Abstract
Molecular abnormalities in metabolic, hormonal and immune pathways are present in peripheral body fluids of a significant subgroup of schizophrenia patients. The authors have tested whether such disturbances also occur in psychiatrically ill and unaffected siblings of schizophrenia patients with the aim of identifying potential contributing factors to disease vulnerability. The subjects were recruited as part of the Genetic Risk and OUtcome of Psychosis (GROUP) study. The authors used multiplexed immunoassays to measure the levels of 184 molecules in serum from 112 schizophrenia patients, 133 siblings and 87 unrelated controls. Consistent with the findings of previous studies, serum from schizophrenia patients contained higher levels of insulin, C-peptide and proinsulin, decreased levels of growth hormone and altered concentrations of molecules involved in inflammation. In addition, significant differences were found in the levels of some of these proteins in siblings diagnosed with mood disorders (n=16) and in unaffected siblings (n=117). Most significantly, the insulin/growth hormone ratio was higher across all groups compared with the controls. Taken together, these findings suggest the presence of a molecular endophenotype involving disruption of insulin and growth factor signaling pathways as an increased risk factor for schizophrenia.
Introduction
Recent investigations have led to identification of abnormal molecular profiles in blood serum and plasma of schizophrenia patients.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 Many of these studies have found changes in molecules linked to insulin resistance and glucose handling in schizophrenia patients, including patients who have not received antipsychotic medications.1,13,14 The affected molecules include neuroendocrine hormones such as insulin, prolactin, pancreatic polypeptide, chromogranin A and growth hormone.4,5,8 Although such effects have been associated previously with response to antipsychotic treatment, potential links between schizophrenia and type 2 diabetes mellitus have been reported for >50 years, even before the development of antipsychotics. Such effects on insulin signaling are consistent with the findings of proteomic profiling analyses, which showed disrupted altered metabolic protein levels in postmortem brain tissues from schizophrenia patients.15, 16, 17
The question of whether these changes are a cause or effect of schizophrenia has not been answered. One approach to resolve this issue is to investigate unaffected relatives of patients. Abnormalities found in siblings of schizophrenia patients may provide clues to the cause of the illness, especially regarding the existence of predisposing factors.18 It has been established that genetic factors contribute to the development of schizophrenia as monozygotic twins show a 40–80% concordance rate and siblings of patients have an approximate 10-fold increased risk for psychosis.19,20 Also, studies have shown that some unaffected relatives of schizophrenia patients exhibit abnormalities in electrophysiological, neurocognitive, symptomatic and behavioral abnormalities found in some psychiatric conditions.21 One small study had reported abnormal glucose responses after oral glucose tolerance tests in six siblings of schizophrenia patients, providing some evidence that supports an association between family-risk for schizophrenia and metabolic disorders.22
Here, we have investigated whether common disturbances in molecular pathways could be identified in the serum from patients and their siblings as potential risk factors for development of schizophrenia. Blood serum samples from all subjects were analyzed by multiplex immunoassays and the resulting molecular fingerprints compared among schizophrenia patients, their siblings and unrelated controls.
Materials and methods
Subjects
The subjects were a subset of the large Dutch ‘Genetic Risk and OUtcome in Psychosis' (GROUP) project23 and consisted of 112 patients, 133 siblings and 87 controls. For the current study, patients were selected with a diagnosis of schizophrenia, schizophreniform or schizoaffective disorder (hereafter referred to as schizophrenia) and were matched closely for age and body mass index (Table 1). Diagnosis was based on Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria and symptom severity was assessed with the Positive and Negative Syndrome Scale.24 The possible presence of subclinical psychosis in asymptomatic siblings and healthy controls was assessed with the Community Assessment of Psychic Experiences.25 Of the siblings, 16 had a DSM-IV diagnosis (symptomatic) and 117 did not (asymptomatic). The presence of psychiatric disorders in 12% of the siblings was consistent with the presence of predisposing factors for mental illness. Control subjects had no first- or second-degree siblings with a psychotic disorder and were also matched closely for age and body mass index. The local ethical committees approved the protocols of the study. The study was conducted according to the Declaration of Helsinki and written informed consent was obtained after complete description of the study to the subjects.
Table 1. Demographic and clinical characteristics of patients, siblings and healthy controls.
| SZ | SS | AS | Controls | |
|---|---|---|---|---|
| Number | 112 | 16 | 117 | 87 |
| Gender (male/female)a | 97/15* | 5/11* | 57/60* | 59/28 |
| Ageb | 24.5±5.3 | 25.9±7.6 | 27.1±8.2 | 26.5±9.6 |
| Boddy mass index (kg m−2)b | 23.9±3.6* | 22.6±3.4 | 22.9±3.4 | 22.3±3.0 |
| PANSS | ||||
| Positive | 11.7±5.2 | — | — | — |
| Negative | 14.7±6.6 | — | — | — |
| General | 26.8±8.8 | — | — | — |
| Total | 52.9±17.5 | — | — | — |
| Diagnosis | ||||
| Schizophrenia and schizophreniform | 93 | — | — | |
| Schizoaffective | 19 | — | — | |
| Eating disorder | 1 | — | — | |
| Depressive disorder | 13 | — | — | |
| Pervasive developmental disorder | 2 | — | — | |
Abbreviations: AS, asymptomatic siblings; PANSS, positive and negative syndrome scale; SS, symptomatic siblings; SZ, schizophrenia.
Chi-square test (*P<0.05 compared against controls).
Wilcoxon rank sum test (*P<0.05 compared against controls).
Multiplex immunoassay
Blood samples were obtained from all patients as available and were not necessarily collected under fasting conditions. Serum was prepared and analyzed using the Human DiscoveryMAP (Myriad RBM, Austin, TX, USA) multiplexed immunoassay platform, which measures the concentrations of 184 proteins and small molecules (Supplementary Table 1), as described previously.9 For each measured serum protein, values measured below the lower limit of detection were replaced by half the lowest concentration determined for the respective analyte. Variation in protein levels were investigated using linear mixed-effects models with regard to established diagnosis, presence of symptoms and family membership. Potential covariates such as age, gender and body mass index were included in the models. For proteins with nonsignificant diagnosis–covariate interactions, additive models were compared against reduced models without diagnosis information to obtain adjusted P-values. Otherwise, diagnosis main effect P-values are shown. All P-values were corrected for multiple hypotheses testing by controlling the false-discovery rate (Q-values), and findings of P<0.05 and Q<0.1 were designated as significant.
Results
Schizophrenia patients
Multiplex immunoassay analysis showed that 10 proteins were present at significantly different levels between schizophrenia patients and controls (Table 2). The changes in proinsulin, insulin and C-peptide provide some validation of the findings due to the precursor product relationship of these proteins.26 Also, the levels of insulin and C-peptide showed a significant correlation (Pearson; r=0.89; P<0.001). These changes in the insulin-related peptides are consistent with those of a previous study using single-plex immunoassays, which found similar results in 66 first-onset schizophrenia patients compared with 68 controls.5 The additional finding of reduced growth hormone levels suggests that both metabolism and growth signaling pathways may be altered in schizophrenia patients. Similar changes in these hormones were also found in a multiplex immunoassay study of 250 first-onset schizophrenia patients compared with 230 controls.9 In line with other studies on schizophrenia patients, most of the remaining proteins had functions associated with the inflammatory response, such as T-lymphocyte-secreted protein I 309, CD5L, interferon-γ-induced protein 10, immunoglobulin M and chromogranin A.3,4,9 However, we did not find significant differences in the levels of other molecules such as cortisol and haptoglobin, which have been found consistently to be altered in other studies of first-onset antipsychotic-naive schizophrenia patients.
Table 2. Serum proteins altered between patients and siblings compared with controls.
| Analyte | Patients vs controls | Siblings vs controls | |||||
|---|---|---|---|---|---|---|---|
| P | Q | Ratio |
Symptomatic |
Asymptomatic |
|||
| |
|
|
|
P |
RC |
P |
RC |
| Insulin | <0.001 | <0.01 | 1.33 | 0.042 | 1.30 | 0.017 | 1.18 |
| C-peptide | <0.001 | 0.01 | 1.13 | 0.004 | 1.24 | 0.017 | 1.09 |
| T-lymphocyte-secreted protein I 309 | 0.002 | 0.04 | 0.80 | 0.011 | 0.79 | 0.001 | 0.83 |
| Growth hormone | 0.006 | 0.08 | 0.58 | 0.006 | 0.65 | 0.032 | 0.81 |
| Proinsulin | 0.006 | 0.08 | 1.17 | 0.043 | 1.19 | 0.614 | 1.00 |
| CD5L | 0.002 | 0.04 | 0.95 | 0.207 | 0.95 | 0.035 | 0.96 |
| Interferon-γ-induced protein 10 | 0.001 | 0.04 | 0.92 | 0.464 | 1.00 | 0.083 | 0.93 |
| Immunoglobulin M | 0.002 | 0.04 | 0.91 | 0.845 | 1.08 | 0.502 | 1.03 |
| Carcinoembryonic antigen | 0.005 | 0.07 | 1.17 | 0.667 | 0.98 | 0.959 | 0.97 |
| Adiponectin | 0.007 | 0.08 | 0.90 | 0.492 | 1.14 | 0.145 | 0.98 |
Abbreviation: RC, ratio vs controls. Serum samples from all subjects were analyzed by multiplex immunoassay analysis. All P-values were corrected for multiple hypotheses testing by controlling the false-discovery rate (Q-values) and findings of P<0.05 and Q<0.1 were considered significant (indicated in italics). Analytes in bold were significantly altered in patients as well as in both symptomatic and asymptomatic siblings.
Siblings
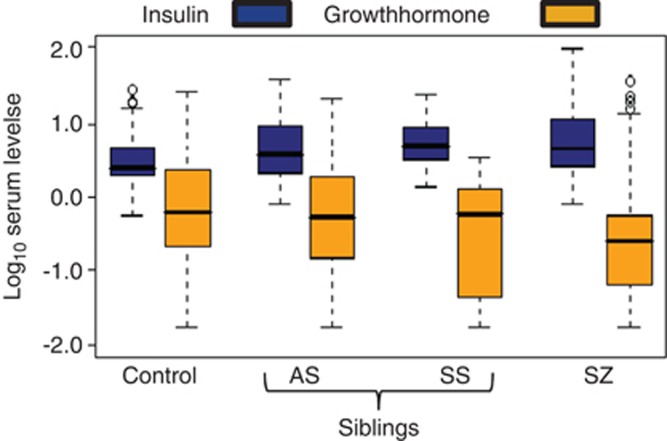
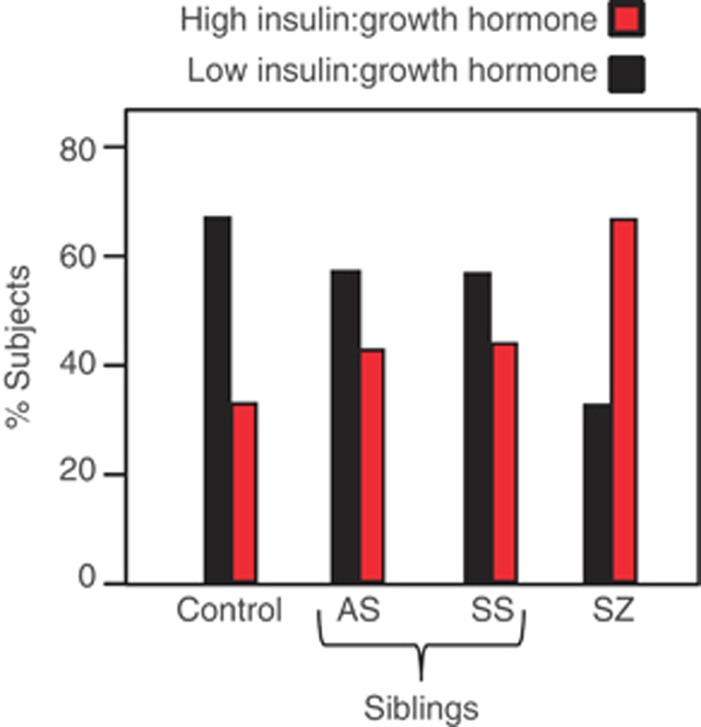
Of 10 altered proteins in schizophrenia patients, 5 of these showed significant differences in symptomatic siblings and 5 were also altered in asymptomatic siblings (Table 2). Four proteins (insulin, C-peptide, T-lymphocyte-secreted protein I 309 and growth hormone) were significantly altered in both symptomatic and asymptomatic siblings. Interestingly, insulin was consistently increased and growth hormone decreased in patients and both sibling groups compared with controls. Likewise, the insulin/growth hormone ratio was increased in patients (ratio=2.2; P<0.001), symptomatic siblings (ratio=1.9; P=0.0214) and asymptomatic siblings (ratio=1.4; P=0.0021) compared with controls (Figure 1). We next determined the proportion of subjects in each group that had high insulin/growth hormone ratios in schizophrenia patients and siblings compared with controls. Insulin/growth hormone values were dichotomized such that patients below the median (9.8 μIU ng−1) were partitioned into low group and those above the median were assigned to the high group. This showed that the highest proportion of subjects with high insulin/growth hormone values were found in the schizophrenia patient group (67%), followed by the symptomatic siblings (45%) and the asymptomatic siblings (43%) (Figure 2). The controls had a significantly lower proportion of high insulin/growth hormone values (30% P<0.001).
Figure 1.
Boxplots showing altered levels of insulin and growth hormone in schizophrenia patients and siblings compared with controls. Serum concentrations of insulin (blue) and growth hormone (GH, yellow) were determined using multiplexed immunoassays for control, asymptomatic siblings (AS), symptomatic siblings (SS) and schizophrenia patients (SZ). The levels of insulin in controls were 4.3±5.0 μIU ml−1 and those for growth hormone were 3.7±6.3 ng ml−1. All values were log10 transformed to account for unequal distribution of the data. Bold horizontal bars reflect median protein levels.
Figure 2.
Histograms showing higher insulin/growth hormone ratios in schizophrenia patients and siblings compared with controls. Proportion of patients with high (red) and low (black) insulin/growth hormone ratios. The high and low categories were defined as subjects with higher and lower insulin:growth hormone ratios compared with the median insulin:growth hormone ratio (9.8 μIU ng−1), respectively.
Discussion
The changes in the insulin-related molecules are consistent with other studies that have implicated glycoregulatory abnormalities in schizophrenia, including impaired fasting glucose tolerance, hyperinsulinemia and insulin resistance.1,5,8,13,15 A potential linkage of these peripheral changes to alterations in brain function have been suggested by 1 H nuclear magnetic resonance spectroscopy profiling studies that found decreased lactate and increased glucose in cerebrospinal fluid from first-onset patients compared with controls.27 Proteomic studies have also found metabolic abnormalities in postmortem brain tissues from schizophrenia patients.15,16,28 Furthermore, brain imaging studies have established that reduced glucose uptake occurs in frontal cortices of some patients with schizophrenia.29 Metabolic perturbations have also been identified in the central nervous system and periphery of bipolar disorder, major depressive disorder and Alzheimer's disease patients, suggesting that similar effects may occur in other psychiatric disorders.
The most notable result of the current study was the finding that altered levels of insulin and growth hormone in schizophrenia patients were also present in siblings with and without a diagnosis of psychiatric disorders. The increase in the insulin/growth hormone ratio would be expected to cause a marked shift in metabolic and growth-regulation pathways. These two hormones can have counteractive effects on some tissues and striking similarities exist between insulin resistance and growth hormone-deficient syndromes.30 Previous studies have shown that subjects with growth hormone deficiency are insulin resistant,31 and long-term growth hormone treatment of adults with a deficiency in this hormone have been shown to improve insulin sensitivity and whole-body glucose metabolism.32 In addition, the results of an early study suggested that the reduced growth hormone levels may be due to schizophrenia-associated increases in dopamine or serotonin activities, which regulate the production of this hormone.33 This is intriguing considering established hypotheses for schizophrenia that have suggested a causal link to increased levels of these neurotransmitters.34 We also found changes in the levels of T-lymphocyte-secreted protein I 309 in all subjects compared with controls, consistent with the effects on immune system dysfunction, which are also observed in schizophrenia9 and metabolic syndrome.35
There are several limitations to consider in this analysis. First, significant differences were not found in the levels of molecules such as cortisol and haptoglobin, which were described in other studies of first-onset antipsychotic-naive schizophrenia patients.9 However, this may be related to the clinical characteristics of the current patients who were all medicated compared with the antipsychotic-naive first-episode patients analyzed previously. Second, it was not possible to measure insulin resistance directly in this study as blood glucose levels were not determined. This is due to the fact that the samples were collected from patients and controls as they became available, and were not necessarily obtained under fasting conditions. Therefore, the measurements used in the current study only give indirect readings and represent approximations of insulin signaling. Third, no conclusion can be drawn about the similarity of the insulin/growth hormone ratios in the symptomatic (n=16) and the asymptomatic sibling (n=117) groups, considering the small sample size of the former. Finally, no records were obtained for any of the subjects under study concerning family histories for occurrence of metabolic conditions such as type 2 diabetes mellitus or metabolic syndrome. This could be informative in future studies concerning the known link between psychiatric disorders and metabolic diseases, as revealed by proteomic and metabolomic studies.36, 37, 38, 39, 40, 41, 42
Overall, the present findings suggest that metabolic and hormonal disturbances such as effects on insulin and growth hormone may represent a vulnerability factor to develop mental disorders. We hypothesize that genetically or environmentally determined dysfunction in these pathways can result in deleterious effects on the brain in predisposed individuals. For example, intermittent glucose deprivation may occur in individuals with sporadic neuronal hyperactivity and associated excessive brain energy demand, which places stress on these pathways. This can also occur in cases of neurodevelopmental deficits affecting blood supply to certain brain regions and peripheral inflammation that deprives the body of glucose. Such effects may be correlated with onset or the remitting relapsing course of psychiatric symptoms. In this light, it is interesting that antipsychotic drugs are known to increase peripheral glucose levels. Indeed, these pro-diabetic effects are viewed as major side effects of these drugs. We propose that these effects may be intrinsically related to the therapeutic mechanism of action by increasing the peripheral blood glucose levels and thereby increasing glucose availability in the brain. Further research should attempt to disentangle the contribution of environmental and genetic factors to the observed molecular alterations. Furthermore, the production of tests based on measurement of these molecules may allow identification of high-risk individuals. This could lead to the implementation of novel disease prevention approaches, which could involve nutrition modification, stress reduction and pharmaco-therapeutic interventions, including the application of well-tolerated drugs that combat insulin resistance.
Acknowledgments
This work was supported by grants from the Stanley Medical Research Institute the Dutch Fund for Economic Structure Reinforcement (FES), under grant agreement number 0908 (NeuroBasic PharmaPhenomics project) and the European Union FP7 SchizDX research programme (grant reference 223427).
Author contributions
SB and NJMvB conceived and planned the experiments. ES carried out the analyses. CM and LdH performed the subject characterization and provided the samples. ES and PCG produced the tables and figures. ES, NJMvB, PCG, RN, CM, LdH and SB wrote the manuscript. PCG carried out the final editing.
ES, PCG and SB are consultants for Myriad-RBM although this does not interfere with ownership regarding data sharing or materials. The remaining authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry. 2004;160:284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Nossova N, Yager T, Tsuang MM, Guo SC, Shyu KG, et al. Assessing the validity of blood-based gene expression profiles for the classification of schizophrenia and bipolar disorder: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005;133B:1–5. doi: 10.1002/ajmg.b.30161. [DOI] [PubMed] [Google Scholar]
- Craddock RM, Huang JT, Jackson E, Harris N, Torrey EF, Herberth M, et al. Increased alpha- defensins as a blood marker for schizophrenia susceptibility. Mol Cell Proteomics. 2007;7:1204–1213. doi: 10.1074/mcp.M700459-MCP200. [DOI] [PubMed] [Google Scholar]
- Domenici E, Willé DR, Tozzi F, Prokopenko I, Miller S, McKeown A, et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS One. 2010;5:e9166. doi: 10.1371/journal.pone.0009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest PC, Wang L, Harris LW, Burling K, Levin Y, Ernst A, et al. Increased levels of circulating insulin-related peptides in first-onset, antipsychotic naive schizophrenia patients. Mol Psychiatry. 2010;15:118–119. doi: 10.1038/mp.2009.81. [DOI] [PubMed] [Google Scholar]
- Levin Y, Wang L, Schwarz E, Koethe D, Leweke FM, Bahn S, et al. Global proteomic profiling reveals altered proteomic signature in schizophrenia serum. Mol Psychiatry. 2010;15:1088–1100. doi: 10.1038/mp.2009.54. [DOI] [PubMed] [Google Scholar]
- Cheng TM, Lu YE, Guest PC, Rahmoune H, Harris LW, Wang L, et al. Identification of targeted analyte clusters for studies of schizophrenia. Mol Cell Proteomics. 2010;9:510–522. doi: 10.1074/mcp.M900372-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest PC, Schwarz E, Krishnamurthy D, Harris LW, Leweke FM, Rothermundt M, et al. Altered levels of circulating insulin and other neuroendocrine hormones associated with the onset of schizophrenia. Psychoneuroendocrinology. 2011;36:1092–1096. doi: 10.1016/j.psyneuen.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Schwarz E, Guest PC, Rahmoune H, Harris LW, Wang L, Leweke FM, et al. Identification of a biological signature for schizophrenia in serum. Mol Psychiatry. 2012;17:494–502. doi: 10.1038/mp.2011.42. [DOI] [PubMed] [Google Scholar]
- Pedrini M, Massuda R, Fries GR, de Bittencourt Pasquali MA, Schnorr CE, Moreira JC, et al. Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J Psychiatr Res. 2012;46:819–824. doi: 10.1016/j.jpsychires.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Beumer W, Drexhage RC, De Wit H, Versnel MA, Drexhage HA, Cohen D, et al. Increased level of serum cytokines, chemokines and adipokines in patients with schizophrenia is associated with disease and metabolic syndrome. Psychoneuroendocrinology. 2012;37:1901–1911. doi: 10.1016/j.psyneuen.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Yang J, Chen T, Sun L, Zhao Z, Qi X, Zhou K, et al. Potential metabolite markers of schizophrenia. Mol Psychiatry. 2013;18:67–78. doi: 10.1038/mp.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nimwegen LJ, Storosum JG, Blumer RM, Allick G, Venema HW, de Haan L, et al. Hepatic insulin resistance in antipsychotic naive schizophrenic patients: stable isotope studies of glucose metabolism. J Clin Endocrinol Metab. 2008;93:572–577. doi: 10.1210/jc.2007-1167. [DOI] [PubMed] [Google Scholar]
- Arranz B, Rosel P, Ramírez N, Dueñas R, Fernández P, Sanchez JM, et al. Insulin resistance and increased leptin concentrations in noncompliant schizophrenia patients but not in antipsychotic-naive first-episode schizophrenia patients. J Clin Psychiatry. 2004;65:1335–1342. doi: 10.4088/jcp.v65n1007. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–697. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Maccarrone G, Wobrock T, Zerr I, Gormanns P, Reckow S, et al. Proteome analysis of the thalamus and cerebrospinal fluid reveals glycolysis dysfunction and potential biomarkers candidates for schizophrenia. J Psychiatr Res. 2010;44:1176–1189. doi: 10.1016/j.jpsychires.2010.04.014. [DOI] [PubMed] [Google Scholar]
- English JA, Pennington K, Dunn MJ, Cotter DR. The neuroproteomics of schizophrenia. Biol Psychiatry. 2011;69:163–172. doi: 10.1016/j.biopsych.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Faraone SV, Lyons MJ. Identification of the phenotype in psychiatric genetics. Eur Arch Psychiatry Clin Neurosci. 1993;243:131–142. doi: 10.1007/BF02190719. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ, et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch Gen Psychiatry. 1999;56:162–168. doi: 10.1001/archpsyc.56.2.162. [DOI] [PubMed] [Google Scholar]
- McGue M, Gottesman II. The genetic epidemiology of schizophrenia and the design of linkage studies. Eur Arch Psychiatry Clin Neurosci. 1991;240:174–181. doi: 10.1007/BF02190760. [DOI] [PubMed] [Google Scholar]
- Olin SC, Mednick SA. Risk factors of psychosis: identifying vulnerable populations premorbidly. Schizophr Bull. 1996;22:223–240. doi: 10.1093/schbul/22.2.223. [DOI] [PubMed] [Google Scholar]
- Rouillon F, Sorbara F. Schizophrenia and diabetes: epidemiological data. Eur Psychiatry. 2005;4:S345–S348. doi: 10.1016/s0924-9338(05)80189-0. [DOI] [PubMed] [Google Scholar]
- Korver N, Quee PJ, Boos HB, Simons CJ, de Haan L. GROUP investigators: Genetic Risk and Outcome of Psychosis (GROUP), a multi site longitudinal cohort study focused on gene-environment interaction: objectives, sample characteristics, recruitment and assessment methods. Int J Methods Psychiatr Res. 2012;21:205–221. doi: 10.1002/mpr.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Hanssen M, Smirnis NK, Avramopoulos DA, Evdokimidis IK, Stefanis CN, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32:347–358. doi: 10.1017/s0033291701005141. [DOI] [PubMed] [Google Scholar]
- Hutton JC. Insulin secretory granule biogenesis and the proinsulin-processing endopeptidases. Diabetologia. 1994;37:S48–S56. doi: 10.1007/BF00400826. [DOI] [PubMed] [Google Scholar]
- Holmes E, Tsang TM, Huang JT, Leweke FM, Koethe D, Gerth CW, et al. Metabolic profiling of CSF: evidence that early intervention may impact on disease progression and outcome in schizophrenia. PLoS Med. 2006;3:e327. doi: 10.1371/journal.pmed.0030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington K, Beasley CL, Dicker P, Fagan A, English J, Pariante CM, et al. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry. 2008;13:1102–1117. doi: 10.1038/sj.mp.4002098. [DOI] [PubMed] [Google Scholar]
- Brown GG, Thompson WK. Functional brain imaging in schizophrenia: selected results and methods. Curr Top Behav Neurosci. 2010;4:181–214. doi: 10.1007/7854_2010_54. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Turyn D. Growth hormone-induced alterations in the insulin-signaling system. Exp Biol Med (Maywood) 2002;227:149–157. doi: 10.1177/153537020222700301. [DOI] [PubMed] [Google Scholar]
- Murray R, Shalet S.Insulin sensitivity is impaired in adults with varying degrees of GH deficiency Clin Endocrinol (Oxford) 200562182–188., 2005. [DOI] [PubMed] [Google Scholar]
- Arafat AM, Möhlig M, Weickert MO, Schöfl C, Spranger J, Pfeiffer AF, et al. Improved insulin sensitivity, preserved beta cell function and improved whole-body glucose metabolism after low-dose growth hormone replacement therapy in adults with severe growth hormone deficiency: a pilot study. Diabetologia. 2010;53:1304–1313. doi: 10.1007/s00125-010-1738-4. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Davidson M, Hirschowitz J, Stern RG, Davis BM, Gabriel S, et al. Nocturnal growth hormone secretion in schizophrenic patients and healthy subjects. Psychiatry Res. 1992;41:155–161. doi: 10.1016/0165-1781(92)90107-e. [DOI] [PubMed] [Google Scholar]
- Huttunen M. The evolution of the serotonin-dopamine antagonist concept. J Clin Psychopharmacol. 1995;15:4 S–10 S. doi: 10.1097/00004714-199502001-00002. [DOI] [PubMed] [Google Scholar]
- Donath MY, Böni-Schnetzler M, Ellingsgaard H, Halban PA, Ehses JA. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol Metab. 2010;21:261–267. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Harris LW, Guest PC, Wayland MT, Umrania Y, Krishnamurthy D, Rahmoune H, et al. Schizophrenia: Metabolic aspects of aetiology, diagnosis and future treatment strategies. Psychoneuroendocrinology. 2012;38:752–766. doi: 10.1016/j.psyneuen.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, McEvoy J, Baillie RA, Lee D, Yao JK, Doraiswamy PM, et al. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol Psychiatry. 2007;12:934–945. doi: 10.1038/sj.mp.4002000. [DOI] [PubMed] [Google Scholar]
- Yao JK, Dougherty GG, Jr, Reddy RD, Keshavan MS, Montrose DM, Matson WR, et al. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol Psychiatry. 2010;15:938–953. doi: 10.1038/mp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Condray R, Dougherty GG, Jr, Keshavan MS, Montrose DM, Matson WR, et al. Associations between purine metabolites and clinical symptoms in schizophrenia. PLoS One. 2012;7:e42165. doi: 10.1371/journal.pone.0042165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Dougherty GG, Jr, Reddy RD, Keshavan MS, Montrose DM, Matson WR, et al. Homeostatic imbalance of purine catabolism in first-episode neuroleptic-naïve patients with schizophrenia. PLoS One. 2010;5:e9508. doi: 10.1371/journal.pone.0009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condray R, Dougherty GG, Jr, Keshavan MS, Reddy RD, Haas GL, Montrose DM, et al. 3-Hydroxykynurenine and clinical symptoms in first-episode neuroleptic-naive patients with schizophrenia. Int J Neuropsychopharmacol. 2011;14:756–767. doi: 10.1017/S1461145710001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Dougherty GG, Reddy RD, Matson WR, Kaddurah-Daouk R, Keshavan MS, et al. Associations between purine metabolites and monoamine neurotransmitters in first-episode psychosis. Front Cell Neurosci. 2013;7:90. doi: 10.3389/fncel.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.