43) as compared with two 10-repeats (n=42) (Table 2, Figure 1, Supplementary Figure). Right amygdala reactivity did not differ between genotypes in either of the two samples (Table 2).
Table 2. Differences in amygdala reactivity between SLC6A3 dichotomized genotype subgroups in the affective face processing task.
| Study population | Hemisphere | F (df) | P |
|---|---|---|---|
| Swedish sample (PET) | |||
| Reactivity (angry–neutral faces) | |||
| DAT | Left | 5.07 (1,44) | 0.029* |
| Right | 0.005 (1,44) | 0.946 | |
| DAT × Diagnosis | Left | 0.01 (1,44) | 0.905 |
| Right | 0.03 (1,44) | 0.855 | |
| American sample (fMRI) | |||
| Reactivity (threatening faces–geometric shapes) | |||
| DAT | Left | 5.13 (1,81) | 0.026* |
| Right | 0.98 (1,81) | 0.324 | |
Abbreviations: DAT, dopamine transporter; fMRI, functional magnetic resonance imaging; PET, positron emission tomography; SAD, social anxiety disorder; SLC6A3, dopamine transporter gene.
For the analysis of variance of the Swedish sample, diagnostic subgroup (SAD and healthy volunteer, respectively) was included as a between group factor. For the analysis of both the Swedish and the US population, sex was included as a covariate. *P<0.05.
Figure 1.
Differences (P<0.05) in left amygdala reactivity between SLC6A3 dichotomized genotype subgroups. (a) The amygdala regions of interest used to extract data in the Swedish sample (PET) are shown overlaid on an anatomical template. (b) Scatter plot showing left amygdala reactivity in the Swedish sample. (c) The amygdala regions of interest used to extract data in the US sample (fMRI). (d) Scatter plot showing left amygdala reactivity in the US sample. 9 carriers, carriers of one or two 9-repeats; 10/10, carriers of two 10-repeats; PET, positron emission tomography; SLC6A3, dopamine transporter gene.
Discussion
The possible association between SLC6A3 VNTR and amygdala reactivity was investigated in two samples, one comprising Swedish subjects with SAD plus Swedish healthy volunteers assessed with PET and another one comprising US healthy volunteers assessed with fMRI. Within both samples, subjects carrying at least one 9-repeat allele displayed significantly higher left amygdala reactivity than carriers of two 10-repeat alleles.
PET and fMRI have different temporal sensitivity, hence different tasks, optimized for each modality, were used. Thus, in the PET study the subjects were shown essentially two long blocks of either angry or neutral faces, whereas the fMRI paradigm was based on a rapid shift between angry/fearful faces and geometrical shapes, respectively. The results thus suggest that both slow and relatively rapid amygdala reactivity is modulated by the studied polymorphism.
Importantly, the purpose of this study was to explore the extent to which the studied polymorphism influences amygdala reactivity in humans using two available samples of convenience, one of which happened to consist partly of subjects suffering from SAD, an anxiety disorder characterized by a marked and persistent fear of humiliation or scrutiny in social or performance situations.27 The study was hence not prompted by an a priori hypothesis that patients and healthy volunteers should differ with respect to the studied association, and it did not have an optimal design for comparing patients and healthy volunteers as the number of healthy subjects exposed to the same PET paradigm as the SAD patients was relatively small. Moreover, a previous study, comprising partly the same subjects, did not reveal any difference between SAD patients and controls with respect to amygdala reactivity assessed using this paradigm.24 This notwithstanding, diagnosis was included as a between group factor in the analysis of covariance by means of which the Swedish data were analyzed, the outcome of the test, however, suggesting that it did not influence the association, which was hence in the same direction in SAD cases and healthy volunteers.
To the best of our knowledge, this is the first study to report on an association between the VNTR polymorphism in the DAT gene and amygdala reactivity. The assumption that variations in DA activity may impact this endophenotype, however, gains support from several previous association studies. For example, the val158met polymorphism in the gene encoding the DA-inactivating enzyme catechol-O-methyl transferase—the met allele of which tentatively promotes tonic but reduces phasic dopaminergic transmission36—has hence been shown to be associated with amygdala reactivity in several studies. These are, however, not unanimous with respect to the direction of the effect or with respect to laterality (for ref see Lonsdorf et al.37). Of note are also a few papers reporting associations between polymorphisms in the DA D2 receptor gene and the same measure.38,39
Amygdala reactivity has often been regarded as an endophenotype tentatively mediating the effect of genes on anxiety-related behavior. In this vein, previous studies showing subjects carrying the short version of the 5-HTTLPR polymorphism in the promoter of the serotonin transporter gene (SCL6A4) to display higher amygdala reactivity than carriers of the long variant2,24,40, 41, 42, 43 have been regarded as consistent with the potential association between the short allele of the 5-HTTLPR and anxiety-related personality traits.44 Given these previous observations, the possible influence of SLC6A3 VNTR on anxiety-related phenotypes seems to be a topic worth exploring; as yet, this issue, however, have been only sparsely studied, and data have been conflicting, with no robust support so far for an association between this polymorphism and anxiety. On the other hand, there are numerous reports suggesting (albeit not unanimously) an association between the 9-repeat allele of the SLC6A3 VNTR with attention deficit hyperactivity disorder (see for example Brown et al.45), which is of interest in this context, as subjects with attention deficit hyperactivity disorder have been reported to display enhanced activation of the left amygdala when exposed to threatening faces,46 and since the increased reactivity is normalized by treatment with central stimulants.47 Also of note is a previous report suggesting 9 carriers to display enhanced detection of novel and behaviorally relevant signals as assessed by measuring auditory N1 potentials.48
It has not been obvious how to explain the previously reported association between 5-HTTLPR and amygdala activity in terms of serotonergic output. Thus, an allele causing reduced reuptake of serotonin is associated with an increase in amygdala reactivity,2 whereas acute administration of a serotonin reuptake inhibitor has been reported to cause both enhanced49 and reduced reactivity,50 and prolonged administration to reduce this measure.51,52 The same could be said of the presently reported association of the SLC6A3 VNTR; thus, although a drug blocking the reuptake of DA (by reversing the direction of DAT) causes enhanced amygdala reactivity,7 we observed subjects carrying a polymorphism presumably leading to increased DAT expression (for refs, see Introduction) to display stronger activation of amygdala during the processing of angry or fearful faces. The reason for these discrepancies is far from obvious, but it should be underlined that the output of a certain transmitter may not always be expressed simply in terms of enhanced or reduced activity. With respect to dopaminergic activity, many authors have, for example, emphasized the importance of separating tonic from phasic activity.36 Moreover, the possibility that an association between a certain polymorphism and a certain trait may be due to an influence exerted during brain development, rather than in the adult organism, should not be ignored. Finally, it should be pointed out that a possible influence of a polymorphism in the DAT gene on amygdala reactivity does not necessarily take place in the amygdala, but can be exerted in another brain region which is also innervated by dopaminergic nerve terminals and which exerts an impact on amygdala mediated by non-dopaminergic neurons.
In conclusion, we demonstrate that variation in the SLC6A3 VNTR polymorphism is of importance for interindividual differences in amygdala function. This observation is in line with previous data suggesting (i) the studied polymorphism to be of functional importance and (ii) amygdala to be under the influence of DA.
Acknowledgments
GlaxoSmithKline, Torsten och Ragnar Söderberg's Foundation, Bertil Hållsten's Foundation, Swedish Brain Power, the Swedish Research Council, the Swedish Council for Working Life and Social Research and the Swedish Brain foundation supported the study. Funding for this work was also provided through NIH grants HL040962 and HL065137 to SBM and MH072837 to ARH, as well as a NARSAD Young Investigator Award to ARH. We thank Gunilla Bourghardt, Inger Oscarsson, Mark Kimak and Patrick Fisher for technical assistance.
Dr Bani, Dr Merlo Pich and Dr Bettica were GlaxoSmithKline employees at the time of the study. The other authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner S, Rosenkranz JA, Grace AA, Barrionuevo G. Dopamine modulates excitability of basolateral amygdala neurons in vitro. J Neurophysiol. 2005;93:1598–1610. doi: 10.1152/jn.00843.2004. [DOI] [PubMed] [Google Scholar]
- Grace AA, Rosenkranz JA. Regulation of conditioned responses of basolateral amygdala neurons. Physiol Behav. 2002;77:489–493. doi: 10.1016/s0031-9384(02)00909-5. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P, Delaveau P, Blin O, Nieoullon A. Dopaminergic contribution to the regulation of emotional perception. Clin Neuropharmacol. 2005;28:228–237. doi: 10.1097/01.wnf.0000185824.57690.f0. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27:1036–1040. doi: 10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Chase TN, Hyde TM, et al. Dopamine modulates the response of the human amygdala: a study in Parkinson's disease. J Neurosci. 2002;22:9099–9103. doi: 10.1523/JNEUROSCI.22-20-09099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Takano A, Asai K, Suhara T, et al. Effects of dopaminergic and serotonergic manipulation on emotional processing: a pharmacological fMRI study. Neuroimage. 2005;27:991–1001. doi: 10.1016/j.neuroimage.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Kienast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Buchholz HG, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008;11:1381–1382. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddley K, Vasiliou AS, Ali FR, Paredes UM, Bubb VJ, Quinn JP. Molecular genetics of monoamine transporters: relevance to brain disorders. Neurochem Res. 2008;33:652–667. doi: 10.1007/s11064-007-9521-8. [DOI] [PubMed] [Google Scholar]
- Shumay E, Chen J, Fowler JS, Volkow ND. Genotype and ancestry modulate brain's DAT availability in healthy humans. PLoS One. 2011;6:e22754. doi: 10.1371/journal.pone.0022754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1:152–156. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, et al. Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry. 2000;157:1700–1703. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- Michelhaugh SK, Fiskerstrand C, Lovejoy E, Bannon MJ, Quinn JP. The dopamine transporter gene (SLC6A3) variable number of tandem repeats domain enhances transcription in dopamine neurons. J Neurochem. 2001;79:1033–1038. doi: 10.1046/j.1471-4159.2001.00647.x. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D'Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3' UTR VNTR: Evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet. 2002;114:975–979. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Miller GM, Madras BK. Polymorphisms in the 3′-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression. Mol Psychiatry. 2002;7:44–55. doi: 10.1038/sj.mp.4000921. [DOI] [PubMed] [Google Scholar]
- van de Giessen E, de Win MM, Tanck MW, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009;50:45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005;46:745–751. [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Riedel M, Muller U, Moller HJ, Ettinger U. Relationship between SLC6A3 genotype and striatal dopamine transporter availability: a meta-analysis of human single photon emission computed tomography studies. Synapse. 2011;65:998–1005. doi: 10.1002/syn.20927. [DOI] [PubMed] [Google Scholar]
- Furmark T, Henningsson S, Appel L, Ahs F, Linnman C, Pissiota A, et al. Genotype over-diagnosis in amygdala responsiveness: affective processing in social anxiety disorder. J Psychiatry Neurosci. 2009;34:30–40. [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. SCID-I: Interview protocol (Swedish version) Pilgrim Press: Stockholm, Sweden; 1998. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 (Suppl 20:22–33. [PubMed] [Google Scholar]
- American Psychiatric Association . DSM-IV. American Psychiatric Association: Washington, DC, USA; 1994. [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial effect. Consulting Psychologists Press: Palo Alto, CA, USA; 1976. [Google Scholar]
- Hunnerkopf R, Strobel A, Gutknecht L, Brocke B, Lesch KP. Interaction between BDNF Val66Met and dopamine transporter gene variation influences anxiety-related traits. Neuropsychopharmacology. 2007;32:2552–2560. doi: 10.1038/sj.npp.1301383. [DOI] [PubMed] [Google Scholar]
- Reif A, Rosler M, Freitag CM, Schneider M, Eujen A, Kissling C, et al. Nature and nurture predispose to violent behavior: serotonergic genes and adverse childhood environment. Neuropsychopharmacology. 2007;32:2375–2383. doi: 10.1038/sj.npp.1301359. [DOI] [PubMed] [Google Scholar]
- O'Gara C, Stapleton J, Sutherland G, Guindalini C, Neale B, Breen G, et al. Dopamine transporter polymorphisms are associated with short-term response to smoking cessation treatment. Pharmacogenet Genomics. 2007;17:61–67. doi: 10.1097/01.fpc.0000236328.18928.4c. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Fox J. Regression Diagnostics. Sage Publications: Newbury Park, CA, USA; 1991. [Google Scholar]
- Belayachi S, Van der Linden M. Feeling of doing in obsessive-compulsive checking. Conscious Cogn. 2010;19:534–546. doi: 10.1016/j.concog.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Golkar A, Lindstom KM, Fransson P, Schalling M, Ohman A, et al. 5-HTTLPR and COMTval158met genotype gate amygdala reactivity and habituation. Biol Psychol. 2011;87:106–112. doi: 10.1016/j.biopsycho.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Lee BT, Lee HY, Han C, Pae CU, Tae WS, Lee MS, et al. DRD2/ANKK1 TaqI A polymorphism affects corticostriatal activity in response to negative affective facial stimuli. Behav Brain Res. 2011;223:36–41. doi: 10.1016/j.bbr.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Blasi G, Lo Bianco L, Taurisano P, Gelao B, Romano R, Fazio L, et al. Functional variation of the dopamine D2 receptor gene is associated with emotional control as well as brain activity and connectivity during emotion processing in humans. J Neurosci. 2009;29:14812–14819. doi: 10.1523/JNEUROSCI.3609-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, et al. Variation of human amygdala response during threatening stimuli as a function of 5'HTTLPR genotype and personality style. Biol Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Deckert J, Hohoff C, Kugel H, et al. 5-HTTLPR biases amygdala activity in response to masked facial expressions in major depression. Neuropsychopharmacology. 2008;33:418–424. doi: 10.1038/sj.npp.1301411. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Brown AB, Biederman J, Valera E, Makris N, Doyle A, Whitfield-Gabrieli S, et al. Relationship of DAT1 and adult ADHD to task-positive and task-negative working memory networks. Psychiatry Res. 2011;193:7–16. doi: 10.1016/j.pscychresns.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167:61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Nagel BJ, Maia TV, Mechling A, Oh M, Wang Z, et al. Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:828–37 e3. doi: 10.1016/j.jaac.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia M, Clemente I, Dominguez-Borras J, Escera C. Dopamine transporter regulates the enhancement of novelty processing by a negative emotional context. Neuropsychologia. 2010;48:1483–1488. doi: 10.1016/j.neuropsychologia.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR. Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 2008;33:3221–3225. doi: 10.1038/npp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, O'Sullivan U, Cowen PJ, Harmer CJ. Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry. 2009;194:535–540. doi: 10.1192/bjp.bp.108.056093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology. 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.