Abstract
Background:
Validated multigene signatures (MGS) provide additional prognostic information when evaluating clinical features of ER+, HER2− early breast cancer. We have studied the quantitative and qualitative impact of MGS on multidisciplinary team (MDT) recommendations.
Methods:
We prospectively recruited 75 ER+, HER2− breast cancer patients. Inclusion was based on biopsy assessment of grade, hormone receptor status, HER2, clinical tumour and nodal status. A fresh tissue sample was sent for MammaPrint (MP), TargetPrint analysis at surgery. Clinical risk was decided by the MDT in the absence of MP results and repeated following the collection of MP results. Decision changes were recorded and a health technology assessment was undertaken to compare cost effectiveness.
Results:
The majority of patients were assigned low to intermediate clinical risk by the MDT. According to MP, 76% were low risk. A very high correlation between local IHC and the TargetPrint assessment was shown. In over a third of patients, discordance between clinical and molecular risk was observed. Decision changes were recorded in half of these cases (18.6%) and resulted in two out of three patients not requiring chemotherapy. The use of MP was also found to be more cost effective.
Conclusions:
The multigene signature MP revealed clinical and molecular risk discordance in a third of patients. The impact of this on MDT recommendations was most profound in cases where few clinical risk factors were observed and enabled some women to forgo chemotherapy. The use of MGS is unlikely to have an impact in either clinically low-risk women or in patients with more than one relative indication for chemotherapy.
Keywords: breast cancer, gene expression profiling, tumour board recommendation, adjuvant systemic treatment
Since 2009, the St Gallen consensus panel has suggested that validated multigene signatures may be helpful in deciding whether, in addition to endocrine therapy, adjuvant chemotherapy (CHT) is indicated for women with ER+/Her2− early breast cancer. The implementation should occur in cases where its use was uncertain after consideration of conventional markers (Goldhirsch et al, 2009).
The 70-gene tumour expression profile MammaPrint (MP) was initially established as a predictor of disease outcome in premenopausal breast cancer (Van't Veer et al, 2002) and was translated into a customised diagnostic breast cancer mini-array, MP, with reliable use in a diagnostic setting (Glas et al, 2006).
A recent independent evaluation of several genomic tests found the available evidence on the analytical and clinical validity of MP to be convincing (Azim et al, 2013). It was shown that in postmenopausal patients at low risk of breast cancer-related death, MP can be of clinical use to accurately select women for adjuvant CHT (Mook et al, 2010).
A community-based observational study confirms the potential of this 70-gene signature to more accurately select breast cancer patients who can forgo adjuvant CHT without compromising outcome. In this study, MP reduced the proportion of high-risk patients as classified by Adjuvant Online by 20% (Drukker et al, 2013).
In Austria, endocrine treatment (ET) in the absence of CHT (Jakesz et al, 2005) even in nodal-positive patients is quite frequent. Several studies of the Austrian breast and colorectal study group showed excellent survival data in both premenopausal (Jakesz et al, 2002; Gnant et al, 2009, 2011) and postmenopausal women (Dubsky et al, 2012) in the absence of adjuvant CHT.
Considering this specific treatment environment that a priori leans heavily on endocrine treatment, we asked how a gene expression analysis like MP would change adjuvant CHT treatment decisions. Before the presented study, we performed a retrospective analyses of 27 patients with low- to intermediate-risk ER+/HER2− early breast cancer treated in a breast care centre in Upper Austria (Krankenhaus Barmherzige Schwestern Linz, Austria). These data clearly provided valuable data concerning the discordance between molecular and clinical risk assessment (n=27; discordance rate: 37%). Unfortunately, we were unable to retrospectively study how molecular vs clinical risk assignment alone influences the multidisciplinary team (MDT) decision concerning the recommendation to administer adjuvant CHT. However, this type of decision analysis is important to pinpoint how and when clinicians should implement molecular tests.
In the herein presented study we prospectively included women who lacked clear indications for CHT from conventional markers. In addition, we took the opportunity to compare molecular measurement of hormone receptors, HER-2 and proliferation using immunohistochemistry (IHC) performed at our institution. Finally, in order to evaluate the economic impact of decision changes, a health technology assessment was performed comparing the hypothetical costs arising from decisions made with and without the multigene signature.
Materials and methods
Ethical and legal aspects
The project was conducted in accordance with the latest revision of the Declaration of Helsinki and the requirements of Good Clinical Practice of the European Community (CPMP/ICH/135/95). The study protocol has been reviewed and approved by the ethics committee of the Medical University of Vienna (EK-No 1116/2009).
Selection of patients and study design
After gaining informed written consent, 75 patients with ER-positive, G1 or G2 primary breast carcinomas with a clinical tumour size between 1 and 3 cm and clinically negative lymph nodes were included into the prospective study. Surgery was performed at the Medical University of Vienna over a 2-year period from April 2010 until November 2012. Only patients considered fit for adjuvant CHT treatment were included in this trial. Patients with a triple-negative phenotype at preoperative core needle biopsy or HER-2 overexpression and/or clinical tumour diameter <1 cm were excluded. Furthermore, stage UICC IV and patients who had undergone preoperative CHT were excluded. Complete resection of all tumour tissue of the breast and regional lymph nodes was mandatory for clinical and molecular risk assignment within the study protocol.
At our institution, between 280 and 300 primary breast cancer patients undergo surgery per year, and hence this study includes ∼8% of all primary breast cancer patients who underwent surgery during this period.
In summary, we analysed a population of women with low to intermediate risk according to the St Gallen criteria 2009 in order to recruit women where the decision to administer adjuvant chemotherapy or not would most likely profit from a further molecular assessment. The study was designed to explore the routine implementation of MP as an additional biomarker into clinical decision making at the MDT level.
Tumour sample collection
A tumour tissue sample of at least 3 × 3 × 3 mm was collected within 60 min of surgical removal, placed in the RNARetain (AsuraGen, Austin, TX, USA) molecular fixative and sent to Agendia (Irvine, CA, USA) for Mammaprint and Targetprint analysis. The responsible pathologist adhered to optimal tissue handling techniques in order to preserve tissue quality for histopathological diagnosis.
Methodology in MDT
Complete patient data including preoperative histology and IHC were subjected to discussion at the Breast Health Care Centre of Vienna (BHCV) MDT according to the local SOP. All data were presented in a strictly standardised format according to BHCV ‘Tumour Board Guidelines'. The MDTs were attended by surgeons, medical oncologists, radiologists, pathologists, radiation oncologists and breast care specialist nurses.
Initial MDT risk stratification decisions (clinical low to high risk) were made in the absence of MP results categorised classically according to pathological features including tumour size, nodal status, grading, IHC of hormone receptors and Ki67. The recommendation of whether or not to deliver adjuvant CHT followed the St Gallen guidelines of 2009 very closely. The later St Gallen guidelines of 2011 were not implemented at BHCV. An MDT risk assessment was performed and a recommendation regarding the addition of CHT to ET was documented.
After obtaining the MP results, the risk stratification and treatment recommendation were repeated and new risk and treatment decisions were recorded.
Histological assessment of IHC and molecular assessment using TargetPrint
Histological analysis of ER, PR and HER2 as well as Ki-67 was performed on all tissue samples. All analyses were conducted at the pathologic department of the Medical University of Vienna that also serves as the central pathology in the Austrian Breast and Colorectal Cancer Study Group (ABCSG). Hormone receptor expression was scored as previously described (Reiner et al, 1990). Briefly, ER+/PgR+ indicates the positive staining of 10–50% of tumour cell nuclei; 51–80% corresponds to ER++/PgR++; and 81–100% of stained nuclei indicate a high degree of hormone receptor expression (ER+++/PgR+++) (Dubsky et al, 2012). The Reiner score was calculated according to expression of ER/PR and intensity of the IHC analyses. The assessment of Ki-67 has previously been described (Bago-Horvath et al, 2011). Briefly, invasive tumour cells in 20 representative HPF ( × 400 magnification) were visually evaluated and only nuclear staining was scored as positive. The results were documented as the percentage of Ki-67-stained nuclei regardless of staining intensity.
MammaPrint and TargetPrint were all performed on fresh tumour samples. Microarray analysis (RNA labelling, microarray hybridisation and scanning) was performed at the centralised Agendia Laboratories blinded for clinical and histological data. RNA was cohybridised with a standard reference to the custom-designed diagnostic chip, each containing oligonucleotide probes for the profiles in triplicate or more (Glas et al, 2006).
The IHC analyses of hormone receptors and Her2neu expression were also correlated with the TargetPrint result. The concordance of the proliferation marker Ki-67 (MIB-1) with MP risk stratification was evaluated.
Cost-effectiveness analysis
In a previous study, a Markov model was constructed with four mutually exclusive health states: disease-free survival, relapse (including local and regional recurrences, secondary primary and contra lateral breast cancer), distant metastases and death. In each strategy, the sensitivity and specificity of the prognostic tests were based on three retrospective validation series (Van De Vijver et al, 2002; Buyse et al, 2006; Bueno-De-Mesquita et al, 2009). Patients were classified as having a true low, true high, false low or false high risk of developing metastases. The same costs and utilities, to calculate the quality-adjusted life years (QALYs), were applied (Retel et al, 2010).
For the current study, the model simulated the course of events for two prognostic tests: the results after following the MP test and the results after following the clinical decision-making process of the MDT (according to St Gallen guidelines of 2009, as described above). We modelled the noncompliance towards the MP test. In case of noncompliance with a discordant test result, the MP result was available (and paid), but not used in the adjuvant treatment decision. It was assumed that patients would thus be treated according to the MDT assessment. The noncompliance rates towards the MP were modelled for the discordant cases: adjuvant treatment decision according to MDT-assessment low risk/MP high risk (4 out of 8 were discordant, 4 adjuvant decisions were changed; 50% noncompliance) and MDT-assessment high risk/MP low risk (11 out of 21, 10 adjuvant decisions were changed; 52% noncompliance).
Statistical analyses
Continuous data were described using median values and ranges (minimum–maximum). Categorical data were described using absolute and relative frequencies. Statistical differences between groups were tested with the t-test for normally distributed continuous data or by the Wilcoxon rank-sum test or nonparametric data. Associations between two continuous variables were assessed by Spearman's correlation coefficient. P⩽0.05 was assumed to be significant. All calculations were performed with SAS (Version 9.2, Cary, NC, USA).
The Markov model for statistical analysis of the cost effectiveness was programmed in Microsoft Excel (Microsoft, Redmond, WA, USA). Future costs and effects were reduced to their present day value by a rate of 4% and 1.5% per annum, respectively. Incremental cost-effectiveness ratios (ICERS) were calculated by dividing the incremental costs by the incremental QALYs. The calculations are performed per year, over a total simulated time period of 20 years.
Results
Demographic data
A total of 75 patients with hormone receptor-positive, primary, early breast cancer were prospectively included in the study with a mean age of 60 years (min 33, max 86), median tumour size of 1.7 cm (min 0.7, max 10). Nearly 90% of tumours were G1 or G2. In 8 patients (10.7%), a G3 tumour was diagnosed in the final histology report (as opposed to the core needle biopsy used for study inclusion). Approximately one-third of patients had positive lymph node status, of which seven women had more than three positive lymph nodes. In all, 74 patients had an ER+ and/or PR+ tumour, and in 1 patient overexpression of HER2 was found (Table 1).
Table 1. Patient characteristics.
| Demographic data (n=75) | ||
|---|---|---|
| Mean age in years (±s.d.) |
60±13 |
|
| Median tumour size in cm (min, max) |
1.7 (0.7, 10) |
|
|
Tumour size |
n |
% |
| T1a |
0 |
0 |
| T1b |
5 |
6.7 |
| T1c |
44 |
58.7 |
| T2 |
21 |
28 |
| T3 |
5 |
6.7 |
| Invasive ductal carcinoma |
60 |
80 |
| Invasive lobular carcinoma |
13 |
17.3 |
| Invasive mucinous carcinoma |
2 |
2.7 |
|
Grading | ||
| G1 |
27 |
36 |
| G2 |
40 |
53.3 |
| G3 |
8 |
10.7 |
|
Lymph nodes | ||
| N0 |
48 |
64 |
| N1 |
20 |
26.7 |
| N2/N3 |
7 |
9.3 |
| PVI |
22 |
29.3 |
| ER+ |
74 |
98.7 |
| Her2neu+ |
1 |
1.3 |
|
Ki-67 | ||
| 10% |
26 |
34.7 |
| 15–25% |
27 |
36 |
| >30% |
22 |
29.3 |
| MammaPrint high risk |
18 |
24 |
| MammaPrint low risk |
57 |
76 |
| Luminal |
73 |
97.3 |
| Basal |
1 |
1.3 |
| ERB2 | 1 | 1.3 |
Abbreviations: ER=oestrogen receptor; PVI=perivascular invasion.
IHC and molecular assessment using TargetPrint and BluePrint
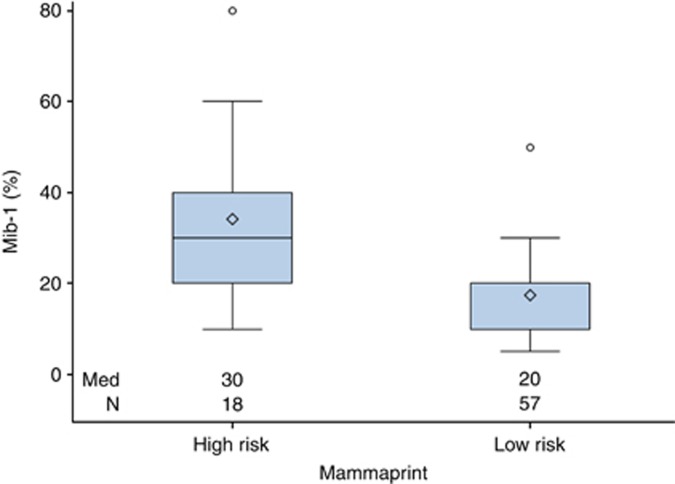
All locally assessed IHC results were compared with the TargetPrint assay (both assessing ER/PgR and Her2). There were highly significant correlations between IHC and target prints (ER: rs=0.47, P<0.0001, PgR: rs=0.72, P<0.0001, Her2: rs=0.29, P=0.0135). Local IHC analysis of Ki67 also highly correlated with MP results (P<0.0001; Table 2 and Figure 1).
Table 2. Concordance of TargetPrint and immunohistochemistry.
| Spearman's correlation | P-value | |
|---|---|---|
| Oestrogen receptor: Reiner score TargetPrint |
0.471 |
<0.0001 |
| Progesterone receptor: Reiner score TargetPrint |
0.715 |
<0.0001 |
| Her2neu− TargetPrint |
0.294 |
0.0135 |
|
Correlation of MIB-1 and MammaPrint | ||
| |
Median (min, max) |
P-value |
| Low risk |
20 (5–50) |
<0.0001 |
| High risk | 30 (10–80) | |
Figure 1.
Correlation of proliferation (MIB-1) and gene expression analysis MammaPrint (MP).
According to BluePrint subtyping, 73 patients had luminal-type breast cancer; 1 was HER2 type, and 1 basal type. The patient with HER2-type cancer also exhibited overexpression in our IHC assessment. This patient was included in the study because of a HER2− preoperative biopsy outside of our centre. The patient with the basal-like tumour had a G2 invasive ductal carcinoma with negative ER (0%), positive PR (50%), Her2 0% and Ki-67 of 40%. The MP and clinical assessment were high risk and she received adjuvant CHT.
MammaPrint results and decision change at MDT
In the prospective cohort, 57 (76%) patients were low risk and 18 patients (24%) were high risk according to the 70-gene analyses (Table 1). In 10 patients (13.3%), there was a decision change towards ET and in 4 patients (5.33%) the MDT decision changed towards the addition of adjuvant CHT (Table 3), after taking molecular risk into account. In total, 18.6% of women underwent a decision change according to the MDT assessment because of discordant molecular and clinical data.
Table 3. Decision change at multidisciplinary team (MDT).
| Clinical risk assignment | Molecular risk assignment | n | Decision change |
|---|---|---|---|
| Clinically low risk |
MammPrint low risk |
36 |
0 |
| Clinically low risk |
MammaPrint high risk |
8 |
4 |
| Clinically high risk |
MammPrint low risk |
21 |
10 |
| Clinically high risk | MammaPrint high risk | 10 | 0 |
In a further 15 patients (20%), we found a discordance between clinically assigned risk and MP results. Four patients with a clinical profile determined to be low risk by the MDT showed a high molecular risk according to the MP test. Eleven patients judged to be clinically high risk were found to be of MP low risk. Thus, in 11 women the clinical decision to administer CHT was not amended despite a low molecular risk profile.
In a descriptive analysis we determined the clinical factors that led to CHT administration despite low-risk molecular profile. Five patients were found to have three or more positive lymph nodes in the pathologic report in addition to at least one other relative risk factor. In three women, tumour size was found to be well beyond 3 cm among other risk factors. In the remaining three patients, two women showed a combination of two positive lymph nodes in combination with G2 and high Ki67. Finally, a single patient showed only a G2 tumour with increased proliferation, but her age at diagnosis was 36 years.
In summary, the MP test was able to identify a relevant group of women (n=21, 28%) with low molecular risk despite clinical or histological risk factors. In 13.3% overall, this led to a decision change that resulted in the removal of CHT from the MDT recommendation.
Cost-effectiveness analysis
A cost-effectiveness analysis was performed in the prospective population comparing the cost of MP analysis with the amount saved by CHT reduction. The total health-care costs per patient were: €31 696 for MP analysis and €35 475 for MDT assessment. The MP yielded more QALYS (11.97 out of 20 years) compared with MDT assessment (11.24 out of 20 years), showing that the use of MP is more effective and less costly than MDT assessment (Table 4). In the case of 100% compliance towards the MP test, the MP test was still found to be more effective and less costly (difference in QALYs: 0.32, difference in costs: €554).
Table 4. Cost effectiveness.
| Clinically low | Clinically high | Total | |
|---|---|---|---|
|
MammaPrint | |||
| Low | 36 | 21 (wherefrom 10 × change to ER, and 11 × noncompliant towards MP) | 57 |
| High |
8 (wherefrom 4 × change to CHT, and 4 × noncompliant towards MP) |
10 |
18 |
| Total | 44 | 31 | 75 |
|
Results |
|||||
|---|---|---|---|---|---|
| Costs | QALYs | Incremental costs | Incremental QALYs | ICER costs/QALY | |
| MammaPrint | €31 696 | 11.97 | −€3779 | 0.73 | Dominanta |
| Clinically | €35 475 | 11.24 |
Abbreviations: CHT=chemotherapy; ER=oestrogen receptor; ICER=incremental cost-effectiveness ratio; MP=MammaPrint; QALY=quality-adjusted life years.
Dominant: the MP is less costly and more effective compared with the clinical strategy.
Discussion
The aim of our study was to explore the effect of the molecular risk profile MP on the decision making of a MDT in ER+/Her2− early breast cancer. Our main focus was to record changes in decision making attributed to risk discordance between the clinical and molecular risk profile. Furthermore, we investigated possible analytical variability between the commercial assays BluePrint and TargetPrint and local immunohistochemistry.
An important finding was the high rate of discordance when comparing molecular and clinical risk. Both a retrospective cohort (data not shown) and the described prospective cohort showed well over one-third of discordant cases (37% and 39%), and this rate is comparable to a similar published case series (Albain et al, 2009). It is conceivable however that analytical variability especially concerning IHC may add to the differential risk assignments. It was therefore reassuring to observe that the gene expression data concerning ER, PGR and Ki-67 were almost identical to our own IHC assessments with highly significant concordance. It is unlikely that differences occurred because of analytical bias between IHC and molecular methods.
Over two-thirds of discordant cases resulted in a downgrade of risk and subsequently led to several MDT decisions without CHT. The protocol for the prospective study foresaw that patients were recruited into the study following clinical assessment and preoperative biopsies. The goal was to preselect patients allowing molecular and full histological data to be discussed without further delay because of shipment and molecular analysis. This led to quite a high percentage of women (16%) who may gain little benefit from a molecular test as they displayed gross lymph node involvement and/or large tumour size and/or poorly differentiated tumours. In these cases, CHT would be indicated in the absence of a molecular assessment – indeed, in several of these women the test has not been fully validated to have prognostic value. In the future, we recommend that the indication for molecular testing should be withheld until full histological assessment of the surgical specimen is completed. Since completion of the study, MP has been made available for formalin-fixed and paraffin-embedded tissue (Sapino et al, 2013).
In comparison with other studies, we had a low number of decision changes at our institution because of a long tradition of ET use in the absence of CHT in ABCSG studies (Jakesz et al, 2005; Gnant et al, 2009). In endocrine-responsive disease, treatment recommendations that exclude CHT (even despite nodal involvement) are more common in Austria than in other European institutions. From the 75 women included in the study, 29 (39%) showed a discordance between their clinical and molecular profile. Interestingly, the MDT changed their decision in only half of these cases (n=14). We would suggest three main reasons for this finding. As mentioned above, several women with clear indications for CHT were included in the study and therefore the MDT did not omit CHT from the recommendations in these five cases.
Furthermore, four women deemed to be low risk by the MDT (and in retrospect indeed no relative indication for CHT) showed high-risk molecular signatures. The predictive value of MP regarding the benefit of CHT is questionable and this was the main reason for not adding cytotoxic treatment in these patients. Prospective data from adjuvant trials are much needed in order to answer this question (Rutgers et al, 2011). Finally, there remains a group of six women where despite adequate prognostic validation of the test and a low-risk molecular profile the MDT recommended CHT – this possibly shows a certain scepticism towards molecular prognosis. It is noteworthy that these decisions were made during the first months of this study and, for the large majority of clinicians present, reflected their first encounter with a fairly new prognostic biomarker (Drukker et al, 2013). In this setting, the cost effectiveness of the MP test was also demonstrated (Retel et al, 2013).
Despite a rigid preselection of clinically low- and intermediate-risk patients, 19% of the patients underwent decision changes because of the molecular signature. Typically, these women displayed risk profiles with one or two discordant pathological variables: for example, Ki-67 may have been clearly elevated but the tumour size was well below 2 cm in the context of high ER, or there was a single positive lymph node with no other risk features present. Although we were able to confirm the cost effectiveness of the MP test ( Retel et al, 2010; Yang et al, 2012; Rouzier et al, 2013) in our study, implementing gene expression analysis specifically in these types of clinical situations patients may lead to further cost reduction as the impact of molecular scores may be most profound. Furthermore, in treatment environments outside of Austria, a higher proportion of patients may receive CHT after clinical consideration. Thus, again the impact of molecular analysis on cost saving may be larger in these countries.
The main weakness of this study arises from the small sample size. Nevertheless, it provided us with the opportunity to study how medical professionals perceived clinical risk, and how the molecular risk assessment altered this perception on a case-by-case basis. This analysis gives clear insight into how molecular testing should be integrated into clinical decision making. Our study shows that the highest rate of success is likely to be found in women who display intermediate- to high-risk clinical profiles with discordant or inconsistent variables that indicate risk.
Acknowledgments
We thank Agendia for providing 75 tests for the prospective study. For the grammatical correction of the text we thank Hannan Al-lamee, MD, from the University of Liverpool.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Albain KS, Paik S, Van't Veer L. Prediction of adjuvant chemotherapy benefit in endocrine responsive, early breast cancer using multigene assays. Breast. 2009;18 (Suppl 3:S141–S145. doi: 10.1016/S0960-9776(09)70290-5. [DOI] [PubMed] [Google Scholar]
- Azim HAJr, Michiels S, Zagouri F, Delaloge S, Filipits M, Namer M, Neven P, Symmans WF, Thompson A, Andre F, Loi S, Swanton C. Utility of prognostic genomic tests in breast cancer practice: The IMPAKT 2012 Working Group Consensus Statement. Ann Oncol. 2013;24:647–654. doi: 10.1093/annonc/mds645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bago-Horvath Z, Rudas M, Dubsky P, Jakesz R, Singer CF, Kemmerling R, Greil R, Jelen A, Bohm G, Jasarevic Z, Haid A, Gruber C, Postlberger S, Filipits M, Gnant M. Adjuvant sequencing of tamoxifen and anastrozole is superior to tamoxifen alone in postmenopausal women with low proliferating breast cancer. Clin Cancer Res. 2011;17:7828–7834. doi: 10.1158/1078-0432.CCR-11-1846. [DOI] [PubMed] [Google Scholar]
- Bueno-De-Mesquita JM, Linn SC, Keijzer R, Wesseling J, Nuyten DS, Van Krimpen C, Meijers C, De Graaf PW, Bos MM, Hart AA, Rutgers EJ, Peterse JL, Halfwerk H, De Groot R, Pronk A, Floore AN, Glas AM, Van't Veer LJ, Van De Vijver MJ. Validation of 70-gene prognosis signature in node-negative breast cancer. Breast Cancer Res Treat. 2009;117:483–495. doi: 10.1007/s10549-008-0191-2. [DOI] [PubMed] [Google Scholar]
- Buyse M, Loi S, Van't Veer L, Viale G, Delorenzi M, Glas AM, D'assignies MS, Bergh J, Lidereau R, Ellis P, Harris A, Bogaerts J, Therasse P, Floore A, Amakrane M, Piette F, Rutgers E, Sotiriou C, Cardoso F, Piccart MJ. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98:1183–1192. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- Drukker CA, Bueno-De-Mesquita JM, Retel VP, Van Harten WH, Van Tinteren H, Wesseling J, Roumen RM, Knauer M, Van 'T Veer LJ, Sonke GS, Rutgers EJ, Van De Vijver MJ, Linn SC. A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int J Cancer. 2013;133:929–936. doi: 10.1002/ijc.28082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubsky PC, Jakesz R, Mlineritsch B, Postlberger S, Samonigg H, Kwasny W, Tausch C, Stoger H, Haider K, Fitzal F, Singer CF, Stierer M, Sevelda P, Luschin-Ebengreuth G, Taucher S, Rudas M, Bartsch R, Steger GG, Greil R, Filipcic L, Gnant M. Tamoxifen and anastrozole as a sequencing strategy: a randomized controlled trial in postmenopausal patients with endocrine-responsive early breast cancer from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol. 2012;30:722–728. doi: 10.1200/JCO.2011.36.8993. [DOI] [PubMed] [Google Scholar]
- Glas AM, Floore A, Delahaye LJ, Witteveen AT, Pover RC, Bakx N, Lahti-Domenici JS, Bruinsma TJ, Warmoes MO, Bernards R, Wessels LF, Van't Veer LJ. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics. 2006;7:278. doi: 10.1186/1471-2164-7-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rucklinger E, Greil R, Marth C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- Gnant M, Mlineritsch B, Stoeger H, Luschin-Ebengreuth G, Heck D, Menzel C, Jakesz R, Seifert M, Hubalek M, Pristauz G, Bauernhofer T, Eidtmann H, Eiermann W, Steger G, Kwasny W, Dubsky P, Hochreiner G, Forsthuber EP, Fesl C, Greil R. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakesz R, Hausmaninger H, Kubista E, Gnant M, Menzel C, Bauernhofer T, Seifert M, Haider K, Mlineritsch B, Steindorfer P, Kwasny W, Fridrik M, Steger G, Wette V, Samonigg H. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer—Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol. 2002;20:4621–4627. doi: 10.1200/JCO.2002.09.112. [DOI] [PubMed] [Google Scholar]
- Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, Hilfrich J, Kwasny W, Menzel C, Samonigg H, Seifert M, Gademann G, Kaufmann M, Wolfgang J. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–462. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- Mook S, Schmidt MK, Weigelt B, Kreike B, Eekhout I, Van De Vijver MJ, Glas AM, Floore A, Rutgers EJ, Van 'T Veer LJ. The 70-gene prognosis signature predicts early metastasis in breast cancer patients between 55 and 70 years of age. Ann Oncol. 2010;21:717–722. doi: 10.1093/annonc/mdp388. [DOI] [PubMed] [Google Scholar]
- Reiner A, Neumeister B, Spona J, Reiner G, Schemper M, Jakesz R. Immunocytochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res. 1990;50:7057–7061. [PubMed] [Google Scholar]
- Retel VP, Joore MA, Drukker CA, Bueno-De-Mesquita JM, Knauer M, Van Tinteren H, Linn SC, Van Harten WH. Prospective cost-effectiveness analysis of genomic profiling in breast cancer. Eur J Cancer. 2013;49:3773–3779. doi: 10.1016/j.ejca.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Retel VP, Joore MA, Knauer M, Linn SC, Hauptmann M, Harten WH. Cost-effectiveness of the 70-gene signature versus St. Gallen guidelines and Adjuvant Online for early breast cancer. Eur J Cancer. 2010;46:1382–1391. doi: 10.1016/j.ejca.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Rouzier R, Pronzato P, Chéreau E, Carlson J, Hunt B, Valentine WJ. Multigene assays and molecular markers in breast cancer: systematic review of health economic. Breast Cancer Res Treat. 2013;139:621–637. doi: 10.1007/s10549-013-2559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutgers E, Piccart-Gebhart MJ, Bogaerts J, Delaloge S, Veer LV, Rubio IT, Viale G, Thompson AM, Passalacqua R, Nitz U, Vindevoghel A, Pierga JY, Ravdin PM, Werutsky G, Cardoso F. The EORTC 10041/BIG 03-04 MINDACT trial is feasible: results of the pilot phase. Eur J Cancer. 2011;47:2742–2749. doi: 10.1016/j.ejca.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Sapino A, Roepman P, Linn SC, Snel MH, Delahaye LJ, Van Den Akker J, Glas AM, Simon IM, Barth N, De Snoo FA, Van 'T Veer LJ, Molinaro L, Berns EM, Wesseling J, Riley LB, Anderson D, Nguyen B, Cox CE. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J Mol Diagn. 2013;16 (2:190–197. doi: 10.1016/j.jmoldx.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Van't Veer LJ, Dai H, Van De Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, Van Der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- Van De Vijver MJ, He YD, Van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, Van Der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Yang M, Rajan S, Issa AM. Cost effectiveness of gene expression profiling for early stage breast cancer: a decision-analytic model. Cancer. 2012;118:5163–5170. doi: 10.1002/cncr.27443. [DOI] [PubMed] [Google Scholar]