Abstract
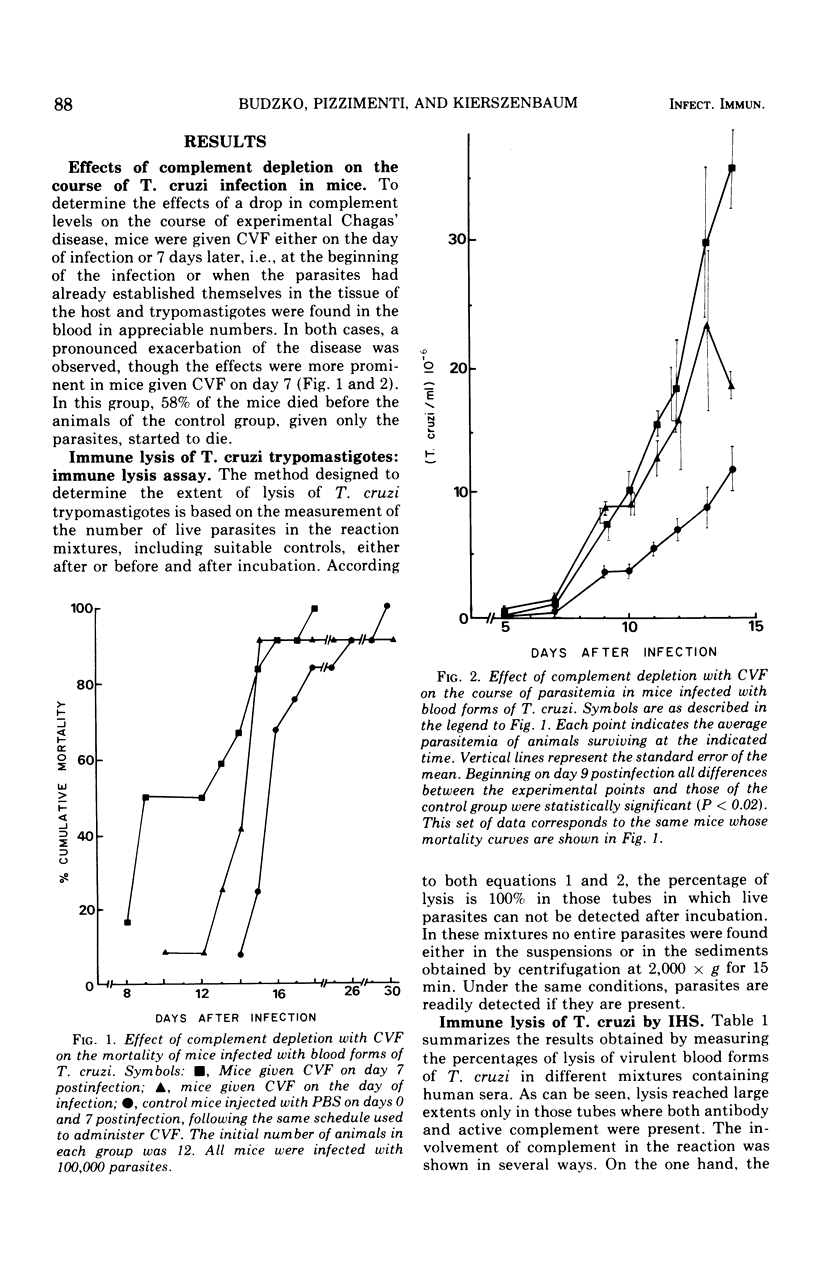
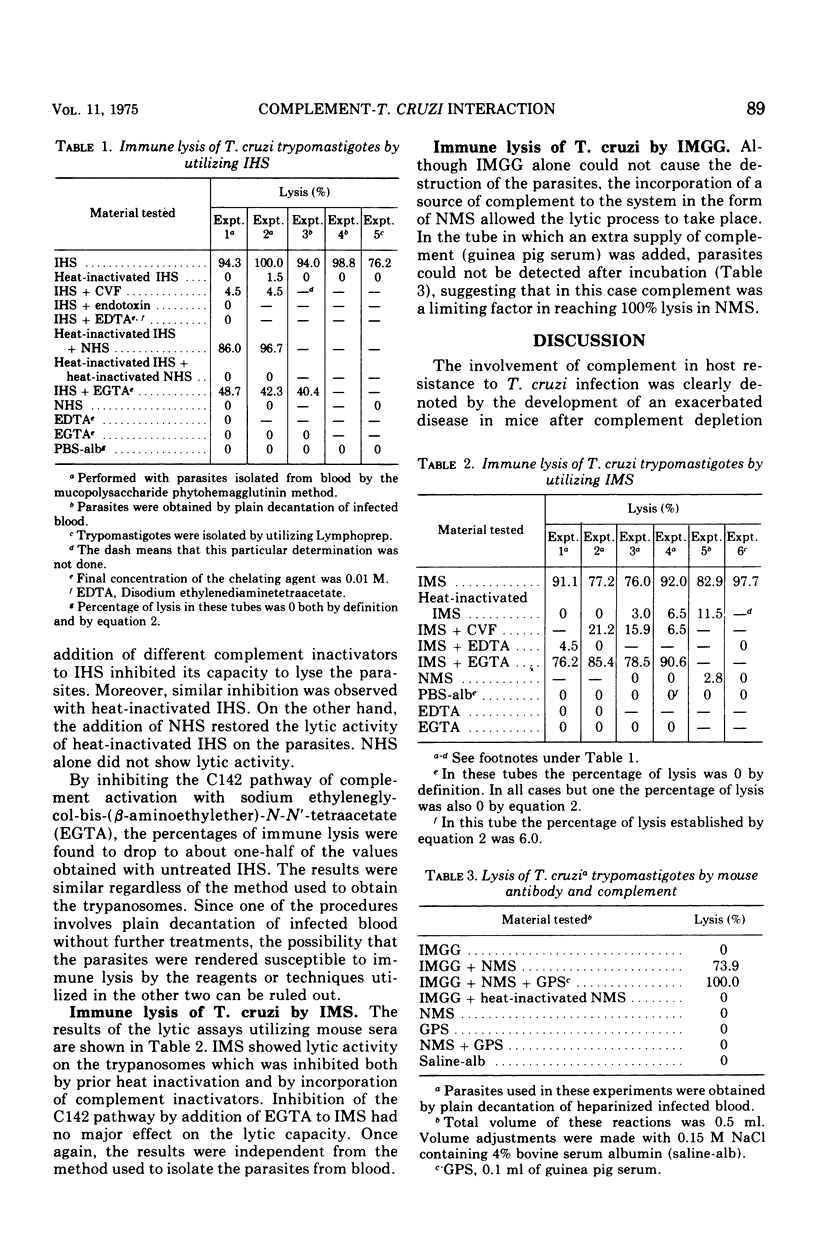
In mice infected with virulent blood (trypomastigote) forms of Trypanosoma cruzi, complement depletion with cobra venom factor caused a marked exacerbation of the disease evidenced by significantly increased parasitemia levels and early mortality as compared with those of untreated infected animals. The effect was greater in mice receiving cobra venom factor on day 7 postinfection, i.e., at the time when the parasites had had time to localize and multiply in the tissues and appeared in the circulation in appreciable numbers. The possibility that complement participates in host defense against T. cruzi infection through a mechanism involving immune lysis was explored in vitro. T. cruzi trypomastigotes were found to undergo immune lysis in sera of patients with chronic Chagas' disease, in sera of immunized mice, and in solutions containing both immune mouse gamma globulin and a source of active complement. This phenomenon failed to take place either in the absence of complement or after complement inactivation by heat or utilizing complement inactivators. The lytic capacity of heated sera was restored by the addition of active complement to the system. During the immune lysis of T. cruzi blood forms, complement was activated in human sera via both the classical and the alternate pathways. In mouse sera, activation followed at least the alternate pathway.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anziano D. F., Dalmasso A. P., Lelchuk R., Vásquez C. Role of complement in immune lysis of Trypanosoma cruzi. Infect Immun. 1972 Nov;6(5):860–864. doi: 10.1128/iai.6.5.860-864.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerisola J. A., Alvarez M., Lugones H., Rebosolán J. B. Sensibilidad de las reacciones serológicas para el diagnóstico de la enfermedad de Chagas. Bol Chil Parasitol. 1969 Jan-Mar;24(1):2–8. [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Kierszenbaum F., Knecht E., Budzko D. B., Pizzimenti M. C. Phagocytosis: a defense mechanism against infection with Trypanosoma cruzi. J Immunol. 1974 May;112(5):1839–1844. [PubMed] [Google Scholar]
- Kierszenbaum F., Saavedra L. E. The effects of bacterial endotoxin on the infection of mice with Trypanosoma cruzi. J Protozool. 1972 Nov;19(4):655–657. doi: 10.1111/j.1550-7408.1972.tb03552.x. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Fjellström K. E. Isolation of the anticomplementary protein from cobra venom and its mode of action on C3. J Immunol. 1971 Dec;107(6):1666–1672. [PubMed] [Google Scholar]
- RIGAS D. A., OSGOOD E. E. Purification and properties of the phytohemagglutinin of Phaseolus vulgaris. J Biol Chem. 1955 Feb;212(2):607–615. [PubMed] [Google Scholar]
- RUBIO M. Actividad lítica de sueros normales sobre formas de cultivo y sanguíneas de Trypanosoma cruzi. Bol Chil Parasitol. 1956 Oct-Dec;11(4):62–69. [PubMed] [Google Scholar]
- Teixeira A. R., Santos-Buch C. A. The immunology of experimental Chagas' disease. I. Preparation of Trypanosoma cruzi antigens and humoral antibody response to there antigens. J Immunol. 1974 Sep;113(3):859–869. [PubMed] [Google Scholar]


