Abstract
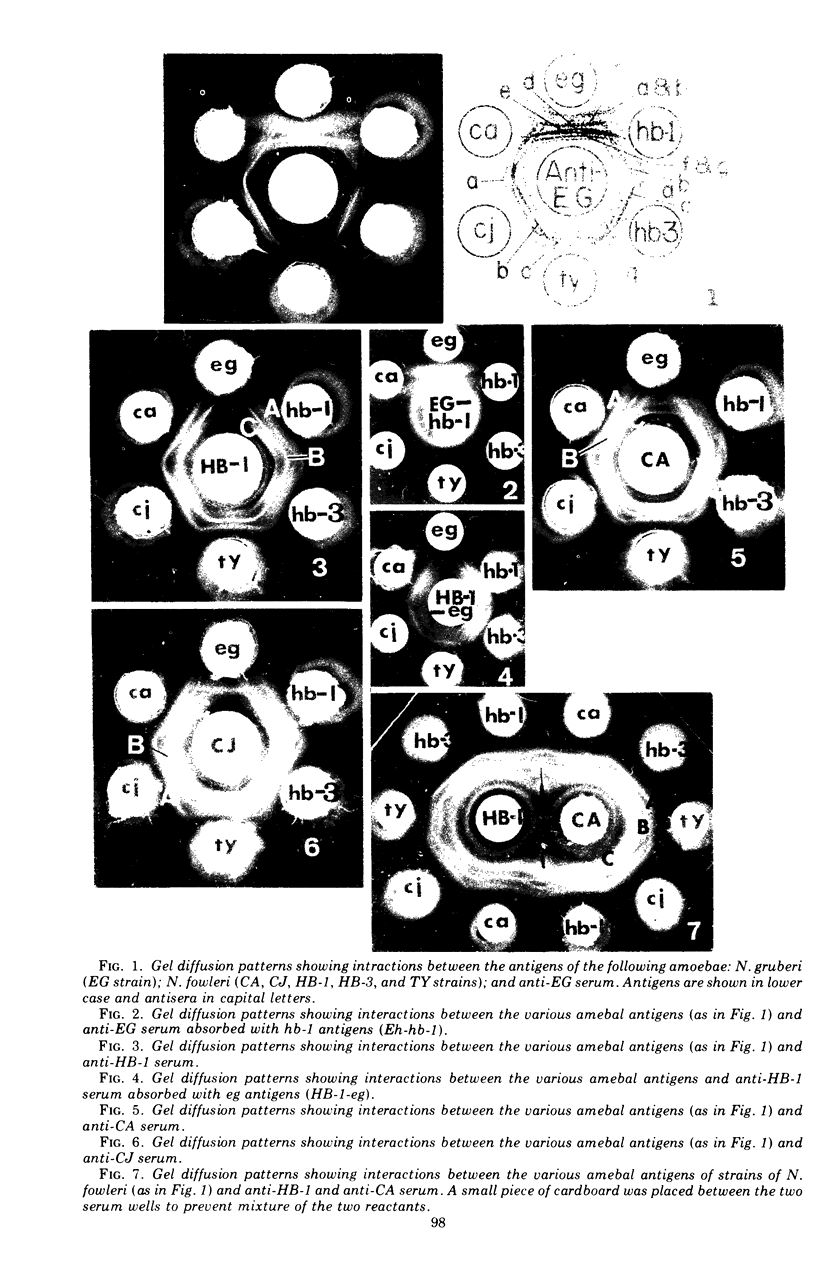
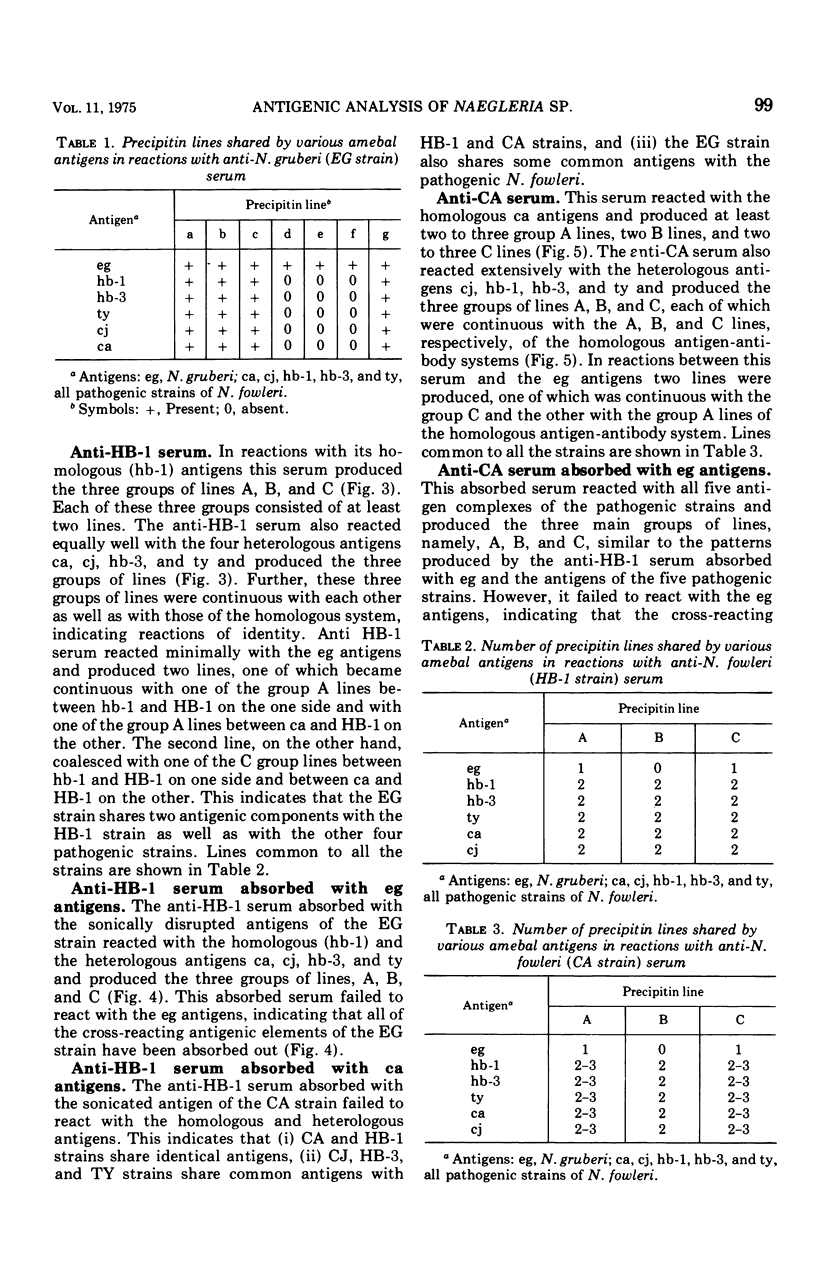
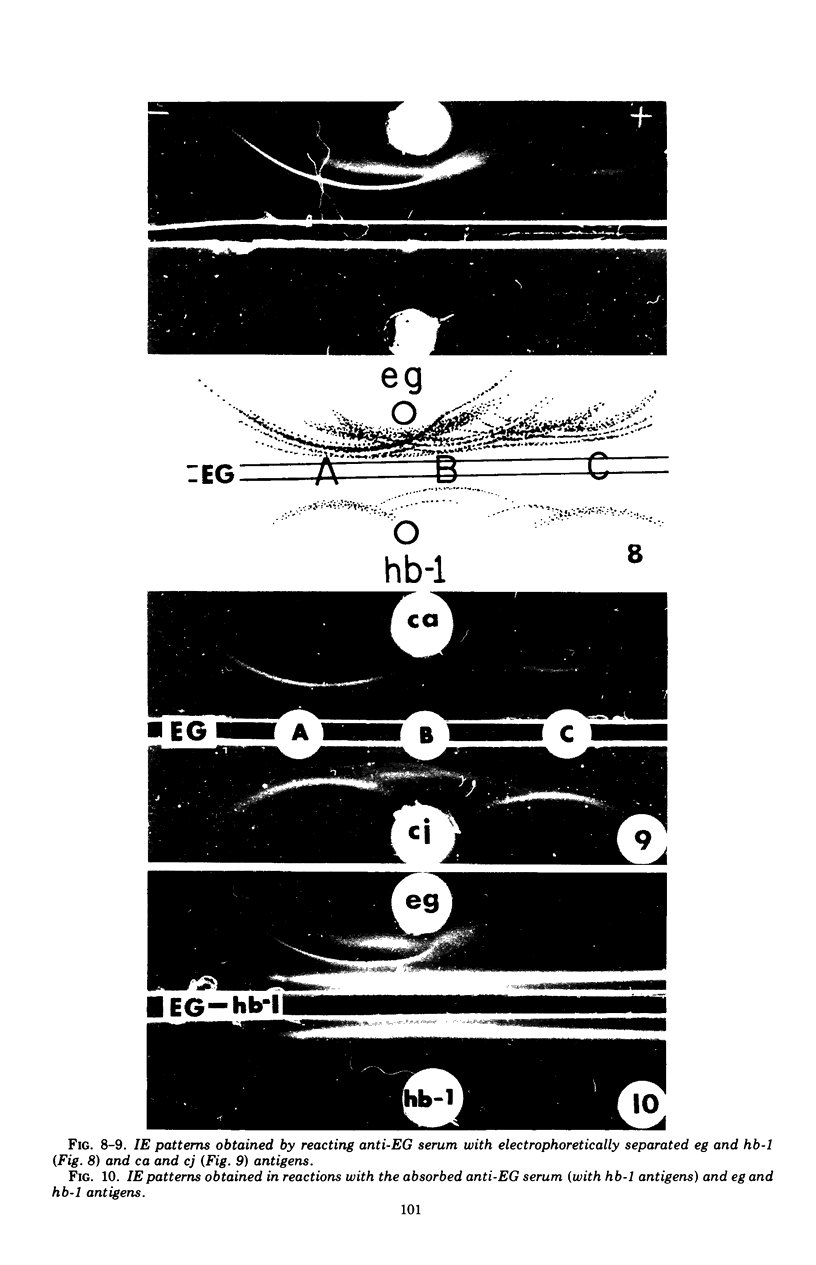
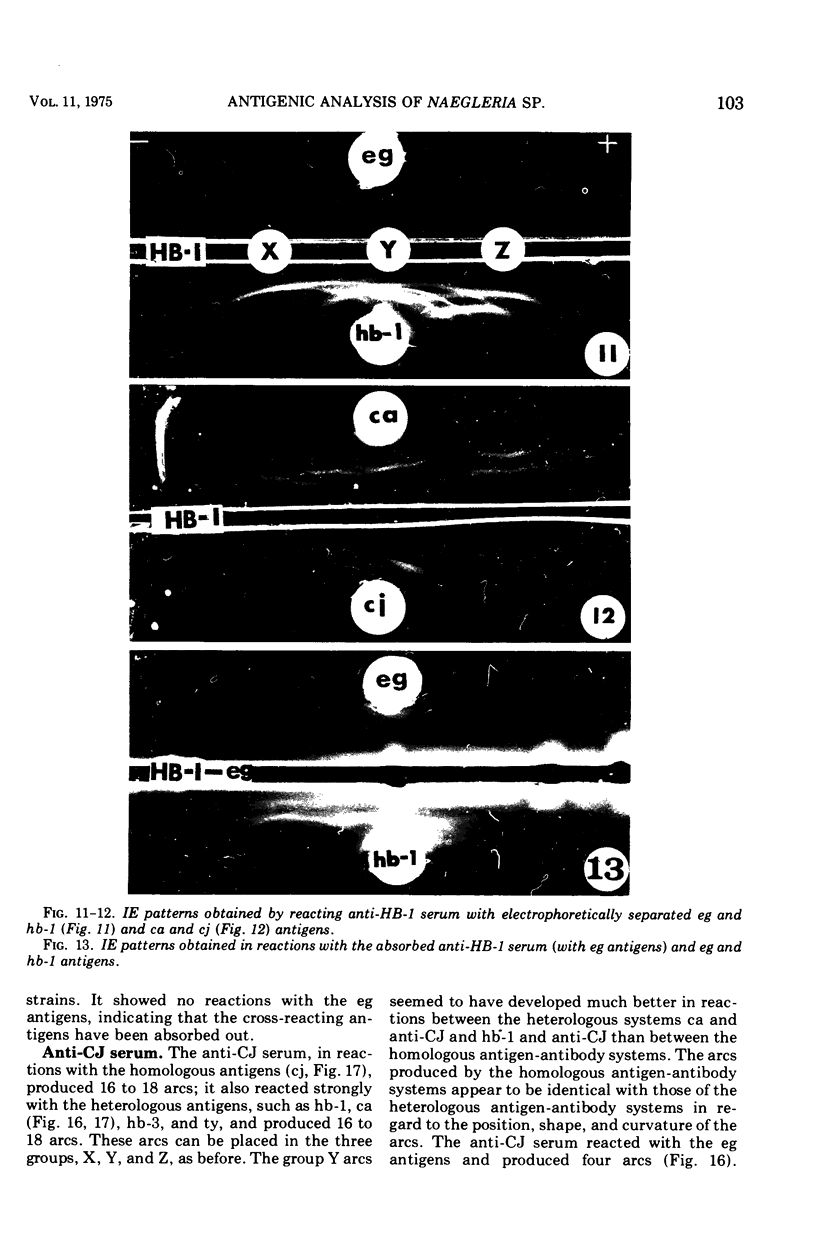
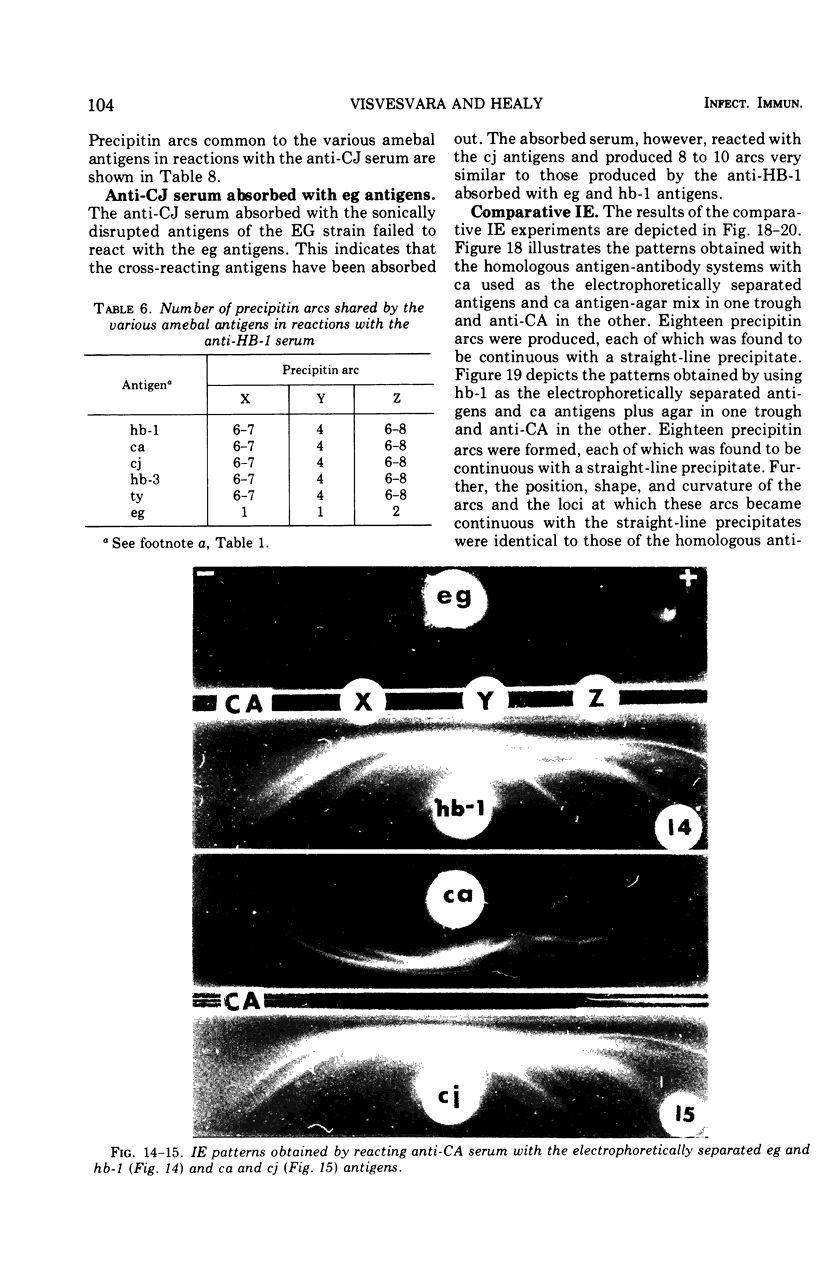
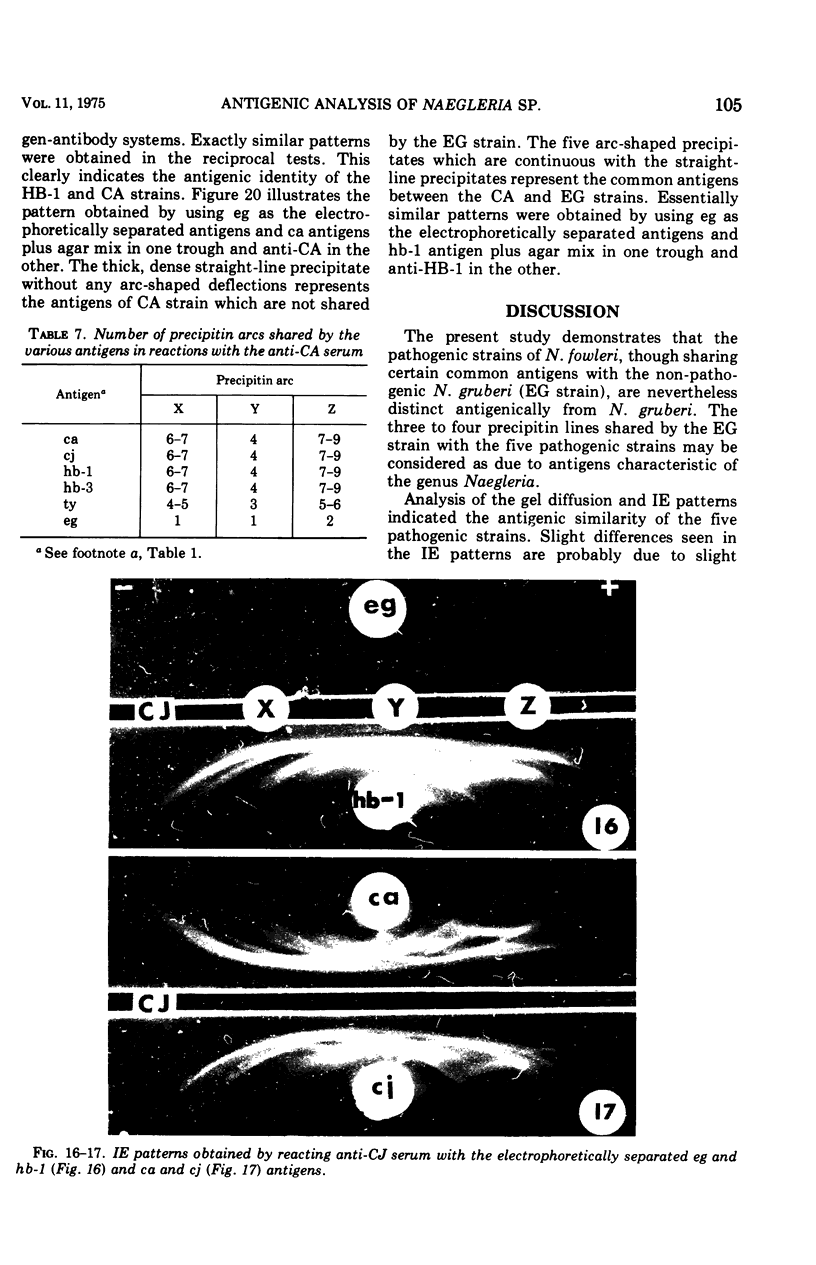
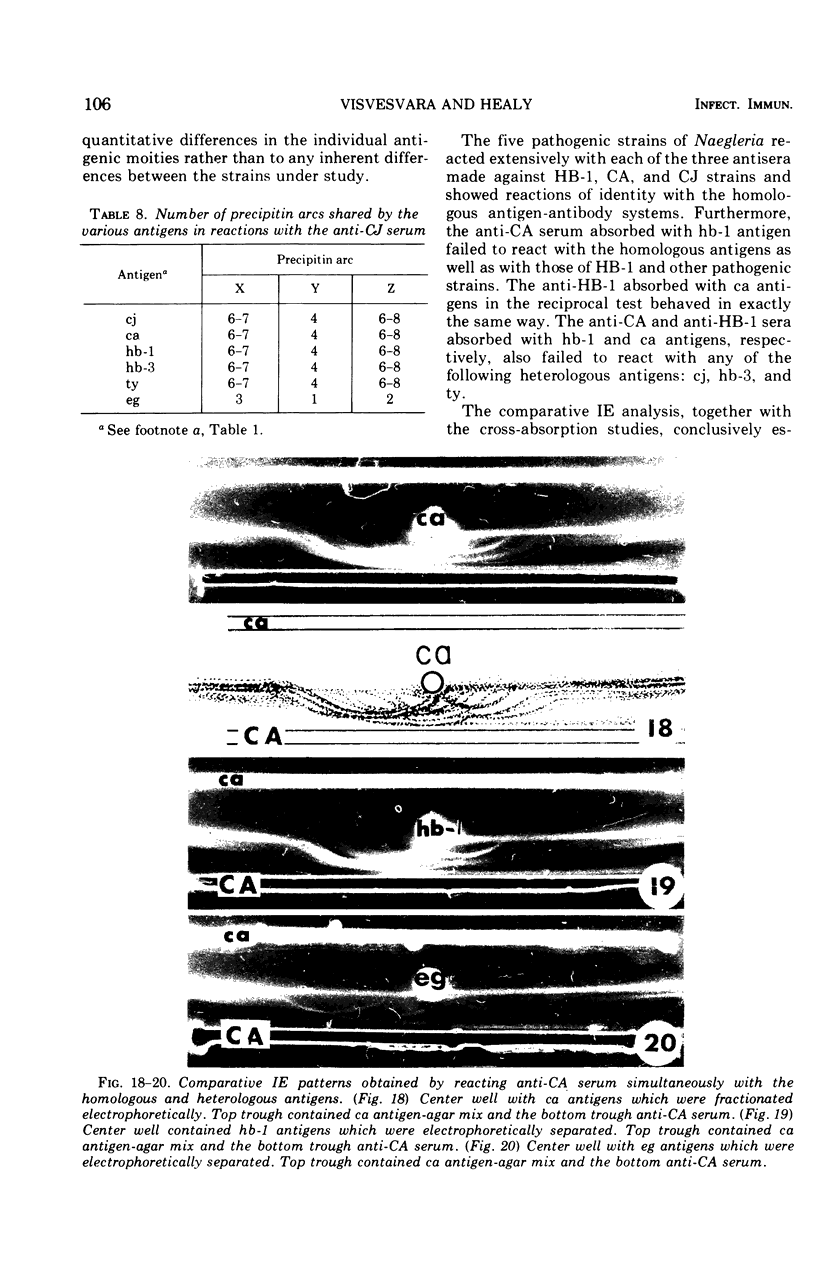
Antigens prepared from each of five strains (CA, CJ, HB-1, HB-3, and TY) of pathogenic Naegleria and the EG strain of nonpathogenic Naegleria gruberi were compared by the gel diffusion and immunoelectrophoresis techniques. Axenically grown amoebae were used as sources of antigens. Antisera were produced in individual rabbits against three strains (CA, CJ, and HB-1) of pathogenic Naegleria and the EG strain of N. gruberi. In the gel diffusion experiment each of the six antigens was reacted with each of the four antisera in agar gel. The results of these experiments revealed that the antigens of N. gruberi reacted strongly with the homologous antiserum but minimally with each of the three heterologous antisera. The antigens of all five pathogenic strains reacted extensively with the anti-CA, anti-CJ, and anti-HB-1 sera and moderately with the anti-EG serum. In the immunoelectrophoresis test each of the six antigens was separated electrophoretically in agar gel and reacted with each of the four antisera. The EG strain reacted extensively with its homologous antiserum and produced multiple precipitin arcs; it reacted minimally with anti-CA, anti-CJ, and anti-HB-1 sera and produced only three arcs. The antigens of all five strains of Naegleria fowleri reacted very strongly with anti-CA, anti-CJ, and anti-HB-1 sera and produced multiple precipitin arcs. They, however, reacted variably with the anti-EG serum and produced three to six precipitin arcs. Comparative immunoelectrophoretic analysis carried out on the CA and HB-1 strains revealed the antigenic identity of these two strains. Based on these results, together with those from the reciprocal absorption experiments, it was concluded that (i) the pathogenic strains of Naegleria, though they shared three to six common antigens with N. gruberi, were nevertheless distinct from it, and (ii) the five pathogenic strains were antigenically close and belonged in the same species. Antigens of Acanthamoeba castellanii, A. culbertsoni, and Entamoeba histolytica were also reacted with the four anti-Naegleria sera in gel diffusion experiments. Results of these tests indicate that these three organisms are antigenically distinct from Naegleria.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K., Jamieson A. Primary amoebic meningoencephalitis. Lancet. 1972 Apr 22;1(7756):902–903. doi: 10.1016/s0140-6736(72)90772-6. [DOI] [PubMed] [Google Scholar]
- Butt C. G., Baro C., Knorr R. W. Naegleria (sp.) identified in amebic encephalitis. Am J Clin Pathol. 1968 Nov;50(5):568–574. doi: 10.1093/ajcp/50.5.568. [DOI] [PubMed] [Google Scholar]
- Carter R. F. Description of a Naegleria sp. isolated from two cases of primary amoebic meningo-encephalitis, and of the experimental pathological changes induced by it. J Pathol. 1970 Apr;100(4):217–244. doi: 10.1002/path.1711000402. [DOI] [PubMed] [Google Scholar]
- Cerva L., Zimák V., Novák K. Amoebic meningoencephalitis: a new amoeba isolate. Science. 1969 Feb 7;163(3867):575–576. doi: 10.1126/science.163.3867.575. [DOI] [PubMed] [Google Scholar]
- Culbertson C. G., Ensminger P. W., Overton W. M. Pathogenic Naegleria sp.--study of a strain isolated from human cerebrospinal fluid. J Protozool. 1968 May;15(2):353–363. doi: 10.1111/j.1550-7408.1968.tb02136.x. [DOI] [PubMed] [Google Scholar]
- Diamond L. S. Techniques of axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae. J Parasitol. 1968 Oct;54(5):1047–1056. [PubMed] [Google Scholar]
- Duma R. J., Rosenblum W. I., McGehee R. F., Jones M. M., Nelson E. C. Primary amoebic meningoencephalitis caused by Naegleria. Two new cases, response to amphotericin B, and a review. Ann Intern Med. 1971 Jun;74(6):923–931. doi: 10.7326/0003-4819-74-6-923. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pacheco G., Tulloch G. S. Microfilariae of Dirofilaria striata in a dog. J Parasitol. 1970 Apr;56(2):248–248. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- SEN A., MUKERJEE S., RAY J. C. Observations on the antigenic make-up of amoebae. Ann Biochem Exp Med. 1961 Oct;21:323–326. [PubMed] [Google Scholar]
- Schuster F. L. Intranuclear virus-like bodies in the amoeboflagellate Naegleria gruberi. J Protozool. 1969 Nov;16(4):724–727. doi: 10.1111/j.1550-7408.1969.tb02333.x. [DOI] [PubMed] [Google Scholar]
- Singh B. N., Das S. R. Studies on pathogenic and non-pathogenic small free-living amoebae and the bearing of nuclear division on the classification of the order amoebida. Philos Trans R Soc Lond B Biol Sci. 1970 Oct 22;259(832):435–476. doi: 10.1098/rstb.1970.0063. [DOI] [PubMed] [Google Scholar]
- WADSWORTH C., HANSON L. A. Comparative analysis of immune electrophoretic precipitates employing a modified immune electrophoretic technique. Int Arch Allergy Appl Immunol. 1960;17:165–177. doi: 10.1159/000229122. [DOI] [PubMed] [Google Scholar]