Abstract
The studies investigating the effects of green tea on blood pressure (BP) have generated inconsistent results. The aim of this study is to quantitatively evaluate the effects of green tea on BP control. PubMed, Embase, and the Cochrane Library (updated to March 2014) were searched for randomized controlled trials evaluating the effects of green tea on BP. Pooled effect of green tea consumption on BP was evaluated using fixed-effects or random-effects model. Thirteen trials comprising a total of 1,367 subjects were included in the current meta-analysis. The overall outcome of the meta-analysis suggested that green tea consumption significantly decrease systolic blood pressure (SBP) level by −1.98 mmHg (95% CI: −2.94, −1.01 mmHg; P < 0.001). Compared with the control group, green tea also showed a significant lowering effect on diastolic blood pressure (DBP) in treatment group (−1.92 mmHg; 95% CI: −3.17, −0.68 mmHg; P = 0.002). Subgroup analyses further suggested that the positive effect of green tea polyphenols on BP was only showed in studies using a low-dose green tea polyphenol, with the long-term intervention duration or ruling out the confounding effects of caffeine. The meta-analysis suggested that green tea consumption had a favorable effect on decrease of BP.
Cardiovascular disease (CVD) is one of the most common public health challenges worldwide. The World Health Organization (WHO) estimates that the cost of not investing in CVD prevention and therapy could amount to as much as $47 trillion worldwide in the next 25 years1, and the consequences will be more severe in developing countries, given that 80% of cardiovascular deaths occur in low and middle income countries1. As a major risk factor for cardiovascular disease, hypertension is the most common chronic condition in American adults. Recent studies have shown that two thirds of hypertensives in the United States are undertreated or untreated2. It has been demonstrated that even people who are free of hypertension (BP < 140/90 mm Hg) at the age of 55 have a 90% lifetime risk for developing hypertension, according to the Framingham data3. In addition, accumulating evidence suggest that health-promoting lifestyle modifications can improve blood pressure (BP) control and even reduce medication needs4. Among the comprehensive lifestyle modifications, dietary adjustment is one of the most effective measures for preventing hypertension. A previous meta-analysis suggested that even small changes in BP caused by dietary modification might have a significant impact on the prevalence of hypertension5.
Green tea, derived from the plant Camellia sinensis, is a popular beverage worldwide and the major source of flavonoid intake in the US diet6. The most prominent effects of tea on human health have been attributed to green tea, and the health-promoting effects of green tea are mainly attributed to catechins, which belong to a family of compounds known as flavonoid-like polyphenols or flavanols7. A recent meta-analysis has revealed that green tea consumption might significantly reduce plasma low density lipoprotein–cholesterol (LDL-C) and total cholesterol concentrations8. In addition, our previous meta-analysis demonstrated that green tea may have a favorable effect on glucose control in adults9. Therefore, one question is pertinent: does green tea also have beneficial effects on BP? Although Hartley et al. conducted a meta-analysis evaluating the effect of green tea on BP, they only included two RCTs when evaluating the effect of green tea on BP10, and thus the results of their study might be interpreted with caution. To date, previous randomized controlled trials (RCTs) investigating the effects of green tea consumption on BP have generated inconsistent results. Thus, we conducted the meta-analysis to explore the effects of green tea on BP based on the PRISMA guidelines.
Results
Results of the literature search
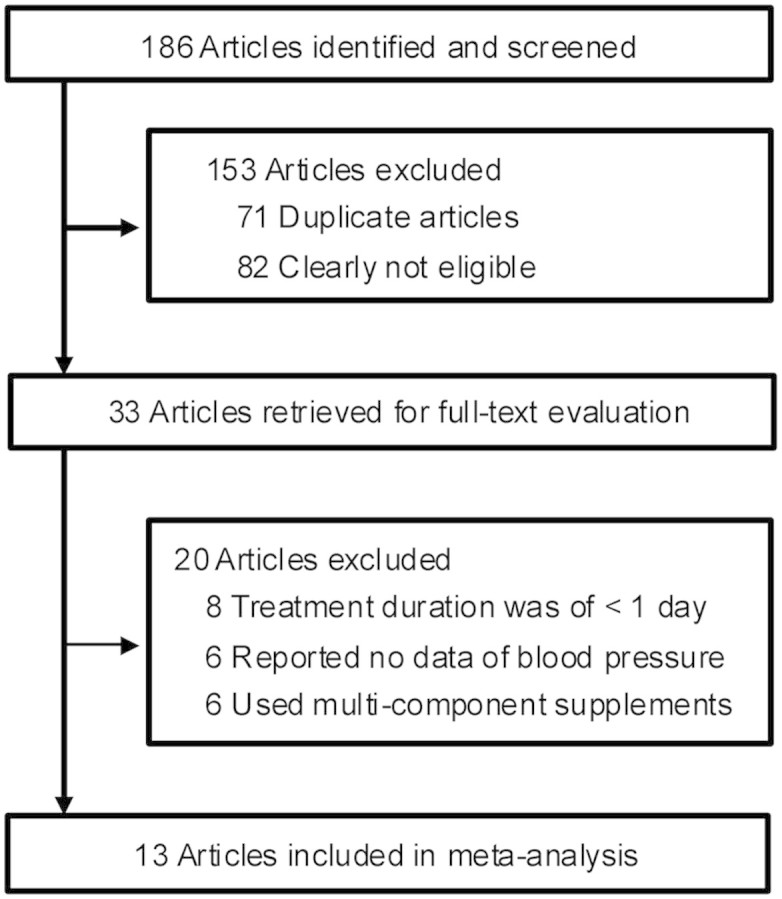
Detailed processes of the relevant study selection are shown in Fig. 1. A total of 186 reports were initially identified, and 153 articles were excluded either because of duplication or because they were clearly not relevant to the current meta-analysis after carefully reviewing the titles and abstracts. Thus, 33 articles remained for an in-depth examination. Among these 33 articles, 20 were further excluded for the following reasons: 8 were due to their short duration (<1 day), 6 were due to no data of SBP or DBP, and 6 were discarded because the green tea extract was given as part of a multi-component supplement. Thus, 13 articles were ultimately selected for inclusion in the meta-analysis11,12,13,14,15,16,17,18,19,20,21,22,23.
Figure 1. Flow diagram showing the number of citations retrieved by individual searches of articles included in the review.
Study characteristics
A summary of the study characteristics included in the meta-analysis is presented in (Table 1). The total number of subjects included in each study ranged from 22 to 240 subjects. The study duration varied from 3 wk to 3 mo (median: 12 wk). Of the 13 trials included in the current meta-analysis, 9 trials focused on adults with prehypertension, 3 trials selected participants with stage 1 hypertension and one trial investigated subjects with Stage 2 hypertension. Most studies (11 of 13) used parallel design. All studies suggested subjects maintain a usual diet, and one study recommended that subjects maintain their physical activities during the study period. Of the 9 studies ruled out the confounding effect of caffeine on BP, 5 used decaffeinated green tea extract as supplements, 2 used decaffeinated green tea beverage in the intervention group, and 2 used placebo matching caffeine in the control group. And four studies used caffeinated green tea as supplements. The green tea polyphenols content ranged from 208 to 1207 mg/d (median: 582.8 mg/d).
Table 1. Characteristics of 13 randomized controlled trials included in analysis.
| Source | No. of subjects | Study design | Population | Duration | Tea group | Control group | Jadad score | Type of dieta |
|---|---|---|---|---|---|---|---|---|
| Fukino, 2005, Japan (11) | 66 | Parallel | Stage 1 hypertension, overweight, 32–73 y of age | 2 mo | DGTB (544 mg polyphenols) | No tea | <4 | Usual diet |
| Diepvens, 2006, Netherlands (12) | 46 | Parallel | Prehypertension, overweight, 19–57 y of age | 87 d | GTE capsule (1,207 mg polyphenols, 236.7 mg caffeine) | Placebo (maltodextrin capsule) | <4 | Low-energy diet |
| Nagao, 2007, Japan (13) | 240 | Parallel | Prehypertension, obese, 25–55 y of age | 12 wk | GTE beverage (582.8 mg polyphenols, 72.3 mg caffeine) | GTE beverage (96 mg catechins, 75.0 mg caffeine) | <4 | Usual diet |
| Hill, 2007, Switzerland (14) | 38 | Parallel | Prehypertension, overweight/obese, 45–70 y of age | 12 wk | DGTE capsule (300 mg polyphenols) | Placebo (lactose capsules) | <4 | Usual diet with excise |
| Fukino, 2008, Japan (15) | 70 | Crossover | Stage 1 hypertension, overweight, 32–73 y of age | 2 mo | GTE beverage (544 mg polyphenols, 102 mg caffeine) | Water | <4 | Usual diet |
| Nagao, 2009, Japan (16) | 43 | Parallel | Prehypertension, T2DM, 64.9 ± 1.6 y of age | 12 wk | GTE beverage (582.8 mg polyphenols, 72.3 mg caffeine) | GTE beverage (96.3 mg catechins, 75.0 mg caffeine) | <4 | Usual diet |
| Frank, 2009, UK (17) | 33 | Parallel | Prehypertension, overweight,18–55 y of age | 3 wk | GTE Capsule (714 mg polyphenols, 114 mg caffeine) | Placebo (maltodextrin) | <4 | Usual diet |
| Brown, 2009, UK (18) | 88 | Parallel | Prehypertension, overweight/obese, 40–65 y of age | 8 wk | DGTE capsule (800 mg polyphenols) | Placebo (lactose capsules) | ≥4 | Usual diet |
| Nantz, 2009, USA (19) | 111 | Parallel | Prehypertension, overweight, 21–70 y of age | 3 mo | DGTB (320 mg polyphenols) | Placebo capsule | ≥4 | Usual diet |
| Basu, 2010, USA (20) | 22 | Parallel | BP < 160/100 mmHg, obesity and metabolic syndrome, 25–63 y of age | 8 wk | Green tea beverage (928 mg polyphenols, 8.96 mg caffeine) | Water | <4 | Usual diet |
| Brown, 2011, UK (21) | 66 | Crossover | Prehypertension, overweight and obese, 40–69 y of age | 6 wk | DGTE capsule (800 mg polyphenols) | Placebo (lactose capsules) | ≥4 | Usual diet |
| Suliburska, 2012, Poland (22) | 46 | Parallel | Prehypertension, obese, 30–60 y of age | 3 mo | DGTE capsule (208 mg polyphenols) | Placebo capsules (cellulose) | ≥4 | Usual diet |
| Bogdanski, 2012, Poland (23) | 56 | Parallel | Stage 1 hypertension, obese, 30–60 y of age | 3 mo | DGTE capsule (208 mg polyphenols) | Placebo capsules (cellulose) | ≥4 | Usual diet |
Abbreviations: DGTB, Decaffeinated green tea beverage; DGTE, decaffeinated green tea extract; EGCG, epigallocatechin gallat; GTE, green tea extract; T2DM, type 2 diabetes mellitus.
aA usual diet was similar to a conventional diet; a low-energy diet contained less amounts of energy than a usual diet.
Data quality
Study quality was assessed by using the Jadad scale and the results were varied. Five trials18,19,21,22,23 were classified as high quality (Jadad score of ≥4) and the remaining 8 trials were low quality (Jadad score of <4). All of the high-quality RCTs reported the use of random number generation or randomization list performed by an independent statistician, and 2 studies used an adequate allocation concealment conducted by a third-party manufacture. Details related to the dropouts were reported in all high quality trials.
Effect of green tea consumption on blood pressure
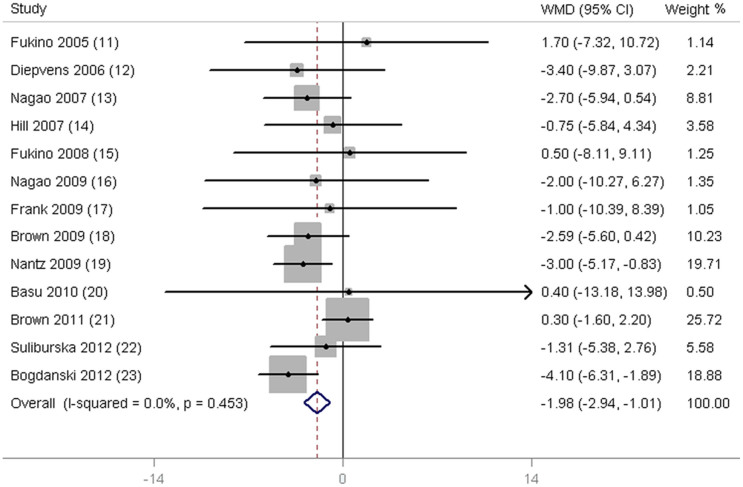
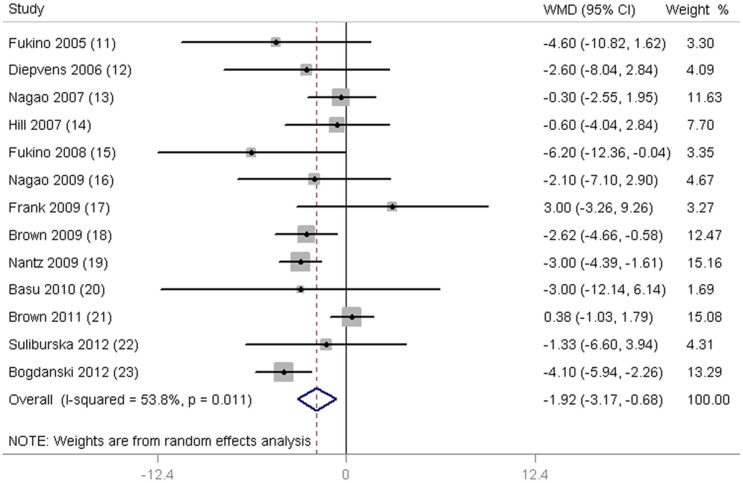
As shown in Fig. 2, a significant reduction in SBP was observed in subjects supplemented with green tea (−1.98 mmHg; 95% CI, −2.94 to −1.01 mmHg; P < 0.001) when compared with control subjects. The mean difference in DBP was reported in 13 trials and was found to be significantly different (−1.92 mmHg; 95% CI, −3.17 to −0.68 mmHg; P = 0.002; Fig. 3). In addition, no significant differences were detected when we used the different model to pooling the data (Supplementary Fig. S1 and Fig. S2 online).
Figure 2. Meta-analysis of effects of green tea on systolic blood pressure (SBP).
Weight was assigned with STATA (Version 11; StataCorp, College Station, TX) by using number of subjects and SD. Sizes of data markers indicate the weight of each study in this analysis. The diamond represents the overall estimated effect and the result was obtained from a fixed-effects model. WMD, weighted mean difference.
Figure 3. Meta-analysis of effects of green tea on diastolic blood pressure (DBP).
Weight was assigned with STATA (Version 11; StataCorp, College Station, TX) by using number of subjects and SD. Sizes of data markers indicate the weight of each study in this analysis. The diamond represents the overall estimated effect and the result was obtained from a random-effects model. WMD, weighted mean difference.
We performed the subgroup analyses (Table 2) to further explore the effects of BP status, study design, green tea polyphenols dose, study duration and quality as well as to evaluate any differences between trials by ruling out confounding effect of caffeine and using caffeinated green tea as supplement. No significant differences were found between the random-effects model and fixed-effects model in the subgroup analyses. Green tea polyphenols consumption was categorized as either high-dose (≥582.8 mg/d) or low-dose (<582.8 mg/d). The duration was divided into a long-term subgroup (≥12 wk) and a short-term subgroup (<12 wk). The subgroup analyses indicated that green tea significantly lowered BP in subjects with Stage 1 hypertension or prehypertension. In parallel- and crossover-design subgroups, significant reductions in BP were observed in the parallel-design subgroup. However, no significant effect was found in the crossover-design subgroup. When we stratified studies according to the type of intervention, the beneficial effects of green tea on BP could only be observed when the confounding effect of caffeine was removed. Significant reductions in SBP and DBP were only observed in the low-dose green tea polyphenols subgroup or the long-term duration subgroup. Finally, when studies were stratified according to Jadad score, significant reductions of BP were found only in those trials with high Jadad scores.
Table 2. Subgroup analyses of blood pressure (BP) stratified by previously defined study characteristics.
| Systolic blood pressure (SBP) | Diastolic blood pressure (DBP) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test of heterogeneity | Test of heterogeneity | |||||||||
| Variables | No. of trials | Net change (95% CI) | P | I2,% | P | No. of trials | Net change (95% CI) | P | I2,% | P |
| BP status | ||||||||||
| Stage 1hypertension | 3 | −3.52 (−5.61, −1.43)a | 0.30 | 16.2 | 0.001 | 3 | −4.30 (−5.99, −2.60)a | 0.81 | <0.001 | <0.001 |
| −3.56 (−5.65, −1.47)b | 0.006 | −4.30 (−5.99, −2.60)b | <0.001 | |||||||
| Prehypertension | 9 | −1.57 (−2.66, −0.48)a | 0.56 | <0.001 | 0.005 | 9 | −1.33 (−2.11, −0.56)a | 0.05 | 49.3 | 0.001 |
| −1.57 (−2.66, −0.48)b | 0.005 | −1.96 (−3.53, −0.39)b | 0.012 | |||||||
| Study design | ||||||||||
| Parallel | 11 | −2.82 (−3.95, −1.69)a | 0.95 | <0.001 | <0.001 | 11 | −2.52 (−3.33, −1.70)a | 0.29 | 15.9 | <0.001 |
| −2.82 (−3.95, −1.69)b | <0.001 | −2.39 (−3.37, −1.41)b | <0.001 | |||||||
| Crossover | 2 | 0.31 (−1.54, 2.16)a | 0.97 | <0.001 | 0.743 | 2 | 0.05 (−1.33, 1.43)a | 0.04 | 76.0 | 0.942 |
| 0.31 (−1.54, 2.16)b | 0.743 | −2.20 (−8.49, 4.10)b | 0.494 | |||||||
| Type of intervention | ||||||||||
| Trials ruling out confounding effect of caffeine | 9 | −2.00 (−2.99, −1.01)a | 0.19 | 28.8 | <0.001 | 9 | −1.84 (−2.56, −1.12)a | 0.01 | 63.0 | <0.001 |
| −2.04 (−3.31, −0.77)b | 0.002 | −1.89 (−3.24, −0.54)b | 0.006 | |||||||
| Trials using green tea contained caffeine | 4 | −1.55 (−5.84, 2.75)a | 0.89 | <0.001 | 0.481 | 4 | −2.16 (−5.36, 1.05)a | 0.23 | 30.5 | 0.187 |
| −1.55 (−5.84, 2.75)b | 0.481 | −2.15 (−6.06, 1.76)b | 0.281 | |||||||
| Duration | ||||||||||
| <12 wk (low median) | 5 | −0.44 (−1.97, 1.09)a | 0.59 | <0.001 | 0.575 | 5 | −0.79 (−1.90, 0.32)a | 0.02 | 65.3 | 0.161 |
| −0.44 (−1.97, 1.09)b | 0.575 | −1.60 (−4.16, 0.96) | 0.220 | |||||||
| ≥12 wk (low median) | 8 | −2.98 (−4.21, −1.74)a | 0.91 | <0.001 | <0.001 | 8 | −2.57 (−3.48, −1.66)a | 0.29 | 17.2 | <0.001 |
| −2.98 (−4.21, −1.74)b | <0.001 | −2.43 (−3.54, −1.32)b | <0.001 | |||||||
| Green tea polyphenols dose | ||||||||||
| <582.8 mg/d (low median) | 6 | −2.87 (−4.23, −1.51)a | 0.55 | <0.001 | <0.001 | 6 | −3.19 (−4.20, −2.18)a | 0.44 | <0.001 | <0.001 |
| −2.87 (−4.23, −1.51)b | <0.001 | −3.19 (−4.20, −2.18)b | <0.001 | |||||||
| ≥582.8 mg/d (high median) | 7 | −1.08 (−2.44, 0.29)a | 0.60 | <0.001 | 0.122 | 7 | −0.60 (−1.58, 0.37)a | 0.23 | 25.9 | 0.227 |
| −1.08 (−2.44, 0.29)b | 0.122 | −0.79 (−2.11, 0.54)b | 0.244 | |||||||
| Jadad score | ||||||||||
| Low (<4) | 8 | −1.76 (−3.92, 0.40)a | 0.97 | <0.001 | 0.110 | 8 | −1.17 (−2.67, 0.32)a | 0.47 | <0.001 | 0.125 |
| −1.76 (−3.92, 0.40)b | 0.110 | −1.17 (−2.67, 0.32)b | 0.125 | |||||||
| High (≥4) | 5 | −2.03 (−3.10, −0.96)a | 0.04 | 60.7 | <0.001 | 5 | −2.04 (−2.84, −1.25)a | 0.001 | 78.1 | <0.001 |
| −2.15 (−3.94, −0.36)b | 0.019 | −2.20 (−4.06, −0.35)b | 0.020 | |||||||
aThe result was obtained from a fixed-effects model.
bThe result was obtained from a random-effects model.
The results of meta-regression analysis did not observe a significant dose-responsive effect between green tea and SBP (P for trend = 0.14) or DBP (P for trend = 0.16). And the sensitivity analyses showed that the pooled effects of green tea on BP were not altered when the analyses were limited to high-quality studies and after imputation using a correlation coefficient of 0.5. Moreover, systematically removing each trial during the sensitivity analyses did not significantly change the overall observed effects of green tea on BP.
Publication bias
Funnel plots (Supplementary Fig. S3 and Fig. S4 online) and Egger's tests showed no significant publication bias in the current meta-analyses of SBP and DBP (Egger's test: P = 0.62 and 0.81).
Discussion
Our meta-analysis showed that green tea consumption significantly lowered BP and this effect was not altered when we pooled the data according to BP status. Significant reductions in SBP and DBP were only observed in the low-dose green tea polyphenols, the long-term duration, parallel-design RCTs, high-quality trials subgroups or when the confounding effect of caffeine was removed.
A previous meta-analysis of observational studies demonstrated that 1 cup/d of green tea was associated with a 10% decrease in the risk of developing coronary artery diseases24. A large population-based, prospective cohort study also found that an average of 2 cups/d of green tea intake significantly decreased the mortality risk of CVD25. The overall result of the current meta-analysis and its subgroup analyses consistently suggested that green tea had a significant lowering effect on BP, which might partially explain the favorable effects of green tea on CVD.
Consistent with this meta-analysis, a recent meta-analysis also found that green tea consumption could improve both SBP and DBP control26. Another meta-analysis suggested that green tea intervention had a favorable effect on SBP, but failed to lower the DBP level27. Contrary to the two studies, this meta-analysis improved the design of the subgroup analyses and investigated the potential influence of more confounding factors such as caffeine, intervention duration of green tea consumption, and study quality on the overall results of the meta-analysis. Our subgroup analyses showed that green tea consumption significantly decreased BP in subjects with Stage 1 hypertension or prehypertension, and the beneficial effects of green tea on lowering BP were more prominent in subjects with Stage 1 hypertension. In addition, the subgroup analyses based on the parallel-design RCTs and high-quality study indicated that green tea has a significant lowering effect on BP. Consistent with the study, in vivo studies have revealed that green tea extract can significantly reduce the increase of BP and enhance endothelial function in hypertensive rats28,29,30. The mechanism underlying the beneficial effect of green tea on BP control may involve the following aspects: 1) Green tea extract can maintain vascular tone by balancing vasoconstricting substances, including angiotensin II (Ang-II), prostaglandins, endothelin-1 (ET-1), and vasodilating substances, such as prostacyclin and various endothelium-derived hyperpolarizing factors (EDHFs)31,32,33,34. 2) Green tea can improve ventricular function and exert beneficial effects via increasing nitric oxide (NO) production from endothelium in PI3-kinase dependent pathways29. Green tea extract is also capable of regulating eNOS activation and ROS production, thereby increasing the production of NO35. 3) Green tea is able to reduce oxidative stress and manage the generation of ROS by inhibiting pro-oxidant enzymes and inducing antioxidant enzymes. Green tea catechins also function circuitously by inhibiting the nuclear factor-kappa B, redox-sensitive transcription factors, and activator protein-1 responsible for oxidative stress36,37,38. Moreover, green tea catechins can induce an anti-inflammatory effect by suppression of several inflammatory factors, such as cytokines, adhesion molecules, and nuclear factor-kappa B35,39.
We observed a significant reduction of SBP and DBP in the studies with a low-dose green tea polyphenols consumption subgroup and the subgroup of long-term intervention. Meanwhile, our study indicates that green tea intake has a significant lowering BP effect only in the trials designed to rule out the confounding effects of caffeine when we stratify the studies according to the type of intervention. This finding may partially explain why a high-dose of green tea consumption did not reduce BP, since a higher dose of green tea contains more caffeine and regular caffeine intake might increase BP, as reported by Noordzij et al40 in their meta-analysis. Additionally, a previous cohort study including 1017 US males with a median age of 33 years suggested that drinking coffee was associated with BP increase and the risk of hypertension41. Despite its potential BP-increasing effect, coffee drinking appeared not to be related to coronary events in a prospective study that included over 45 000 US male42. Therefore, the effects of high-dose green tea containing caffeine on cardiovascular disease events need to be further evaluated by more high-quality and large sample size studies. Finally, a significant reduction in BP was found in the high Jadad score subgroup that possessed less bias, but was not shown in the low Jadad score subgroup.
Although the relatively larger number of pooled participants provides stronger statistical power to evaluate the treatment effect, this meta-analysis has several inevitable limitations. First, of the 13 studies, 5 studies were high-quality RCTs, whereas the remaining 8 studies were of low quality. The slight inconsistent results of the subgroup analyses conducted to explore the effects of green tea on BP may be attributed to the differences in study quality and an overall limited number of high-quality studies. The 2010 Consolidated Standard of Reporting Trials (CONSORT) is used worldwide to improve the reporting of RCTs, and also provides the professional guidance on the study design and conducting43. To improve the study qualities, future RCTs should be conducted in accordance with the CONSORT statement, and particularly provide the enough information on the five items in Jadad scoring criterion which are essential for subsequent quality evaluation in the meta-analysis. Second, BP was not the primary outcome in part of the RCTs selected in this meta-analysis and the null findings of secondary outcomes may not be published. Third, the results of meta-regression analysis did not show a significant dose-responsive effect between green tea and SBP or DBP. Therefore, it is difficult to determine the optimal dose for a dietary program as part of a health policy aimed at improving hypertension health. In addition, most of the selected studies (11 of 13) did not report the information on sample size calculation. We could not directly evaluate the rationality of sample sizes in these studies, because the appropriate sample size for the RCT is based on the power calculation, α value, SD value and expected difference according to the specific study design44. The investigators should properly calculate sample sizes before starting the future RCTs and report the details in the publication.
In conclusion, this meta-analysis indicates that green tea consumption significantly lowers BP. The significant decrease of BP in low-dose green tea subgroup, long-term intervention duration subgroup or the subgroup ruling out the confounding effects of caffeine needs to be evaluated by more high-quality RCTs specifically designed to evaluate the effects of green tea on BP.
Methods
Search strategy
PubMed (updated to March 2014; http://www.ncbi.nlm.nih.gov/pubmed/), Embase (updated to March 2014; http://www.embase.com/), the Cochrane Library (updated to March 2014; http://www.cochrane.org/) database, and reference lists and reviews were searched for RCTs examining the effects of green tea on BP in humans. The structured search strategies used the following terms: green tea, green tea extract, catechin, catechins, EGCG, camellia sinensis, or tea polyphenols [All Fields] which were paired with blood pressure [All Fields]. The searching was restricted to the reports of randomized controlled trials conducted in human subjects.
Study selection
Studies were included in the meta-analysis if they met the following criteria: 1) subjects ingested green tea beverage or extract for ≥1 wk; 2) the study was a RCT conducted in human subjects with either a parallel or crossover design; 3) the baseline and endpoint values for systolic blood pressure (SBP), diatolic blood pressure (DBP), or their difference with either SD, SEM or 95% CI were available for each group in the study; 4) the green tea or green tea extract was not given as part of a multi-component supplement; 5) the study used a concurrent control group for green tea or green tea extract treatment group and the only difference between the control and treatment group was green tea or green tea extract.
Data extraction and quality assessment
The data were collected by using a standardized and pre-piloted data extraction form that included the following categories: 1) study characteristics including authors, publication year, sample size, study design, study duration, dose, type of intervention and type of diet; 2) population information including age and baseline health status; and 3) net changes in SBP or DBP.
The quality of RCTs was evaluated by using the following criteria: 1) randomization (the study was described as randomized); 2) double blinding (participant masking and researcher masking); 3) reporting the number of withdrawals and reasons for withdrawal; 4) allocation concealment; and 5) generation of random numbers (using computer, random numbers table, shuffled cards or tossed coins, etc). RCTs scored one point for each area addressed in the study design with a possible score ranging between 0 and 5 (highest level of quality)45. The studies that received a score of ≥4 were deemed to be of high quality, whereas those that received a score of <4 were considered low quality.
Statistical analysis
Our meta-analysis was performed using STATA (Version 11; StataCorp, College Station, TX). The treatment effects were defined as weighted mean difference and 95% CIs calculated for net changes in BP values. The statistic heterogeneity was assessed by using Cochran's test (P < 0.1). The I2 statistic was also calculated, and an I2 > 50% indicated significant heterogeneity across studies46. A random-effects model was used if significant heterogeneity was shown among the trials. Otherwise, the results were obtained from a fixed-effects model. To provide more information on the data synthesis, we showed the results calculated from both the two models.
We excluded percent changes in mean and SD values when we extracted data for the meta-analysis. SD values were calculated from standard errors, 95% CIs, P values, or t-statistics when they were not directly available. In addition, change-from-baseline SD values were imputed as suggested by Follmann et al47, assuming a correlation coefficient of 0.5.
Publication bias was assessed with funnel plots and Egger's test. Previously defined subgroup analyses were performed to examine the potential source of heterogeneity within these studies and included BP status, study design, type of intervention, duration, green tea polyphenol dose, and Jadad score. Additional sensitivity analyses were also performed according to the Handbook for Systematic Review of Interventions of Cochrane software (Version 5.0.2; The Cochrane Collaboration, Oxford, United Kingdom).
Author Contributions
Conceived and designed the experiments: X.L.P., K.L. and M.T.M. Analyzed the data: X.L.P., R.Z. and K.L. Contributed reagents/materials/analysis tools: X.L.P., K.L., R.Z., X.P.Y., X.H.Y. and M.T.M. Wrote the first draft of the manuscript: X.L.P., R.Z. and K.L. Reviewed, edited and approved the manuscript: X.L.P., R.Z., B.W., K.L. and M.T.M.
Supplementary Material
Supplementary information
Acknowledgments
This study was supported by the “12th Five year Plan” for National Key Technology Research and Development Program (grant number: 2012BAI35B02).
References
- Laslett L. J. et al. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 60, S1–49 (2012). [DOI] [PubMed] [Google Scholar]
- Chiong J. R. Controlling hypertension from a public health perspective. Int J Cardiol. 127, 151–156 (2008). [DOI] [PubMed] [Google Scholar]
- Vasan R. S. et al. Residual lifetime risk for developing hypertension in middle-aged women and men: The Framingham Heart Study. JAMA. 287, 1003–1010 (2002). [DOI] [PubMed] [Google Scholar]
- James P. A. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 311, 507–520 (2014). [DOI] [PubMed] [Google Scholar]
- Lewington S., Clarke R., Qizilbash N., Peto R. & Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360, 1903–1913 (2002). [DOI] [PubMed] [Google Scholar]
- Song W. O. & Chun O. K. Tea is the major source of flavan-3-ol and flavonol in the U.S. diet. J Nutr. 138, 1543S–1547S (2008). [DOI] [PubMed] [Google Scholar]
- Khan N. & Mukhtar H. Tea polyphenols for health promotion. Life Sci. 81, 519–533 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X. X. et al. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr. 94, 601–610 (2011). [DOI] [PubMed] [Google Scholar]
- Liu K. et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 98, 340–348 (2013). [DOI] [PubMed] [Google Scholar]
- Hartley L. et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 6, CD009934 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukino Y., Shimbo M., Aoki N., Okubo T. & Iso H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J Nutr Sci Vitaminol (Tokyo). 51, 335–342 (2005). [DOI] [PubMed] [Google Scholar]
- Diepvens K., Kovacs E. M., Vogels N. & Westerterp-Plantenga M. S. Metabolic effects of green tea and of phases of weight loss. Physiol Behav. 87, 185–191 (2006). [DOI] [PubMed] [Google Scholar]
- Nagao T., Hase T. & Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring). 15, 1473–1483 (2007). [DOI] [PubMed] [Google Scholar]
- Hill A. M. et al. Can EGCG reduce abdominal fat in obese subjects. J Am Coll Nutr. 26, 396S–402S (2007). [DOI] [PubMed] [Google Scholar]
- Fukino Y. et al. Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. Eur J Clin Nutr. 62, 953–960 (2008). [DOI] [PubMed] [Google Scholar]
- Nagao T. et al. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity (Silver Spring) 17, 310–317 (2009). [DOI] [PubMed] [Google Scholar]
- Frank J. et al. Daily consumption of an aqueous green tea extract supplement does not impair liver function or alter cardiovascular disease risk biomarkers in healthy men. J Nutr. 139, 58–62 (2009). [DOI] [PubMed] [Google Scholar]
- Brown A. L. et al. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. Br J Nutr. 101, 886–894 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantz M. P., Rowe C. A., Bukowski J. F. & Percival S. S. Standardized capsule of Camellia sinensis lowers cardiovascular risk factors in a randomized, double-blind, placebo-controlled study. Nutrition 25, 147–154 (2009). [DOI] [PubMed] [Google Scholar]
- Basu A. et al. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr. 29, 31–40 (2010). [DOI] [PubMed] [Google Scholar]
- Brown A. L. et al. Health effects of green tea catechins in overweight and obese men: a randomised controlled cross-over trial. Br J Nutr. 106, 1880–1889 (2011). [DOI] [PubMed] [Google Scholar]
- Suliburska J. et al. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res. 149, 315–322 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanski P. et al. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 32, 421–427 (2012). [DOI] [PubMed] [Google Scholar]
- Wang Z. M. et al. Black and green tea consumption and the risk of coronary artery disease: a meta-analysis. Am J Clin Nutr. 93, 506-515 (2011). [DOI] [PubMed] [Google Scholar]
- Kuriyama S. et al. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA. 296, 1255–1265 (2006). [DOI] [PubMed] [Google Scholar]
- Khalesi S. et al. Green tea catechins and blood pressure: a systematic review and meta-analysis of randomised controlled trials. Eur J Nutr. in process; 10.1007/s00394-014-0720-1 (2014). [DOI] [PubMed] [Google Scholar]
- Onakpoya I., Spencer E., Heneghan C. & Thompson M. The effect of green tea on blood pressure and lipid profile: A systematic review and meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 24, 823–836 (2014). [DOI] [PubMed] [Google Scholar]
- Ikeda M. et al. Preventive effects of green tea catechins on spontaneous stroke in rats. Med Sci Monit. 13, BR40–45 (2007). [PubMed] [Google Scholar]
- Potenza M. A. et al. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab. 292, E1378–1387 (2007). [DOI] [PubMed] [Google Scholar]
- Negishi H. et al. Black and green tea polyphenols attenuate blood pressure increases in stroke-prone spontaneously hypertensive rats. J Nutr. 134, 38–42 (2004). [DOI] [PubMed] [Google Scholar]
- Bhardwaj P. & Khanna D. Green tea catechins: defensive role in cardiovascular disorders. Chin J Nat Med. 11, 345–353 (2013). [DOI] [PubMed] [Google Scholar]
- Aird W. C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 100, 158–173 (2007). [DOI] [PubMed] [Google Scholar]
- Galley H. F. & Webster N. R. Physiology of the endothelium. Br J Anaesth. 93, 105–113 (2004). [DOI] [PubMed] [Google Scholar]
- Schiffrin E. L. A critical review of the role of endothelial factors in the pathogenesis of hypertension. J Cardiovasc Pharmacol. 38 Suppl 2, S3–6 (2001). [DOI] [PubMed] [Google Scholar]
- Babu P. V. & Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 15, 1840–1850 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera C., Artacho R. & Gimenez R. Beneficial effects of green tea--a review. J Am Coll Nutr. 25, 79–99 (2006). [DOI] [PubMed] [Google Scholar]
- Frei B. & Higdon J. V. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 133, 3275S–84S (2003). [DOI] [PubMed] [Google Scholar]
- Agarwal A., Prasad R. & Jain A. Effect of green tea extract (catechins) in reducing oxidative stress seen in patients of pulmonary tuberculosis on DOTS Cat I regimen. Phytomedicine. 17, 23–27 (2010). [DOI] [PubMed] [Google Scholar]
- Lin Y. L. & Lin J. K. (-)-Epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-kappaB. Mol Pharmacol. 52, 465–472 (1997). [PubMed] [Google Scholar]
- Noordzij M. et al. Blood pressure response to chronic intake of coffee and caffeine: a meta-analysis of randomized controlled trials. J Hypertens. 23, 921–928 (2005). [DOI] [PubMed] [Google Scholar]
- Klag M. J. et al. Coffee intake and risk of hypertension: the Johns Hopkins precursors study. Arch Intern Med. 162, 657–662 (2002). [DOI] [PubMed] [Google Scholar]
- Grobbee D. E. et al. Coffee, caffeine, and cardiovascular disease in men. N Engl J Med. 323, 1026–1032 (1990). [DOI] [PubMed] [Google Scholar]
- Bian Z. X. & Shang H. C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 154, 290–291; author reply 291–292 (2011). [DOI] [PubMed] [Google Scholar]
- Wittes J. Sample size calculations for randomized controlled trials. Epidemiol Rev. 24, 39–53 (2002). [DOI] [PubMed] [Google Scholar]
- Moher D. et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses. Lancet 352, 609–613 (1998). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ. 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follmann D., Elliott P., Suh I. & Cutler J. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 45, 769–773 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information