Abstract
Over the past ten years, considerable progress has been made in our understanding of the mechanistic enzymology of the Radical-SAM enzymes. It is now clear that these enzymes appear to be involved in a remarkably wide range of chemically challenging reactions. This review article highlights mechanistic and structural aspects of the methylthiotransferases (MTTases) sub-class of the Radical-SAM enzymes. The mechanism of methylthio insertion, now observed to be performed by three different enzymes is an exciting unsolved problem.
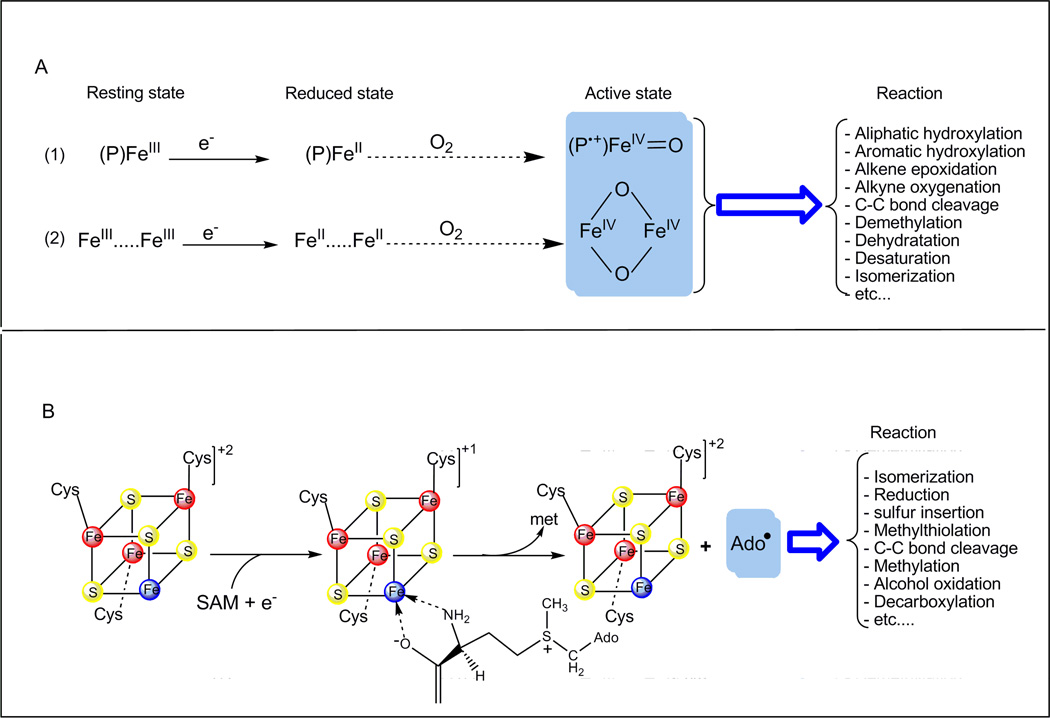
The selective transformation of inert C-H bonds in order to introduce functional groups has been considered to be one of the most challenging problem in bioinorganic chemistry during the last two decades. Indeed, this transformation is fundamentally important to life and immensely useful in industry [1]. It is now established that the functionalization of the C-H bonds is intricately linked to the presence of a metal ion and appears to fall mainly into two broad categories. The first one uses the reductive activation of molecular oxygen by metal ions –most notably iron- with the involvement of high-valent iron–oxo intermediates (Scheme 1A) [2–4]. In this case, the chemistry is restricted to aerobic organisms and the generally accepted mechanism is associated with that established for cytochrome P-450 and referred to as the haem paradigm (Scheme 1A-1). This paradigm is also applicable for a great variety of non-haem iron enzymes. The highly challenging hydroxylation reaction of methane to methanol, as performed by the soluble di-iron-containing methane monooxygenase, is a prototype of such chemistry (Scheme 1A-2) [5]. The second category uses the reductive activation of S-Adenosylmethionine (SAM) by a special [4Fe-4S]2+/1+ cluster to generate an elusive but highly oxidizing 5’-deoxyadenosyl radical (Ado•) intermediate [6]. The high reactivity of the Ado• radical is then be transferred to the substrate through (stereo)selective abstraction of a hydrogen atom of the C-H target, which facilitates progression of chemically difficult reactions (Scheme 1B) [7]. This chemistry occurs in both aerobic and anaerobic organisms and is achieved by the Radical-SAM superfamily of enzymes [8].
Scheme 1. The two strategies for production of highly oxidant species involved in the C-H bond activation.
(A) Mechanisms proposed for O2 activation to yield high-valent-iron-oxo by haem iron enzymes (1) and non-haem iron enzymes (2). P, porphyrin.
(B) Mechanism for the reductive cleavage of SAM by [4Fe-4S] cluster of Radical-SAM enzymes to yield 5’-deoxyadenosyl radical Ado˙. SAM is ligated through the amino and carboxylate groups to the unique iron (bleu) in the [4Fe-4S]+1 cluster. e−, electron; SAM, S-adenosylmethionine; met, methionine.
A bioinformatic survey published in 2002 initially revealed more than 600 different sequences of putative Radical-SAM enzymes in the database [8]. Since then this number has grown steadily and includes many thousands of sequences. Radical-SAM enzymes share a conserved CysXXXCysXXCys sequence, whose cysteines are the ligands of the [4Fe-4S] cluster. However, two variations have been reported in the cluster-binding motif: a CysXXCysXXXXCys motif near the C-terminus of the ThiC enzyme, which is required to convert 5-aminoimidazole ribonucleotide (AIR) into 4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate (HMP-P) [9], and a CysXXXXXCysXXCys motif in the HmdB protein. Which is involved in the maturation of the cofactor for the hydrogen forming methylene-H4-methanopterin dehydrogenase (HmdA) [10].
Since the discovery of this superfamily, many excellent reviews have been published and different subfamilies have been proposed [7, 11, 12]. Indeed, investigations of this topic have illuminated key details of the radical generation processes and now, many new highly challenging Radical-SAM enzymes are under characterization by different groups. In light of these new developments, we decided to focus the present review on three related enzymes families that catalyze a similar methylthio transfer reaction. The MiaB family catalyzes formation of the 2-methylthio-6-isopentenyl adenosine (ms2i6A) in some tRNAs. The MtaB/e-MtaB family catalyzes formation of 2-methylthio-6-threonylcarbamoyl adenosine (ms2t6A) in other tRNAs. Finally, the RimO family catalyzes formation of a methylthiolated aspartate residue, ms-D89, in the ribosomal protein S12. These enzymes define a methylthiotransferase (MTTase) family, which is a subclass of the large Radical-SAM enzyme superfamily [8]. Its members thus catalyze the chemically challenging C-H to C-SCH3 bond conversion, through a radical mechanism that remains poorly understood. Furthermore, these reactions are part of an active new field of research related to epigenetics, because they all involve post-transcriptional and post-translational and modifications that increase the expand the chemical repertoire of proteins beyond the canonical amino-acids and nucleobases [12].
The purpose of this review is to discuss the mechanistic and structural information gained from recent investigation of these fascinating radical-based enzymes and to point out new avenues of investigation which may help to understand some unresolved questions such as: (i) how primary free radicals can be generated in one protein and directed to specific sites in a macromolecular target, (ii) how intermediate free radicals are controlled to generate the desired product, and (iii) how all these radicals avoid deleterious redox-quenching reactions releasing reactive oxygen species.
A- Phylogenetic and sequence Analysis of the MTTase Family
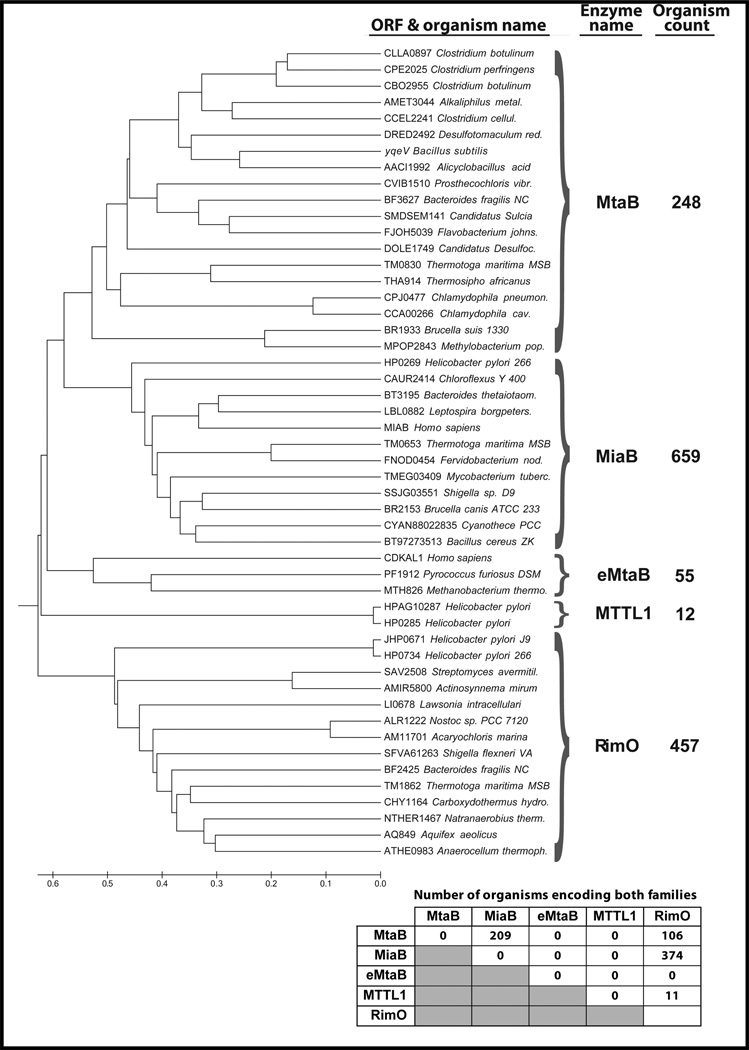
A previous bioinformatics analysis showed that there are five families of homologous MTTases encoded in vertebrate and bacterial cells [13]. The conclusions from this analysis have been confirmed in a recent re-analysis of a larger database containing over 971 fully sequenced eubacterial and archaebacterial genomes (Figure 1). The substrate and products for four of these families has been identified.
Figure 1. Phylogenetic analysis of MTTase families.
Cladogram showing representatives from all MTTase famlies identified via comprehensive sequence analysis of 971 eubacterial and archaeabacterial genomes. Divisions between families were established based on simultaneous encoding of members of two families in a single genome. The total count of such occurrences is indicated in the table at the bottom left. MTTases were identified via an all-versus-all BLAST analysis of the full set of genomes, which yielded 813 sequences containing all three characteristic domains (UPF0004, Radical-SAM, and TRAM). Sequences occurring simultaneously in a single genome were used for an initial clustering, which was confirmed by back-tracing of the branches of the phylogenetic tree to ensure that splits between families are supported by such simultaneous occurrences. No more than one member of each family is encoded in a single genome (as indicated by the numbers on the diagonal in the table).
The first family or clade contains E. coli MiaB, which stands for methythioisopentenyadenosine synthesis enzyme B, and its homologs are found in eukaryotic and eubacterial but not archeaebacaterial organisms. The second family contains B. subtilis MtaB, which stands for methythiothreonylcarbamoyladenosine enzyme B, and its homologs are found exclusively in eubacterial organisms. This family shows slightly more sequence diversity than the others, as evidenced by having a branch-point located closer to the root of the phylogenetic tree in Fig. 1. This diversity might reflect a greater degree of functional diversification, e.g., in substrate specificity or regulatory interactions. The third characterized sequence family is called e-MtaB. This family has been demonstrated to catalyze the same reaction as MtaB despite its sequence divergence (Figure 1). It is found in higher eukaryotes and archaebacteria and has the murine CDKAL-1 gene as a prototype [13]. These first three MTTase enzyme families all act on tRNA substrates. The fourth characterized family contains the T. maritima and E. coli enzyme RimO, which modifies aspartate residue 89 (*D89) in ribosomal protein S12 [14, 15]. This MTTase family and the ribosomal protein modification that it catalyzes are found exclusively in eubacteria. The fifth and final MTTase family has been named for the time being the methylthiotransferase-like 1 (MTTL1) family. This family is restricted to four species of ε–proteobacteria, including all species of Helicobacter pylori included in the analysis. This family has yet to be characterized enzymologically. The genomes in which it is found do not encode any MiaB or MtaB homologs, although most encode a RimO homolog. However, it is approximately as far diverged from the MtaB and MiaB families as they are from RimO, and it is more diverged from them than they are to each other. Therefore, phylogenetic analysis does not provide a preferred hypothesis concerning the chemical nature of its substrate.
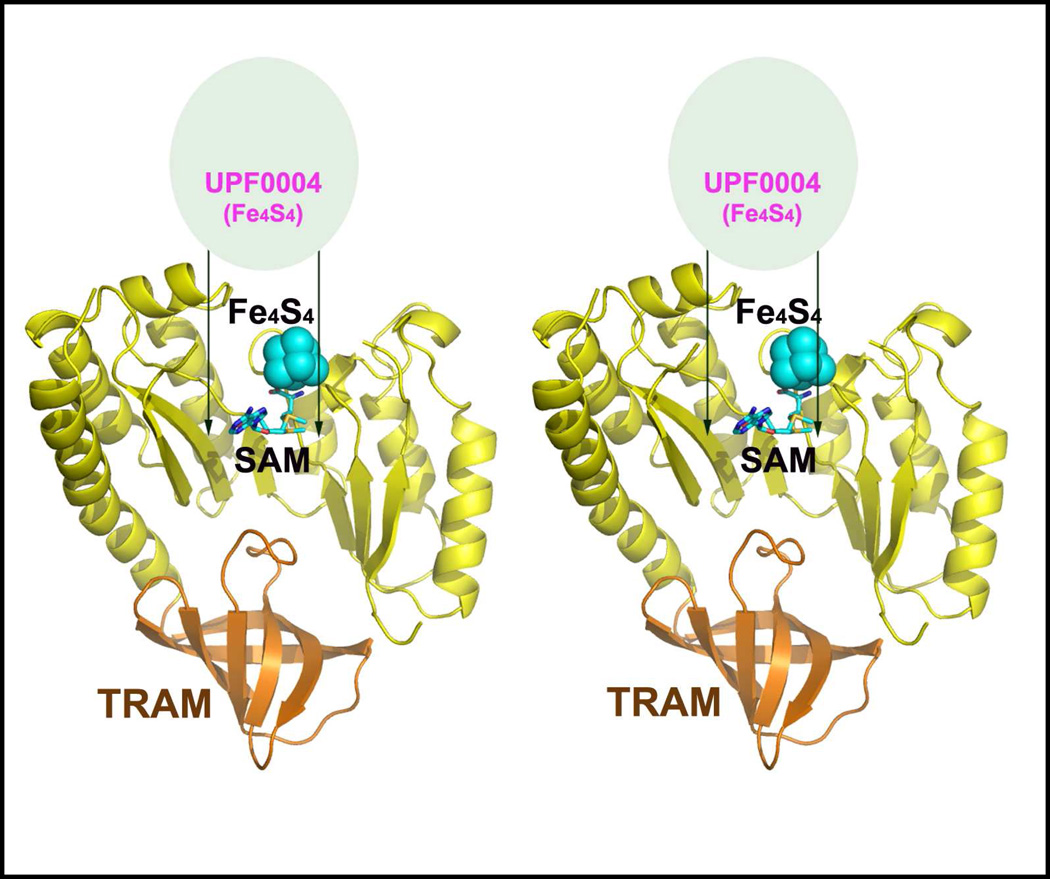
Sequence analysis of MiaB, the first MTTase to be experimentally characterized, suggested a modular structure [16]. This inference has been proven by the recent structure determination of apo-RimO from Thermotoga maritima (Figure 2) [15]. All MTTases comprise an N-terminal UPF0004 (uncharacterized protein family 0004) domain which is ~ 135 residues in length, a central Radical-SAM domain which is ~ 235 residues in length and contains the Radical-SAM cysteine triad, and a C-terminal TRAM (TRM2 and MiaB) domain which is ~ 60 residues in length (Scheme 2). The UPF0004 domain has not been structurally characterized, but it contains invariant cysteines in a CysX34–36CysX28–37Cys motif, which is likely to ligate the second [4Fe-4S] recently demonstrated to be present in Radical-SAM MTTases [15–17].
Figure 2. Structural organization of MTTases.
The stereopair shows the inferred position of the UPF0004 domain (light green ellipse) relative to a ribbon diagram of the Radical-SAM (yellow) and TRAM (orange) domains from the crystal structure of a truncated apo construct of Thermotoga maritima RimO [15]. The approximate locations where the [4Fe-4S] cluster (cyan spheres) and SAM (stick representation) bind to the Radical-SAM domain were inferred by alignment of the homologous regions of the Radical-SAM domain from the crystal structure of holo MoaA [20]. The second [4Fe-4S] cluster present in enzymatically active RimO is likely to be bound by the invariant cys residues in the UPF004 domain. (See text.) Based on the location of the N-terminus of the Radical-SAM domain, which follows the UPF0004 domain in the primary sequence, the [4Fe-4S] cluster bound to the UPF0004 domain is likely to proximal to the Radical-SAM active site. Carbon is colored cyan, nitrogen blue, oxygen red, and sulfur yellow in the SAM molecule.
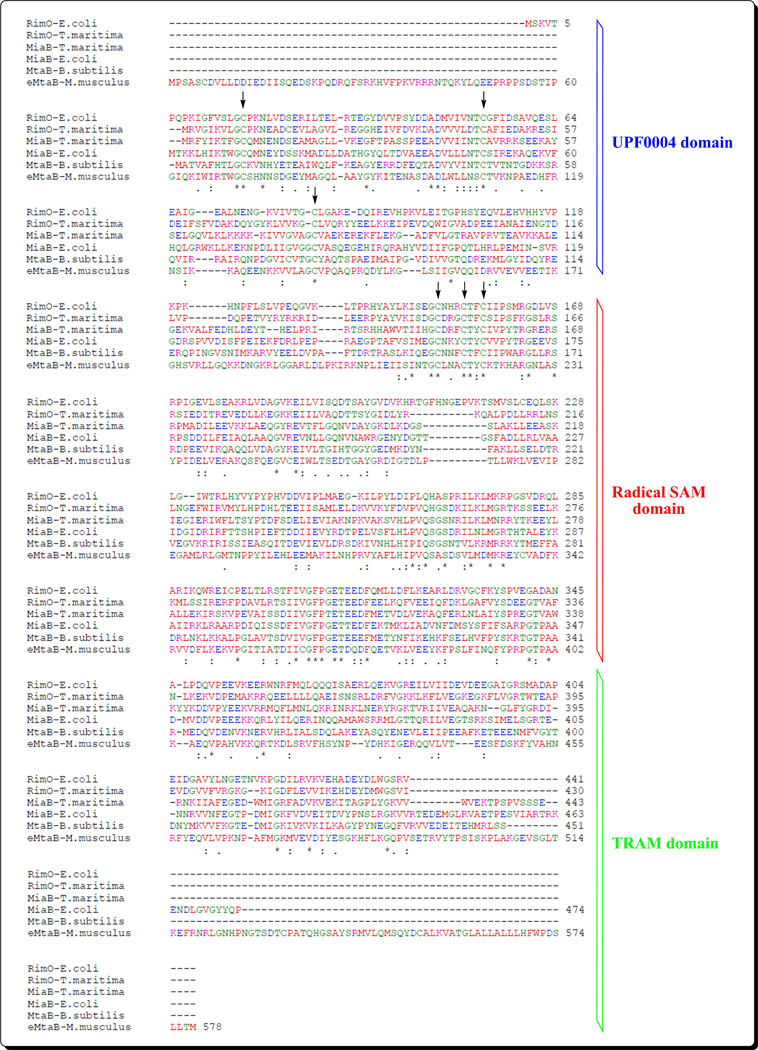
Scheme 2. Multiple sequence alignment of six MTTases.
Two from RimO_(E. coli K-12 and T. maritima DSMZ3109), two from MiaB (E. coli K- 12 and T. maritima DSMZ3109), one from MtaB (B. subtilis MGNA-001), and one from e-MtaB (M. musculus). The alignment was performed with ClustalW at the EBI site. Totally conserved residues are indicated by stars, and conserved cysteine residues are indicated by arrows. The three domains UPF0004, Radical-SAM, and TRAM are shown on the right.
B- Structural organization of MTTase enzymes
To date, structures have been determined for the Radical-SAM domains from nine different enzyme families [18]. All of them contain an α/β core with topological similarity to the well-known TIM barrel fold, in which eight successive α/β supersecondary structural units form a topologically closed β-barrel. The SCOP database (http://scop.mrc-lmb.cam.ac.uk/scop) classifies Radical-SAM domains as either complete or incomplete TIM barrel folds depending on whether the barrel is topologically closed or open. In a complete or closed 8-stranded β-barrel, as observed in the BioB enzyme, the first β-strand hydrogen-bonds to the last to form a topologically complete barrel. In an incomplete or open β-barrel, as observed in the MoaA and RimO (Figure 2) enzymes, the barrel is opened up and flattened out so that the first and last β-strands no longer contact one another. The enzymes with open β-barrel structures often have fewer than eight α/β supersecondary structural units. They also tend to have a larger number of additional secondary structural elements, some of which typically fold back to complete the active site adjacent to the catalytic [4Fe-4S] cluster. The conserved CysXXXCysXXCys motif that ligates the [4Fe-4S] cluster is located in the loop between the β-strand and α-helix in the first α/β supersecondary structural unit in the primary sequence of the domain.
Although these general architectural features are shared among all Radical-SAM enzymes, there is remarkably little conservation in active-site stereochemistry between enzymes with different activities. For example, only a single aromatic amino-acid residue (phe or tyr) is conserved in the active sites of the MoaA, HemN, and RimO enzymes, even though all have a similar incomplete barrel structure. In the SAM-bound crystal structures of MoaA and HemN, the sidechain of this residue makes van der Waals interactions with adenine base of SAM in a perpendicular interaction geometry. The lack of additional conserved residues in the active site suggests that the catalytic properties of Radial-SAM enzymes are determined primarily by the intrinsic chemical properties of the SAM complex with the [4Fe-4S] cluster. In the available crystal structures, the carboxylate and amino groups of the methionine moiety are ligated in a consistent geometry by one of the Fe atoms in the cluster [19–21]. In some structures, the sulphur of this moiety also makes van der Waals contacts to the cluster. The general lack of sequence conservation in the surrounding amino acids between enzymes with different activities suggests that their evolution is controlled by constraints related to substrate recognition rather than catalytic efficiency.
In the MTTase family of Radical-SAM enzymes, the other two conserved domains are located in close proximity to the catalytic [4Fe-4S] cluster in the Radical-SAM domain (Figure 2). The crystal structure of apo RimO shows the exact location of the TRAM domain, which interacts with the loops on the opposite side of the open β-barrel from the loops ligating the [4Fe-4S] cluster. Given the established role of some TRAM domains in binding RNA, this domain is likely to help mediate binding of macromolecular tRNA substrates to MiaB and e-MtaB/MtaB. The TRAM domain in RimO is generally acidic, consistent with being adapted to help mediate binding of its basic macromolecular substrate, ribosomal protein S12.
A structure is not yet available for the UPF0004 domain, either alone or as part of an intact MTTase enzyme. However, the location of the N-terminus of the Radical-SAM domain in apo RimO suggests that the UPF0004 domain is also likely to interact with the catalytic [4Fe-4S] cluster in the Radical-SAM domain (Figure 2). Therefore, conserved [4Fe-4S] cluster bound to this domain could potentially participate directly in catalyzing the methylthiolation reaction performed by the MTTase enzymes in the Radical-SAM superfamily.
C- Mechanisms of the methylthiolation reactions
- tRNA as a substrate
One of the best characterized biosynthetic pathways for tRNA hypermodification is the one leading to ms2i6A37 (Figure 3-A). In E. coli, this hypermodified adenine is found next to the anticodon motif at position 37 of tRNAs reading triplets starting with uridine, except tRNAser(GGA) [22]. In some bacteria, such as Salmonella typhimirium, ms2i6A37 is further modified into ms2io6A37 (also called 2-methytlhio-cis-ribozeatin), catalyzed by the MiaE enzyme [23].
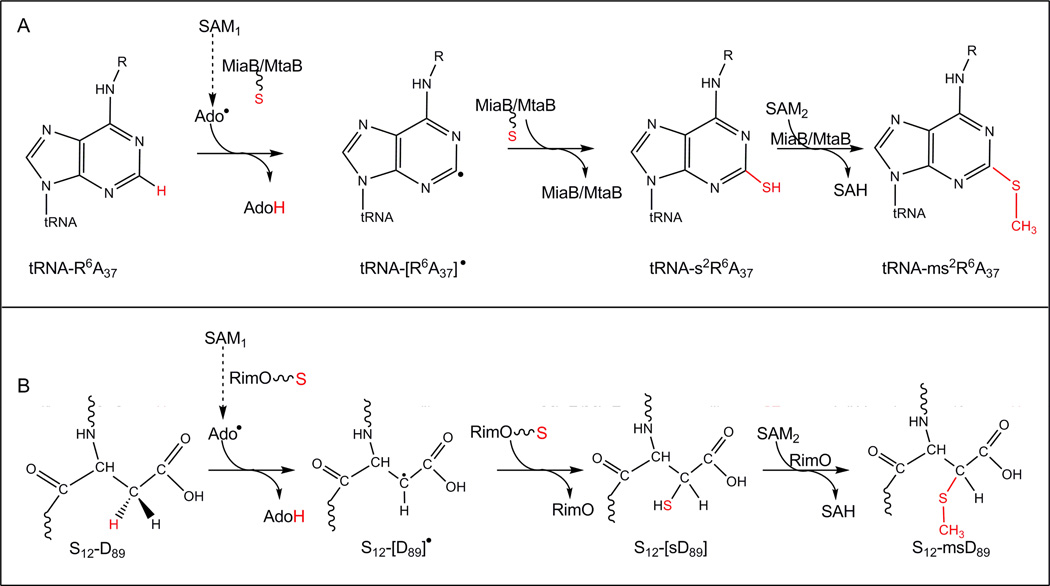
Figure 3. Radical-based mechanisms in post-transcriptional and posttranslational modifications.
(A) methylthiolation of aromatic carbons C2, R = -CH2-CH=C(CH3)2 for ms2i6A biosynthesis or -CO-NH-CH(CH(CH3)OH-COOH for ms2t6A production.
(B) 3-methythio aspartate formation.
Ado˙; 5’-deoxyadenosyl radical, AdoH; 5’-deoxyadenosyl, SAM; S-adenosylmethionine, SAH; S-adenosylhomocystein.
The first committed step in the biosynthesis of ms2i(o)6A37 is catalyzed by MiaA and involves the transfer of a dimethylallyl group from dimethylallyl diphosphate (DMAPP) to the exocyclic N6 of adenosine 37 [24]. The MiaA enzyme does not contain any cofactor but requires the presence of Mg2+ cation [25]. The second step in the biosynthesis pathway, which is catalyzed by MiaB, involves the methylthiolation of i6A37 at carbon 2 [16, 26]. Both in vivo and in vitro experiments indicate that the methylthiolation reaction depends on the presence of the isopentenyl group at position 6 (Figure 3-A) [26, 27]. Given the strong similarity in the tRNA modification mechanism used by all MTTases, it seems likely that the presence of the threonylcarbamoyl group at position 6 similarly will be an essential prerequisite for the methythiolation reaction catalyzed by MtaB/e-MtaB (Figure 3-A).
The fundamental chemical step common to all radical SAM enzymes is the reductive cleavage of SAM to generate the Ado• radical, which then abstracts a hydrogen atom from substrate to enable its transformation into product (scheme 1B). In the case of MTTases, there are two additional steps needed to complete the reaction. The first of these is the insertion of a sulfur atom into the activated substrate, in order to generate a putative thiolated intermediate “s2x6A” (x = i or t), while the second is the methylation of the “s2x6A” intermediate by a second molecule of SAM. The methylation reaction is proposed to occur through a standard SN2 reaction mechanism, as in SAM-dependent methyltransferases.
The “s2i6A” compound was first suggested to accumulate as an intermediate in the way to ms2i6A during experiments conducted under conditions of methionine starvation which was known to produce methyl-deficient tRNA [28]. Incubation of this intermediate with E. coli crude extracts and [methyl-14C]-SAM under aerobic conditions resulted in incorporation of the radiolabelled derivative and the conversion of the uncharacterized intermediate into ms2i6A [28]. This reaction sequence was also supported by in vitro experiments demonstrating that MiaB is a bifunctional enzyme involved in both thiolation and methylation of i6A [26].
The sulfurating mechanism of MiaB is believed to be common with other Radical SAM enzymes catalyzing sulfuration reactions such as biotin synthase (BioB) and lipoic acid synthase (LipA) [29, 30]. This inference is supported by the observation that they all contain two Fe-S clusters. One [4Fe-4S] cluster is involved in SAM reduction and cleavage, whereas the other, a [4Fe-4S] in all cases except in BioB which contains a [2Fe-2S] cluster, is proposed to provide the sulfur for the sulfuration step [30]. This step is a critical feature of the mechanism that has been debated extensively in the literature but still remains to be confirmed. Experimental investigation of this step is complicated by the fact that no more than a single catalytic cycle can be achieved in vitro even in the presence of an excess of exogenous sulphide (or a mixture of cysteine and cysteine desulfurase). Inactivation of the enzyme during the first cycle of turnover is consitent with the N-terminal [4Fe-4S] cluster of MiaB found in the UPF0004 domain functioning as a sacrificial S-donor during single turnover experiments [16, 30]. A similar role has been suggested for the [2Fe-2S] cluster in BioB and the second [4Fe-4S] cluster in LipA [30]. The direct sulphur donor could be a derivative of that cluster, as suggested in the case of BioB, for which the decay of the [2Fe-2S] cluster in the C-terminal domain during catalysis was found to be much faster than biotin formation [31]. In this case, the nature of this derivative and of the chemistry converting the precursor Fe-S cluster into this sulfurating species would be to be elucidated. Whether the sulfur transfer is direct or indirect, because the MTTases probably function catalytically in vivo, it is absolutely essential to find the mechanism of regeneration of an active sulfurating species after each cycle as well as the nature of the pool of sulphur atoms used for this regeneration. Elucidating thses features of the enzyme mechanism represents one of the major issues to be addressed in the future research on MTTases.
There are other mechanistic issues to consider regarding methylthiolation reactions. First, the methylation reaction could occur on the second cluster prior to insertion of a pre-formed methylthio group into the primary substrate. This alternative to the mechanistic hypothesis to the one described above also deserves consideration. Second, activation of the substrate theoretically implies abstraction of a H atom from C2 of the aromatic base generating, an energetically unfavorable σ-radical. To circumvent this problem Booker et. al. have shown in the case of the enzymes RlmN and Cfr, which catalyze a methylation at C2 and C8 of a nucleic base of rRNA, that the reaction proceeds via addition of an intermediate radical at C2, a thermodynamically more favourable process [32, 33]. In the case of methylthiolation reactions discussed here, this possibility clearly merits consideration. On the other hand, whereas the C-H bond dissociation energies (BDEs) in methane (+439 kJ/mol) and in AdoH (+433 kJ/mol) are much lower than the C-H BDE in benzene (+472 kJ/mol), that of the C2 position in pyridine is +439 kJ/mol [34, 35]. This lower BDE reflects the strongly stabilizing nature of the adjacent lone pair on the unpaired spin. Therefore, the adenosine-C2 radical, with two adjacent nitrogen atoms, might be significantly more stable than anticipated, which makes a standard H atom abstraction at C2 by Ado° as a reasonable possibility. Finally, anot her interesting question to investigate is the mechanism of recognition of the same cofactor, SAM, by one polypeptide at what is likely to be two different sites serving two completely different enzymatic purposes. This situation appears to be unique in biochemistry and represents a fascinating illustration of how the MTTase enzymes controls their reactivity towards the same cofactor
- Ribosomal protein S12 as a substrate
The initially reported amino-acid sequence of protein S12 from E. coli, determined from peptide mapping and subsequent sequencing by Edman degradation, failed to define the identity of the amino acid at position 89 [36]. Later, the DNA sequence of the gene that encodes protein S12 revealed that position 89 is an aspartate residue [37]. In 1996 Kowalak et al. showed that protein S12, analyzed by MALDI-TOF MS technique, displayed a molecular mass of 13,652.0 Da, 46.1 Da larger than the predicted masse from its gene sequence (13,605.9 Da) [38]. The authors proposed that the modification associated with D89 was consistent with a thioether structure (−SCH3) probably at the β-carbon of the aspartyl residue [38]. Ten years latter, Anton et al. identified the yliG gene in E. coli as encoding the enzyme, designated RimO, responsible for the methylthiolation of the S12 protein [14]. Shortly afterwards, RimO proteins from E. coli and Thermotoga maritima were purified by two different groups, and in both studies shown to bind two [4Fe-4S] clusters per polypeptide and to catalyze the methylthiolation of a peptide substrate containing an Asp residue in the appropriate sequence context [15, 17]. RimO has not been characterized as extensively as MiaB. However, the putative mechanism of the reaction also invovles H atom abstraction at the β carbon on the aspartate residue by the 5’-deoxyadenosyl radical, followed by sulfuration of the substrate radical to generate an intermediate thiol compound, and finally by nucleophilic methylation using SAM as demonstrated by isotopic labelling experiments [17]. Thus RimO, like MiaB, catalyzes two successive chemical reactions, both SAM-dependent (Figure 3-B). First, it catalyzes a radical C-H to C-S bond conversion that is likely to share a common sulfurating mechanism with MiaB, BioB and LipA. This mechanism presumably uses the second [4Fe-4S] cluster in the enzyme as the source for sulphur delivery. Second, it functions as a SAM-dependent methyltransferase, although there is not an established SAM-binding site responsible for this activity. Obviously, the same question raised in the case of MiaB is also relevant for RimO, i.e., whether methylation of sulfur occurs before or after sulfation of the primary substrate. Since the thiolation and methylation activities have not yet been successfully decoupled for any MTTase via mutagenesis, both pathways remain possible.
D- Conclusions and future prospects
Many Radical-SAM enzymes have been identified and characterized to varying extents by different groups during the last decade. Nonetheless, important gaps remain in our understanding of these enzymes. In particular, the Radical-SAM enzymes that catalyze C-S bond formation continue to fascinate chemists and require further investigation. For these enzymes, it is not yet known how they insert the sulfur atom into their substrates, essentially because none of these proteins (BioB, LipA, MiaB, and RimO) operate catalytically in vitro (i.e., they are all limited to single cycle of turnover). However, it has been established that beside the [4Fe-4S] Radical-SAM cluster responsible for substrate activation, all of these proteins contain a second Fe-S center. In this division of the Radical-SAM family, there is as yet no exception to the requirement for two iron-sulfur clusters, although variations have been observed regarding the type of clusters bound to the proteins. This observation combined with the inability to observe more than a single cycle of turnover in vitro has led to the general dogma that the bridging sulfur atoms of the second cluster are the source of sulfur atoms during the thiolation reaction in all these enzymes.
On the other hand, it should be noted that Radical-SAM enzymes not involved in sulfur insertion often contain two clusters that absolutely required for the activity, including the enzymes TYW1 that participates in the biosynthesis of wybutosine [39, 40], MoaA that particpiates in the formation of molybdopterin cofactors [20], and BtrN that catalyzes the third step in the biosynthesis of the 2-deoxystreptamine-containing aminoglycoside antibiotic butirosin B [41]. It has been shown that the second cluster in MoaA binds to and activates the GTP substrate; a similar role in substrate activation was proposed for the BtrN enzyme. On the basis of these recent developments, our laboratory currently is testing a similar hypothesis for the MiaB enzyme, namely that the subsite irons in the [4Fe-4S]2+/1+ cluster in the UPF0004 domain may participate in the activation of the co-substrate (“S”, sulfur-containing species) before being incorporated into their substrates. If this hypothesis is validated, it has far-reaching implications because it should open new approaches in the quest for an in vitro catalytic system capable of multiple turnovers. The availability of such an efficient in vitro catalytic system for the biosynthesis of essential sulfur-containing cofactors like biotin would have a major impact on mechanistic studies of Radical-SAM enzymes as well as related areas of economic importance including the nutrition, food-processing and biotechnology industries.
Highlights.
-
>
Activation of the C-H bonds is intricately linked to the presence of a metal ion.
-
>
SAM-dependent radical-based modification of biological macromolecules.
-
>
The methylthiolation reaction mediated by the Radical-SAM enzymes.
-
>
Phylogenetic and sequence Analysis of the MTTase Family.
-
>
Structural organization of MTTase enzymes.
Acknowledgements
We gratefully acknowledge financial support for this project by Région-Rhône-Alpes grant (CIBLE-2008 SA fellowship), ANR-2010 grant (blan-737-01) and NIH Protein Structure Initiative grants U54-GM074958 and U54-GM094597 to the Northeast Structural Genomics Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eisenstein O, Balcells D, Clot E. C-H Bond Activation in Transition Metal Species from a Computational Perspective. Chem. Rev. 2010;110:749–823. doi: 10.1021/cr900315k. [DOI] [PubMed] [Google Scholar]
- 2.Kovaleva EG, Lipscomb JD. Versatility of biological non-heme Fe(II) centers in oxygen activation reactions. Nature Chemical Biology. 2008;4:186–193. doi: 10.1038/nchembio.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krebs C, Fujimori DG, Walsh CT, Bollinger JM. Non-heme Fe(IV)-oxo intermediates. Acc. Chem. Res. 2007;40:484–492. doi: 10.1021/ar700066p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Que L, Costas M, Mehn MP, Jensen MP. Dioxygen activation at mononuclear nonheme iron active sites: Enzymes, models, and intermediates. Chem. Rev. 2004;104:939–986. doi: 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- 5.Lippard SJ. Hydroxylation of C-H bonds at carboxylate-bridged diiron centres. Philosophical Transactions of the Royal Society a-Mathematical Physical and Engineering Sciences. 2005;363:861–877. doi: 10.1098/rsta.2004.1532. [DOI] [PubMed] [Google Scholar]
- 6.Fontecave M, Mulliez E, Ollagnier-de-Choudens S. Adenosylmethionine as a source of 5 '-deoxyadenosyl radicals. Curr. Opin. Chem. Biol. 2001;5:506–511. doi: 10.1016/s1367-5931(00)00237-4. [DOI] [PubMed] [Google Scholar]
- 7.Frey PA, Hegeman AD, Ruzicka FJ. The radical SAM superfamily. Crit. Rev. Biochem. Mol. Biol. 2008;43:63–88. doi: 10.1080/10409230701829169. [DOI] [PubMed] [Google Scholar]
- 8.Sofia HJ, Chen G, Hetzler BG, Reyes-Spindola JF, Miller NE. Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 2001;29:1097–1106. doi: 10.1093/nar/29.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downs DM, Martinez-Gomez NC. ThiC is an [Fe-S] cluster protein that requires AdoMet to generate the 4-amino-5-hydroxymethyl-2-methylpyrimidine moiety in thiamin synthesis. Biochemistry. 2008;47:9054–9056. doi: 10.1021/bi8010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters JW, McGlynn SE, Boyd ES, Shepard EM, Lange RK, Gerlach R, Broderick JB. Identification and Characterization of a Novel Member of the Radical AdoMet Enzyme Superfamily and Implications for the Biosynthesis of the Hmd Hydrogenase Active Site Cofactor. J. Bacteriol. 2010;192:595–598. doi: 10.1128/JB.01125-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marquet A, Bui BTS, Smith AG, Warren MJ. Iron-sulfur proteins as initiators of radical chemistry. Nat. Prod. Rep. 2007;24:1027–1040. doi: 10.1039/b703109m. [DOI] [PubMed] [Google Scholar]
- 12.Atta M, Mulliez E, Arragain S, Forouhar F, Hunt JF, Fontecave M. S-Adenosylmethionine-dependent radical-based modification of biological macromolecules. Curr. Opin. Struct. Biol. 2010;20:684–692. doi: 10.1016/j.sbi.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Arragain S, Handelman SK, Forouhar F, Wei FY, Tomizawa K, Hunt JF, Douki T, Fontecave M, Mulliez E, Atta M. Identification of Eukaryotic and Prokaryotic Methylthiotransferase for Biosynthesis of 2-Methylthio-N-6-threonylcarbamoyladenosine in tRNA. J. Biol. Chem. 2010;285:28425–28433. doi: 10.1074/jbc.M110.106831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anton BP, Saleh L, Benner JS, Raleigh EA, Kasif S, Roberts RJ. RimO, a MiaB-like enzyme, methylthiolates the universally conserved Asp88 residue of ribosomal protein S12 in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1826–1831. doi: 10.1073/pnas.0708608105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arragain S, Garcia-Serres R, Blondin G, Douki T, Clemancey M, Latour JM, Forouhar F, Neely H, Montelione GT, Hunt JF, Mulliez E, Fontecave M, Atta M. Post-translational Modification of Ribosomal Proteins structural and functional characterization of RimO from thermotoga maritima, a radical s-adenosylmethionine methylthiotransferase. J. Biol. Chem. 2010;285:5792–5801. doi: 10.1074/jbc.M109.065516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez HL, Pierrel F, Elleingand E, Garcia-Serres R, Huynh BH, Johnson MK, Fontecave M, Atta M. MiaB, a bifunctional radical-S-adenosylmethionine enzyme involved in the thiolation and methylation of tRNA, contains two essential [4Fe-4S] clusters. Biochemistry. 2007;46:5140–5147. doi: 10.1021/bi7000449. [DOI] [PubMed] [Google Scholar]
- 17.Booker SJ, Lee KH, Saleh L, Anton BP, Madinger CL, Benner JS, Iwig DF, Roberts RJ, Krebs C. Characterization of RimO, a New Member of the Methylthiotransferase Subclass of the Radical SAM Superfamily. Biochemistry. 2009;48:10162–10174. doi: 10.1021/bi900939w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drennan CL, Vey JL. Structural Insights into Radical Generation by the Radical SAM Superfamily. Chem. Rev. 2011;111:2487–2506. doi: 10.1021/cr9002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkovitch F, Nicolet Y, Wan JT, Jarrett JT, Drennan CL. Crystal structure of biotin synthase, an S-adenosylmethionine-dependent radical enzyme. Science. 2004;303:76–79. doi: 10.1126/science.1088493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanzelmann P, Schindelin H. Binding of 5 '-GTP to the C-terminal FeS cluster of the radical S-adenosylmethionine enzyme MoaA provides insights into its mechanism. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6829–6834. doi: 10.1073/pnas.0510711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Layer G, Moser J, Heinz DW, Jahn D, Schubert WD. Crystal structure of coproporphyrinogen III oxidase reveals cofactor geometry of Radical SAM enzymes. EMBO J. 2003;22:6214–6224. doi: 10.1093/emboj/cdg598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grosjean H, Nicoghosian K, Haumont E, Soll D, Cedergren R. Nucleotide-Sequences of 2 Serine Transfer-RNAs with a Gga Anticodon - the Structure-Function-Relationships in the Serine Family of Escherichia-Coli Transfer-Rnas. Nucleic Acids Res. 1985;13:5697–5706. doi: 10.1093/nar/13.15.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathevon C, Pierrel F, Oddou JL, Garcia-Serres R, Blondin G, Latour JM, Menage S, Gambarelli S, Fontecave M, Atta M. tRNA-modifying MiaE protein from Salmonella typhimurium is a nonheme diiron monooxygenase. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13295–13300. doi: 10.1073/pnas.0704338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore JA, Poulter CD. Escherichia coli dimethylallyl diphosphate:tRNA dimethylallyltransferase: A binding mechanism for recombinant enzyme. Biochemistry. 1997;36:604–614. doi: 10.1021/bi962225l. [DOI] [PubMed] [Google Scholar]
- 25.Chimnaronk S, Forouhar F, Sakai J, Yao M, Tron CM, Atta M, Fontecave M, Hunt JF, Tanaka I. Snapshots of Dynamics in Synthesizing N-6-Isopentenyladeno sine at the tRNA Anticodon. Biochemistry. 2009;48:5057–5065. doi: 10.1021/bi900337d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pierrel F, Douki T, Fontecave M, Atta M. MiaB protein is a bifunctional radical-S-adenosylmethionine enzyme involved in thiolation and methylation of tRNA. J. Biol. Chem. 2004;279:47555–47563. doi: 10.1074/jbc.M408562200. [DOI] [PubMed] [Google Scholar]
- 27.Vold BS, Lazar JM, Gray AM. Characterization of a Deficiency of N6-(Delta-2-Isopentenyl)-2-Methylthioadenosine in the Escherichia-Coli Mutant Trpx by Use of Antibodies to N6-(Delta-2-Isopentenyl)Adenosine. J. Biol. Chem. 1979;254:7362–7367. [PubMed] [Google Scholar]
- 28.Agris PF, Armstrong DJ, Schafer KP, Soll D. Maturation of a Hypermodified Nucleoside in Transfer-RNA. Nucleic Acids Res. 1975;2:691–698. doi: 10.1093/nar/2.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fontecave M, Ollagnier-de-Choudens S, Mulliez E. Biological radical sulfur insertion reactions. Chem. Rev. 2003;103:2149–2166. doi: 10.1021/cr020427j. [DOI] [PubMed] [Google Scholar]
- 30.Booker SJ, Cicchillo RM, Grove TL. Self-sacrifice in radical S-adenosylmethionine proteins. Curr. Opin. Chem. Biol. 2007;11:543–552. doi: 10.1016/j.cbpa.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jameson GNL, Cosper MM, Hernandez HL, Johnson MK, Huynh BH. Role of the [2Fe-2S] cluster in recombinant Escherichia coli biotin synthase. Biochemistry. 2004;43:2022–2031. doi: 10.1021/bi035666v. [DOI] [PubMed] [Google Scholar]
- 32.Booker SJ, Grove TL, Benner JS, Radle MI, Ahlum JH, Landgraf BJ, Krebs C. A Radically Different Mechanism for S-Adenosylmethionine-Dependent Methyltransferases. Science. 2011;332:604–607. doi: 10.1126/science.1200877. [DOI] [PubMed] [Google Scholar]
- 33.Booker SJ, Boal AK, Grove TL, McLaughlin MI, Yennawar NH, Rosenzweig AC. Structural Basis for Methyl Transfer by a Radical SAM Enzyme. Science. 2011;332:1089–1092. doi: 10.1126/science.1205358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo Y-R. Comprehensive Handbook of Chemical Bond Energies. CRC Press; 2007. [Google Scholar]
- 35.Zipse H, Hioe J. Radical stability and its role in synthesis and catalysis. Organic & Biomolecular Chemistry. 2010;8:3609–3617. doi: 10.1039/c004166a. [DOI] [PubMed] [Google Scholar]
- 36.Funatsu G, Yaguchi M, Wittmannliebold B. Primary Structure of Protein-S12 from Small Escherichia-Coli Ribosomal-Subunit. FEBS Lett. 1977;73:12–17. doi: 10.1016/0014-5793(77)80004-5. [DOI] [PubMed] [Google Scholar]
- 37.Post LE, Nomura M. DNA-Sequences from the Str Operon of Escherichia-Coli. J. Biol. Chem. 1980;255:4660–4666. [PubMed] [Google Scholar]
- 38.Kowalak JA, Walsh KA. beta-methylthio-aspartic acid: Identification of a novel posttranslational modification in ribosomal protein S12 from Escherichia coli. Protein Sci. 1996;5:1625–1632. doi: 10.1002/pro.5560050816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki Y, Noma A, Suzuki T, Senda M, Senda T, Ishitani R, Nureki O. Crystal structure of the radical SAM enzyme catalyzing tricyclic modified base formation in tRNA. J. Mol. Biol. 2007;372:1204–1214. doi: 10.1016/j.jmb.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Goto-Ito S, Ishii R, Ito T, Shibata R, Fusatomi E, Sekine S, Bessho Y, Yokoyama S. Structure of an archaeal TYW1, the enzyme catalyzing the second step of wye-base biosynthesis. Acta Crystallographica Section D-Biological Crystallography. 2007;63:1059–1068. doi: 10.1107/S0907444907040668. [DOI] [PubMed] [Google Scholar]
- 41.Krebs C, Grove TL, Ahlum JH, Sharma P, Booker SJ. A Consensus Mechanism for Radical SAM-Dependent Dehydrogenation? BtrN Contains Two [4Fe-4S] Clusters. Biochemistry. 2010;49:3783–3785. doi: 10.1021/bi9022126. [DOI] [PMC free article] [PubMed] [Google Scholar]