Abstract
Phosphorus has been identified as an important determinant of nutrition-related biological variation. The macronutrients protein (P) and carbohydrates (C), both alone and interactively, are known to affect animal performance. No study, however, has investigated the importance of phosphorus relative to dietary protein or carbohydrates, or the interactive effects of phosphorus with these macronutrients, on fitness-related traits in animals. We used a nutritional geometry framework to address this question in adult field crickets (Gryllus veletis). Our results showed that lifespan, weight gain, acoustic mate signalling and egg production were maximized on diets with different P : C ratios, that phosphorus did not positively affect any of these fitness traits, and that males and females had different optimal macronutrient intake ratios for reproductive performance. When given a choice, crickets selected diets that maximized both lifespan and reproductive performance by preferentially eating diets with low P : C ratios, and females selected diets with a higher P : C ratio than males. Conversely, phosphorus intake was not regulated. Overall, our findings highlight the importance of disentangling the influences of different nutrients, and of quantifying both their individual and interactive effects, on animal fitness traits, so as to gain a more integrative understanding of their nutritional ecology.
Keywords: nutritional geometry, phosphorus, carbohydrate, protein, field cricket, diet choice
1. Introduction
Variation in dietary quality and quantity are pervasive and powerful determinants of animal fitness [1,2]. The question of how diet influences fitness, and the study of the countervailing adaptations that animals have evolved to buffer variation in fitness against dietary variation, are important and active research areas with implications that reverberate across the biological sciences. Critical to these questions is the realization that food components interact in their influence on animals; the benefits of nutrients are usually not independent, but contingent on the levels of other nutrients in the diet [2]. Such interactions are ubiquitous and powerful mediators of the relationship between diet and fitness, and are implicit in the foundational concept of nutrient balance [2].
The limiting step in developing this perspective, however, has been the lack of frameworks for investigating interactive effects of food components on animals. A minimal requirement is that any framework should be multi-dimensional to provide a basis for conceptualizing nutrition with respect to multiple diet components and their interactions [3]. In recent years, two such frameworks have become established, namely, ecological stoichiometry (ES) [1,4] and nutritional geometry (NG) [2,5,6]. These frameworks are both centred on the concept of nutritional balance, but they differ in several respects. For the present purposes, the most pertinent difference is that NG is centred on organismal traits, whereas ES is primarily centred on ecological processes [3,7]. Similarly, the nutritional variables that are modelled in NG are selected according to their relevance to organismal traits (e.g. nutritional regulatory systems and fitness-relevant responses) whether they are macromolecular nutrients (such as proteins, carbohydrates and lipids) or elements (such as calcium or mineral salts) [8–12]. By contrast, ES models the relationships among chemical elements, because these are general to all levels of biological organization and can be tracked stoichiometrically in their flow through biological systems [13,14], although macromolecules have occasionally been investigated [15].
Both NG and ES have made appreciable contributions to the understanding of how nutritional interactions influence biological systems [1,2]. There are, however, differences in the specific nutritional relationships that have been identified as primary in explaining nutrition-related biological variation. Notably, NG has identified the energetic macronutrients proteins, lipids and carbohydrates as powerful drivers in most systems [2,5,6,9–12]. Conversely, ES has identified the elements carbon, nitrogen and phosphorus as pivotal [1,13,16,17]. These perspectives converge to some extent, because nitrogen represents an element-level proxy for amino acids and carbon is usually regarded as a metric for the energy-providing macronutrients, principally lipids and carbohydrates [1]. A point where continuity has not been established, however, is in relation to the third element, phosphorus. Phosphorus is essential for all organisms, as it is an important constituent of many biomolecules, including phospholipids in cellular membranes, proteins, nucleic acids (RNA and DNA) and adenosine triphosphate [1]. Phosphorus has been identified as a fundamental limiting factor in many stoichiometric studies [13,16,17], yet has been the subject of no studies within the NG paradigm. This is an important omission, because despite their respective organismal and ecological foci, both NG and ES aim to establish continuity across the full range of biological organization [3,4]. To achieve this, we need to learn how behavioural and physiological regulatory mechanisms and animal performance respond to orthogonal variation in dietary phosphorus, protein and carbohydrates.
To examine this question, we used NG to perform experiments in which protein, carbohydrate and phosphorus were varied systematically in the diets of field crickets (Gryllus veletis). Although little is known about the feeding habits of wild field crickets, they are omnivorous, feeding on diverse plants and insects [18–20] that vary considerably in nutrient content [13,21–23]. Such dietary breadth suggests that crickets have evolved homeostatic regulatory systems for buffering performance against variation in nutrient intake [24], and in this respect they provide a good model system for investigating complex nutritional regulatory challenges. Field crickets are also ideal for investigating how diet influences sexually selected traits. Males produce acoustic mate attraction signals by rubbing their forewings together [25]. Overall signalling effort is influenced by diet [10,26,27] and females typically phonolocate towards higher-effort signallers [26,28].
Using no-choice diet trials, we first examined (i) how dietary nutrient balance during adulthood influences adult fitness traits, (ii) whether diet during adulthood influences trade-offs between fitness traits within each sex and (iii) whether any influence of diet on these traits is sex-specific (experiment 1). As females allocate more nutrients to gametes and males allocate more nutrients and energy to sexual displays [7,29,30], we predicted that reproductive proxies (egg production and signalling) would be optimized in different regions of the nutritional landscape for the sexes. Conversely, we predicted that lifespan and weight gain would be optimized in similar regions of the nutritional landscape, as the metabolic processes maintaining these fitness traits are unlikely to differ between the sexes. Within each sex, we further predicted that diet would influence trade-offs between fitness traits.
We then investigated adult diet choice by providing crickets with multiple foods containing varying nutrient ratios to ascertain sex-specific regulation of phosphorus intake (experiment 2) and protein and carbohydrate intakes (experiment 3). We predicted that the sexes would differ in their diet preference as a consequence of their different reproductive strategies. Based on previous studies of insect diet choice [10,12,24], we expected that protein and carbohydrate intake would be tightly regulated. By contrast, the observation that bodily phosphorus content often covaries with dietary phosphorus levels in terrestrial insects implies that neither the intake nor post-ingestive retention of phosphorus should be strongly regulated, if at all [17,31].
2. Material and methods
(a). Subjects
A laboratory population of G. veletis was formed in June 2010 and raised following established protocols (see the electronic supplementary material for details). Our study was conducted in accordance with the guidelines of the Canadian Council on Animal Care, and no field collecting permits were required. Juveniles were separated by sex at the penultimate instar and monitored daily for adult eclosion. Upon eclosion, adults were transferred into separate 520 ml clear plastic containers with a screened lid, and provided with shelter, ad libitum water and experimental food(s).
(b). Measuring initial body size
We measured two proxies of initial body size: body weight (mg) and pronotum height (mm). Crickets were weighed prior to diet trials using a Denver Instruments analytical balance (Pinnacle Series model PI-114; precision ± 0.1 mg), and then photographed in a dorsal position using a Zeiss Discovery V12 stereo dissecting microscope, from which pronotum height was measured (AxioVision v. 4.8, Carl Zeiss; magnification approx. 5×, resolution approx. 1.60 µm). Because weight and pronotum height were significantly positively correlated (males: F1,423 = 674.791, p < 0.001; females: F1,355 = 667.108, p < 0.001), they were reduced to a single composite measure of initial body size using principal component analysis in JMP (v. 10; SAS Institute Inc., Cary, NC).
(c). Experiment 1: influence of dietary nutrient balance on fitness traits
(i). Experimental foods
Experimental foods were created following established protocols (see the electronic supplementary material) [32]. Foods consisted of five different protein (P) : carbohydrate (C) ratios (3P : 1C, 1P : 1C, 1P : 3C, 1P : 5C, 1P : 8C), each varying at three phosphorus (Ph) levels (0.45, 1.45, 2.45% Ph by mass). The resulting 15 nutrient mixtures were each diluted to three cellulose levels (14, 45, 76% cellulose), for a total of 45 unique foods (electronic supplementary material, table S1). Dilution with non-nutritional cellulose was necessary to ensure that some crickets consumed low levels of nutrients to allow resolution in the lower-intake regions of the nutritional landscape in order to spread out nutrient intake along ‘nutritional rails’ (sensu [5]). A nutritional rail is a line in the nutritional landscape whose slope represents the ratio of nutrients in a particular diet. After accounting for dilution with non-nutritional cellulose, the absolute percentage phosphorus (by mass) in the 45 experimental foods ranged from 0.07 to 1.87% (electronic supplementary material, table S1). This range was chosen to reflect natural variation in the diets of crickets in the wild [18–20], including insect matter (0.4–1.4% phosphorus [23]) and plant matter (0.02–1.04% phosphorus [13]). The range of dietary P : C ratios was chosen based on a previous study in which life-history and fitness traits were mapped across a wide range of P : C ratios and concentrations [10].
Crickets were provided weekly with fresh food. Weekly protein, carbohydrate and phosphorus intakes were calculated from total food intake (difference in weight of food dish before and after consumption) and known food compositions (see the electronic supplementary material). Food dishes (50 × 9 mm Petri dishes with upturned 15 × 15 mm plastic lids glued at the centre) containing food were dried in a drying oven for 48 h at 30°C prior to weighing and were weighed using a Denver Instruments analytical balance after removing faeces with fine forceps. Control food containers without crickets were housed in 520 ml plastic containers with water and were dried with experimental food containers. If control food containers increased in weight, experimental food containers were dried for an additional 24 h.
(ii). Measuring male and female fitness traits
Crickets were weighed weekly and checked every 48 h to determine date of death. As female G. veletis begin producing eggs (S.J.H. 2011, personal observations) and males begin producing sexual signals [33] at approximately one week post-adult eclosion, reproduction proxy measurements began during the second week of adulthood. Females were given the opportunity to mate with a randomly selected stock male once weekly for 6 h, from day 7 to 28 of adulthood. Fresh sand for oviposition was provided, and weekly egg production was counted after sifting the sand through a 300 µm sieve.
Male acoustic signalling was monitored for 48 h bouts, beginning on day 7 of adulthood and continuing weekly thereafter until day 29 of adulthood. Signalling was monitored using the Electronic Acoustic Recording System II (see the electronic supplementary material). The following average daily signalling parameters were statistically analysed: pulse duration (ms), interpulse duration (ms), chirp duration (ms), interchirp duration (ms), number of pulses per chirp, pulse rate (no. pulses s−1), chirp rate (no. chirps min−1), carrier frequency (Hz), amplitude (dB) and time spent signalling (min d−1).
Since G. veletis has approximately a two-month breeding season in the wild (S.J.H. 2010, personal observation), we terminated experiment 1 nine weeks post-adult eclosion; crickets still alive at this time (12% of males and 32% of females) were euthanized via freezing. We tested 425 males and 357 females, with 7–10 individuals per experimental food treatment for each sex.
(iii). Statistical analyses
To determine how nutrient intake influences adult weight gain, lifespan, egg production and acoustic mating signals, we used a multivariate surface-response approach to estimate and visualize the linear, quadratic and correlational effects of protein, carbohydrates and phosphorus on these fitness traits [34]. Responses were analysed using general linear models in SPSS (v. 20; SPSS Inc., Chicago, IL), simplified by removing non-significant quadratic and correlational variables [35], and response surfaces were visualized using non-parametric thin-plate splines using R (v. 2.15.2; http://www.r-project.org). We used a sequential model-building approach (ANCOVA) and partial F-tests in SPSS to compare response surfaces within and between sexes to determine whether the effects of protein, carbohydrate and phosphorus intake differed between response variables within a sex, or differed for the same response variables between the sexes [12]. Full details of the statistical methods can be found in the electronic supplementary material.
(d). Experiments 2 and 3: influence of nutrient ratio on diet selection
(i). Experimental foods
Using methods outlined above, three foods were created for experiment 2 (electronic supplementary material, table S2) containing different phosphorus contents (0.45, 1.45 or 2.45% Ph in total nutrients) but identical P : C ratios (food A = 23P : 23C : 0.21Ph; B = 23P : 23C : 0.68Ph; C = 23P : 23C : 1.14Ph). Approximately 10 crickets of each sex were individually provided simultaneously with either two foods that differed only in phosphorus content (food pair 1 = A and B; pair 2 = B and C; pair 3 = A and C) or two identical foods as a control (pair 4 = B and B). We tested a total of 43 females and 44 males. Diet trials lasted 12 days, with fresh food provided every 72 h. To minimize the influence of spatial and temporal variation in greenhouse environmental conditions on our results, we used a randomized block design to determine treatment (food pair) distribution order, and we alternated the position of paired food types within each container every 72 h.
For experiment 3, five foods were created (electronic supplementary material, table S3) containing identical phosphorus contents (all with 1.45% Ph in total nutrients): two pure protein (food D = 46P : 0C : 0.68Ph; E = 76P : 0C : 1.12Ph), two pure carbohydrate (F = 0P : 46C : 0.68Ph; G = 0P : 76C : 1.12Ph) and one control with equal protein and carbohydrate (H = 23P : 23C : 0.68Ph). Approximately 10 crickets of each sex were individually provided simultaneously with one pure protein and one pure carbohydrate food (food pair 5 = D and F; pair 6 = E and G; pair 7 = D and G; pair 8 = E and F) or two identical foods as a control (pair 9 = H and H). We tested a total of 54 females and 54 males. Diet trials were performed in the same manner as described for experiment 2.
(ii). Statistical analyses
To determine whether crickets preferentially consumed more of one food type within each treatment pair and whether the consumption of each food type differed between the sexes, we used general linear mixed models in JMP (v. 10; SAS Institute Inc.). We included total intake of each food as the dependent variable, individual identity and block as random effects, initial body size PC1 as a covariate, and food, sex and the interaction term sex × food as fixed effects. The interaction sex × food was removed from all final models as it was not significant [35]. The PC1 axes explained 75.5% (eigenvalue = 1.51) and 78.7% (eigenvalue = 1.57) of the observed size variation of males and females, respectively, in experiment 2. Similarly, PC1 axes for experiment 3 explained 83.6% (eigenvalue = 1.67) and 72.7% (eigenvalue = 1.45) of the observed variation in size of males and females, respectively.
To examine the strength of nutrient intake regulation across treatment pairs differing in total nutrient concentration, we compared the total intake of phosphorus across treatment pairs 1–3 using an analysis of variance (ANOVA) and pairwise Tukey's HSD contrasts, and compared the total intake of carbohydrate and protein across food pairs 5–8 using a multivariate analysis of variance (MANOVA) and pairwise Tukey's HSD contrasts. Significant differences in nutrient intake across food pairs would indicate weak or no regulation of nutrient intake, whereas no significant differences would indicate strong nutrient intake regulation.
The average dietary phosphorus consumed was found by calculating mean percentage phosphorus intake (as a percentage of total nutrients) across treatment pairs 1–3. Similarly, average P : C consumed was found by calculating mean P : C across diet pairs 5–8. Controls were not used in mean calculations as these pairs did not allow the expression of diet choice. ANCOVAs were used to determine whether the average dietary phosphorus or P : C consumed differed between the sexes.
3. Results
(a). Experiment 1: influence of dietary nutrient balance on fitness traits
(i). Male response surfaces
Protein and carbohydrate intake influenced male weight gain, lifespan and mate signalling (electronic supplementary material, table S4, and figures S2 and S3; figure 1a–c), whereas phosphorus intake only significantly influenced mate signalling (electronic supplementary material, table S4 and figure S3). Smaller males gained more weight relative to body size than larger males (electronic supplementary material, table S4; β = −6.904 ± 1.667, p < 0.001), and weight gain increased linearly with protein intake (β = 0.266 ± 0.048, p < 0.001). Lifespan was positively influenced by carbohydrate intake (electronic supplementary material, table S4; β = 0.028 ± 0.004, p < 0.001). A significant negative quadratic relationship with carbohydrate intake (electronic supplementary material, table S4; β = −7.4 × 10−5 ± 1.6 × 10−5, p < 0.001) indicates that lifespan increased with moderate carbohydrate intake, but declined at higher carbohydrate intakes. Time spent signalling was positively influenced by the intake of both protein (electronic supplementary material, table S4; β = 0.032 ± 0.004, p < 0.001) and carbohydrate (β = 0.041 ± 0.005, p < 0.001). Interchirp duration was positively influenced by phosphorus intake (electronic supplementary material, table S4; β = 0.333 ± 0.126, p = 0.009), and negatively influenced by initial body size (β = −0.371 ± 0.134, p = 0.006), protein intake (β = −0.023 ± 0.004, p < 0.001) and carbohydrate intake (β = −0.025 ± 0.004, p < 0.001). Chirp rate was negatively influenced by phosphorus intake (electronic supplementary material, table S4; β = −1.648 ± 0.641, p = 0.010), and positively influenced by protein intake (β = 0.107 ± 0.019, p < 0.001) and carbohydrate intake (β = 0.121 ± 0.020, p < 0.001). All other male sexual signalling parameters (except for chirp duration) were primarily influenced by initial body size, and carbohydrate and protein intakes (electronic supplementary material, table S4 and figure S2).
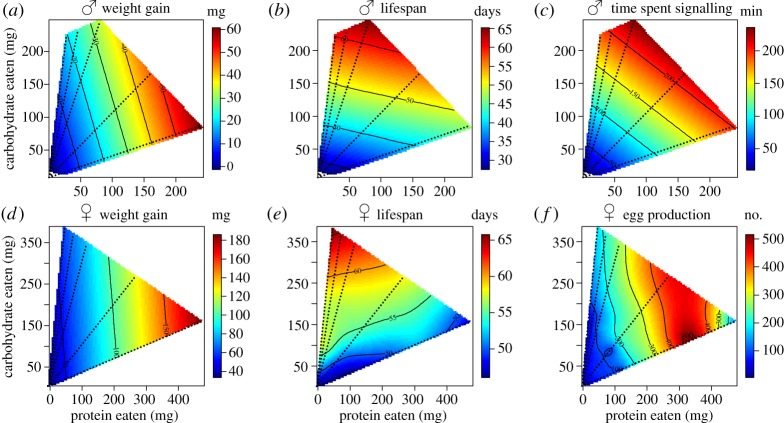
Figure 1.
(a–f) Response surfaces illustrating the effects of dietary protein (P) and carbohydrate (C) intake on fitness traits in male and female G. veletis. Dotted lines represent nutritional rails defined by the five P : C ratios available in experimental foods, and the colour scale depicts the magnitude of the dependent response variable. Response surfaces for male signalling traits other than time spent signalling can be found in the electronic supplementary material, figures S2 and S3.
Diet influenced trade-offs between male fitness traits (figure 1a–c; electronic supplementary material, figures S2 and S3, and table S6). Weight gain was maximized on approximately a 3P : 1C nutrient ratio and lifespan on approximately a 1P : 3C nutrient ratio. By contrast, most acoustic mate signalling parameters increased with increasing total protein and carbohydrate intake, regardless of nutrient ratio, and could therefore be maximized on both low P : C diets that maximize lifespan or high P : C diets that maximize weight gain.
(ii). Female response surfaces
Protein and carbohydrate intake influenced female weight gain, lifespan and egg production (electronic supplementary material, table S4; figure 1d–f), whereas phosphorus did not significantly influence any female fitness trait (electronic supplementary material, table S4). Smaller females gained more weight relative to body size than larger females (electronic supplementary material, table S4; β = −8.267 ± 2.355, p = 0.001) and weight gain increased linearly with protein intake (β = 0.311 ± 0.045, p < 0.001). Lifespan was significantly positively correlated with increasing carbohydrate intake (electronic supplementary material, table S4; β = 0.057 ± 0.015, p < 0.001). Females with a large initial body size laid more eggs (electronic supplementary material, table S4; β = 0.546 ± 0.161, p = 0.001) and egg production increased linearly with protein intake (β = 0.080 ± 0.007, p < 0.001). A significant negative quadratic relationship with protein intake (electronic supplementary material, table S4; β = −1.3 × 10−4 ± 2.2 × 10−5, p < 0.001) indicates that egg production increased with moderate protein intake and declined with higher protein intakes. Diet influenced trade-offs between female fitness traits (figure 1d–f; electronic supplementary material, table S6). Weight gain and egg production were maximized on a 3P : 1C nutrient ratio, whereas lifespan was maximized on a 1P : 8C nutrient ratio.
(iii). Between-sex comparison of response surfaces
The influence of nutrient intake on fitness traits was sex-specific (electronic supplementary material, table S7). Male and female lifespan (figure 1b,e) significantly differed in the linear and quadratic effects of nutrient intake (electronic supplementary material, table S7). Although both male and female lifespan increased with carbohydrate intake, female lifespan increased at a steeper slope with carbohydrate intake, and only male lifespan showed a quadratic relationship with carbohydrate intake (electronic supplementary material, table S4). Male and female reproductive proxies (figure 1c,f) significantly differed in the linear effects of nutrient intake (electronic supplementary material, table S7); both time spent signalling and egg production increased linearly with protein intake, with time spent signalling also being influenced by carbohydrate intake (electronic supplementary material, table S4). Male and female weight gain (figure 1a,d) did not significantly differ in the linear or quadratic effects of nutrient intake (electronic supplementary material, table S7). All statistics are presented in the electronic supplementary material, tables S4 and S7.
(iv). Intake regulation
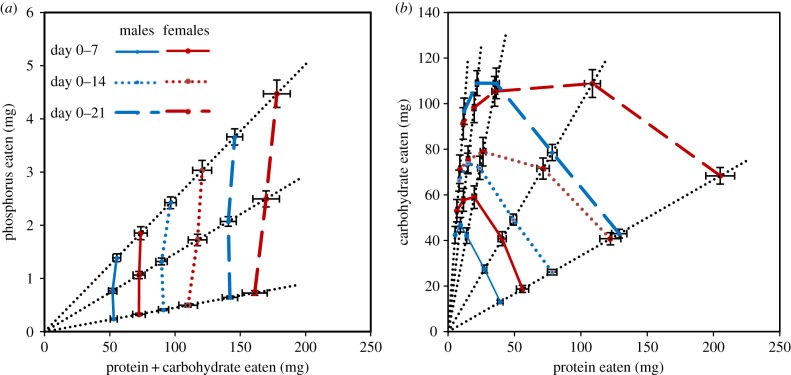
Male and female cumulative intake of phosphorus was plotted against macronutrient (P + C) intake over the first 21 days of adulthood to examine nutrient intake regulation when given no choice of foods (figure 2a). Phosphorus intake increased proportionately with its concentration relative to macronutrients (P + C), indicating complete priority of macronutrient intake regulation over phosphorus and providing no evidence for regulation of phosphorus intake. When the cumulative intake of protein was plotted against carbohydrate intake over the first 21 days of adulthood (figure 2b), the resulting intake array indicated that both protein and carbohydrates contributed to regulation of food intake, with evidence for a decrease in intake when crickets were provided with the most extreme high-carbohydrate diets (figure 2b).
Figure 2.
Mean (±s.e.) cumulative intakes across the first 21 days of adulthood for (a) phosphorus relative to protein and carbohydrate (P + C), and (b) protein relative to carbohydrate. Black dotted lines represent nutritional rails defined by (a) the three phosphorus levels and (b) the five different P : C ratios available in experimental foods (electronic supplementary material, table S1).
(b). Experiment 2: influence of dietary phosphorus on diet selection
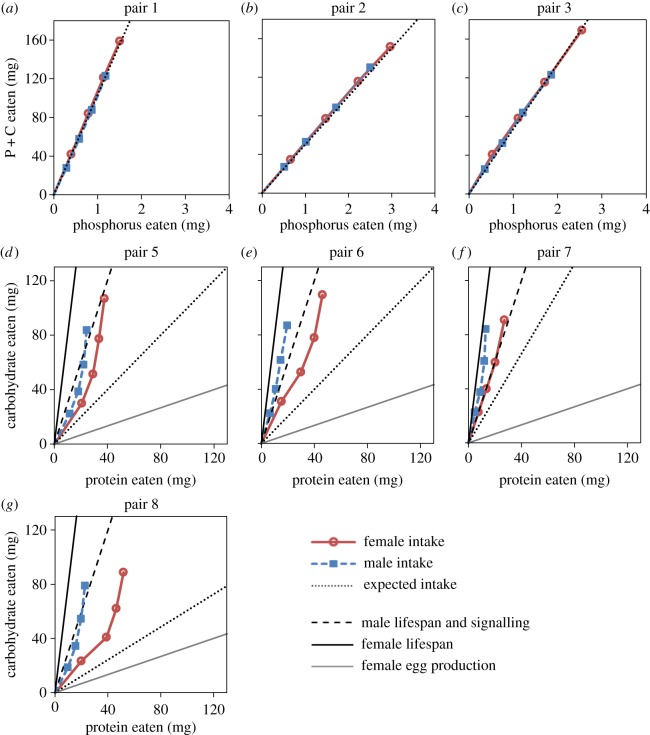
When given a choice, crickets did not preferentially consume high-phosphorus over low-phosphorus diets. There was no difference in total intake between foods in any of the treatment pairs (electronic supplementary material, figure S4 and table S8; pairs 1–4, all p ≥ 0.071). Equal consumption of both foods resulted in the amount of phosphorus eaten closely matching the expected phosphorus intake if crickets were eating randomly between diets (figure 3a–c). Initial body size had no effect on total intake of foods (electronic supplementary material, table S8; pairs 1–4, all p ≥ 0.120). In two of the four treatment pairs, females consumed significantly more than males irrespective of food or body size (electronic supplementary material, table S8; pair 1, p = 0.009; pair 4, p = 0.007). Total phosphorus intake significantly differed across treatment pairs 1–3 (ANOVA: F2,127 = 12.514, p < 0.001), with significant pairwise Tukey's HSD contrasts for all comparisons (p ≤ 0.007) except for treatment pairs 2 and 3 (p = 0.138), indicating weak or no regulation of phosphorus intake. Adjusted mean (±s.e.) percentage phosphorus consumed for males and females was 1.45 ± 0.08 and 1.47 ± 0.08, respectively, and did not differ between the sexes after controlling for initial body size (ANCOVA: F1, 62 = 0.020, p = 0.888).
Figure 3.
Cumulative intakes over 72 h intervals for (a–c) phosphorus relative to protein + carbohydrate (P + C) intake in experiment 2, and (d–g) protein relative to carbohydrate intake in experiment 3 for males (blue) and females (red). Black dotted lines indicate the intake ratio expected if crickets were eating foods indiscriminately. Black dashed, black solid and grey solid lines indicate the intake ratios in experiment 1 that maximized male lifespan and signalling, female lifespan and female egg production, respectively.
(c). Experiment 3: influence of dietary P : C ratio on diet selection
When given a choice, crickets consumed more of the pure carbohydrate food in all treatment pairs (electronic supplementary material, figure S5 and table S8; figure 3d–g; pairs 5–8, all p ≤ 0.001), resulting in an ingested P : C ratio < 1. Unequal consumption of foods differing in P and C content resulted in divergence from the P : C intake expected if crickets were eating randomly between diets (figure 3d–g). Initial body size was significantly positively correlated with total consumption in two of the five treatment pairs (electronic supplementary material, table S8; pair 6, p = 0.007; pair 8, p = 0.006). In one of the five treatment pairs, females consumed significantly more than males irrespective of food or body size (electronic supplementary material, table S8; pair 6, p = 0.012). Total intake of protein and carbohydrate did not significantly differ across treatment pairs 5–8 (MANOVA: Pillai's trace = 0.040, F6,336 = 1.134, p = 0.342), indicating strong regulation of P : C intake. Females consumed higher P : C ratios than males (ANCOVA: F1,83 = 6.401, p = 0.013); the adjusted mean (±s.e.) protein to carbohydrate ratio across treatment pairs 5–8 was 0.44 ± 0.06 (1.3P : 3C) for females and 0.24 ± 0.06 (1P : 4.1C) for males.
4. Discussion
(a). Experiment 1: influence of dietary nutrient balance on fitness traits
Animals often experience a mismatch between their nutrient requirements and the composition of available foods in nature, where diet quality and quantity affect the pool of resources available for allocation towards different fitness traits [36]. Many ES studies suggest that the key to fitness is phosphorus availability [13,16,17]. By contrast, many NG studies suggest that the availability of carbohydrates, lipids and proteins are key to fitness [2,5,6]. Given that food components interact in their influence, and such interactions are powerful mediators of the relationship between diet and fitness, we investigated (using the NG framework) how fitness trait expression was constrained by concomitant variation in phosphorus, protein and carbohydrates. Our results revealed that weight gain, reproductive performance and lifespan in adult field crickets (G. veletis) were maximized on diets with different protein to carbohydrate ratios (P : C), and that the optimal nutrient intake ratio for reproductive performance was sex-specific. Surprisingly, phosphorus intake influenced very few of these fitness traits, and there was no evidence that phosphorus intake was regulated. When not provided with a choice of foods, both sexes prioritized protein and carbohydrate intake over phosphorus intake, whereas protein and carbohydrate intake were both regulated relatively equally (figure 2b, convex arc intake array; sensu [5]), with a decrease in intake evident with the lowest P : C foods.
Similar to previous studies [10,22,37], we demonstrated that G. veletis cannot simultaneously maximize all fitness traits, and thus experienced trade-offs between fitness traits depending on the nutritional composition of available foods. Adult weight gain and egg production were maximized on high-protein diets (3P : 1C), whereas adult lifespan was maximized on high-carbohydrate diets (males: 1P : 3C; females: 1P : 8C) and acoustic mate signalling effort was maximized with increased food intake regardless of nutrient ratio.
Males and females are expected to differ in their nutrient requirements as a result of contrasting reproductive strategies; females allocate more resources towards gamete production, whereas males allocate more resources towards attracting mates [7,29,30]. The effects of nutrient intake on proxies of reproductive success significantly differed between the sexes; egg production was maximized on a 3P : 1C nutrient ratio, whereas most parameters of male sexual signalling were maximized by increasing total carbohydrate or protein intake regardless of dietary nutrient ratio. These results support our prediction that male and female crickets maximize reproductive success in different areas of the nutritional landscape, consistent with most previous studies. For example, 1P : 1C diets are necessary for maximal egg production and high-carbohydrate diets are necessary for maximal signalling effort in Teleogryllus commodus [10], whereas high protein diets result in maximal signalling effort in male Gryllus campestris crickets [37]. Gryllus veletis males in the current study maximized mate signalling with either high protein or carbohydrate intake, suggesting that signalling is a function of caloric intake rather than intake of carbohydrates or protein alone. Dependence on caloric intake reflects the high energetic costs of acoustic signalling [38,39].
Dietary trade-offs between lifespan, weight gain and acoustic signalling effort have important implications for cricket fitness, as older and/or larger males attract more females and have higher mating success [40,41]. Furthermore, females typically prefer males displaying higher-energy signals [39,42]. Given that higher-energy signals are more costly to produce [38,39] and are influenced by nutritional status [10,26,27,43], these signals may provide females with reliable information on indirect fitness benefits of mate choice (e.g. good genes for foraging in offspring). However, our assumption that increasing body mass signifies a fitness gain may be invalid, as increasing weight was associated with lethargy and reduced longevity in our study, possibly resulting from urate crystal formation inside fat body cells (see the electronic supplementary material). This supports recent criticisms of the use of residual mass as an estimate of body condition without distinguishing the components that contribute to body mass [44].
As female crickets tend to prefer males who signal with faster chirp rates [28,45], our finding that phosphorus intake results in slower chirp rates (through increased interchirp duration) suggests high phosphorus intake may potentially have negative fitness consequences. Our finding that phosphorus did not positively influence adult G. veletis fitness traits was unexpected given previous dietary studies. For instance, increasing the phosphorus intake of juvenile invertebrates positively influences growth, survival and later reproduction [31,36]. Similar to our current study, Bertram et al. [27] and Visanuvimol & Bertram [46] examined the influence of adult dietary phosphorus content on cricket fitness traits. However, in contrast to our current study, those studies found that increasing phosphorus in the diets of adult crickets promoted egg production [46] and mate signalling effort [27]. We only detected negative fitness consequences for phosphorus intake even though the range of total dietary phosphorus content used in this study (0.07–1.87%) encompasses the range used in the aforementioned studies (0.2–1%; [27,46]). A plausible explanation for the discrepancy between this and similar studies is that the lifetime phosphorus requirements of our study crickets may have been fulfilled during development, as our juvenile crickets were reared on phosphorus-rich (1.1% total Ph) diets, whereas the diets of juvenile crickets in previous studies were unknown for the first four to five instars following hatching [27,46]. However, we also reared juveniles on protein- and carbohydrate-adequate diets, suggesting that there might be fundamental differences in the critical ontogenetic periods and time scales over which macronutrients and phosphorus are required, with macronutrients dictating the biological responses at a finer temporal resolution than phosphorus. This would have important implications for ecological models of nutrient limitation, and warrants further attention.
As phosphorus is a component of various dietary biomolecules (e.g. protein: casein = 0.8% and albumen = 0.12% phosphorus), previous findings that phosphorus positively influences fitness may have been confounded by correlations between levels of dietary phosphorus and protein. Our study carefully accounted for the phosphorus contained within all nutrient sources, such that phosphorus was varied independently of carbohydrates and protein. It is also unlikely that our findings resulted from using a metabolically in-digestible phosphorus source since our CaHPO4 source has been used in studies, wherein cricket fitness was significantly influenced by phosphorus [27,46].
(b). Experiments 2 and 3: influence of nutrient ratio on diet selection
When faced with nutritional imbalances, organisms may compensate behaviourally by altering the amount they eat or selecting alternative food sources [32]. We predicted that G. veletis would behaviourally regulate the intakes of protein and carbohydrate [10,12,47], but not phosphorus, because previous insect studies have shown that bodily phosphorus content covaries with dietary phosphorus levels [17,31]. Our results support these predictions: crickets consumed significantly more carbohydrate than protein when given the choice. Further, crickets did not differ in their total carbohydrate or protein intake across food treatment pairs, suggesting tight regulation of nutrient intake. Carbohydrate and protein regulation is facilitated by receptors in mouthparts and tarsi, which become more sensitive when certain blood metabolite levels are low, stimulating feeding [2,48]. Conversely, crickets indiscriminately fed on different phosphorus diets and total phosphorus intake differed across food treatment pairs, indicating that phosphorus intake is not regulated.
As males and females have contrasting reproductive strategies [7,29,30], and male and female reproductive proxies were maximized in different areas on the nutritional landscape in this study, we predicted that the sexes would differ in their diet choice. As predicted, males and females ate significantly different protein to carbohydrate ratios (females: 1.33Pc : 3C; males: 1P : 4.1C), but did not significantly differ in the percentage of phosphorus consumed. Female preference for higher-protein diets probably reflects a higher protein requirement for egg production [30]. The self-selected P : C ratio ingested presumably reflects the ratio of nutrients required for optimal somatic and metabolic functioning in adult G. veletis (‘intake target’, sensu [5]). The 1P : 4.1C nutrient ratio self-selected by males in experiment 3 is similar to the 1P : 3C ratio that would simultaneously maximize lifespan and signalling in experiment 1 (figure 3d–g). Conversely, the 1.33P : 3C nutrient ratio selected by females in experiment 3 lies between the 1P : 8C and 3P : 1C ratios that maximized lifespan and egg production, respectively (figure 3d–g). Similar to previous findings [10], our results suggest that females select a P : C ratio that represents a compromise between the different nutritional requirements of egg production and lifespan, whereas male lifespan and mate attraction signalling can be maximized without such compromising.
5. Conclusion
Our study is the first to use the NG framework to examine the relative contributions of dietary phosphorus and macronutrients (protein and carbohydrates) to intake regulation and fitness traits. Although other studies have shown that phosphorus influences invertebrate fitness [17,27,31,36,46], these studies were not designed to vary phosphorus orthogonally to protein and carbohydrates, and did not quantify the relative individual and interactive contributions of each nutrient to the animals' responses. By varying nutrients independently, we were able to measure the individual as well as interactive effects of phosphorus, protein and carbohydrate on a suite of fitness traits in male and female crickets. Our findings reveal that variation in phosphorus in the diets of adult field crickets is less important in influencing fitness traits when it is constrained by concomitant variation in dietary protein and carbohydrates, as would be the case for the foods available to adult crickets in the wild. By contrast, adult cricket fitness traits were strongly influenced by protein and carbohydrate intake, irrespective of dietary phosphorus levels. Importantly, dietary macronutrient content influenced trade-offs between fitness traits, such that not all traits could be simultaneously maximized with any given diet. The response of fitness traits to dietary nutrient intake was also sex-specific: while both lifespan and weight gain were maximized in similar regions of the nutritional landscape, the sexes optimized reproductive traits in different regions of the nutritional landscape. Given these strong effects of macronutrients relative to phosphorus on performance, it is not surprising that adult crickets regulated the intake of protein and carbohydrate but not phosphorus. Overall, our findings highlight the importance of disentangling the influences of different nutrients, and quantifying both their individual and interactive effects, on animal fitness traits, so as to gain a more integrative understanding of their nutritional ecology. Important priorities for future research are to investigate the effects of protein, carbohydrate and phosphorus on G. veletis over the entire life cycle, and to establish the generality of our results across a taxonomically and ecologically diverse range of organisms.
Supplementary Material
Acknowledgements
We thank Sandra South for her advice on creating experimental diets, and Dr Richard Inger and one anonymous reviewer for their constructive comments on the manuscript.
Data accessibility
All raw data for experiments 1–3 are accessible at Dryad: doi:10.5061/dryad.5m8p3.
Funding statement
This work was funded through research grants from the Natural Sciences and Engineering Research Council (NSERC) of Canada, Canadian Foundation for Innovation, Ontario Research Fund and Carleton University Research Fund to S.M.B., and NSERC Canadian Graduate Scholarship and Ontario Graduate Scholarship to S.J.H. J.-G.J.G. was funded by NSERC, S.J.S. by an Australian Research Council Laureate Fellowship, and D.R. was part-funded by Gravida: the National Research Centre for Growth and Development, New Zealand.
References
- 1.Sterner RW, Elser JJ. 2002. Ecological stoichimetry: the biology of elements from molecules to the biosphere. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Simpson SJ, Raubenheimer D. 2012. The nature of nutrition: a unifying framework from animal adaptation to human obesity. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Raubenheimer D, Simpson SJ, Mayntz D. 2009. Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct. Ecol. 23, 4–16. ( 10.1111/j.1365-2435.2008.01522.x) [DOI] [Google Scholar]
- 4.Elser JJ. 2006. Biological stoichiometry: a chemical bridge between ecosystem ecology and evolutionary biology. Am. Nat. 168, S25–S35. ( 10.1086/509048) [DOI] [PubMed] [Google Scholar]
- 5.Raubenheimer D, Simpson SJ. 1993. The geometry of compensatory feeding in the locust. Anim. Behav. 45, 953–964. ( 10.1006/anbe.1993.1114) [DOI] [Google Scholar]
- 6.Simpson SJ, Raubenheimer D. 1993. A multi-level analysis of feeding behaviour: the geometry of nutritional decisions. Phil. Trans. R. Soc. Lond. B 342, 381–402. ( 10.1098/rstb.1993.0166) [DOI] [Google Scholar]
- 7.Morehouse NI, Nakazawa T, Booher CM, Jeyasingh PD, Hall MD. 2010. Sex in a material world: why the study of sexual reproduction and sex-specific traits should become more nutritionally-explicit. Oikos 119, 766–778. ( 10.1111/j.1600-0706.2009.18569.x) [DOI] [Google Scholar]
- 8.Raubenheimer D, Simpson SJ. 2006. The challenge of supplementary feeding: can geometric analysis help save the kakapo? Notornis 53, 100–111. [Google Scholar]
- 9.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JWO, Taylor PW, Soran N, Raubenheimer D. 2008. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl Acad. Sci. USA 105, 2498–2503. ( 10.1073/pnas.0710787105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J, Clissold F, Raubenheimer D, Bonduriansky R, Brooks RC. 2008. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Curr. Biol. 18, 1062–1066. ( 10.1016/j.cub.2008.06.059) [DOI] [PubMed] [Google Scholar]
- 11.Mayntz D, Nielsen VH, Sørensen A, Toft S, Raubenheimer D, Hejlesen C, Simpson SJ. 2009. Balancing of protein and lipid intake by a mammalian carnivore, the mink, Mustela vison. Anim. Behav. 77, 349–355. ( 10.1016/j.anbehav.2008.09.036) [DOI] [Google Scholar]
- 12.South SH, House CM, Moore AJ, Simpson SJ, Hunt J. 2011. Male cockroaches prefer a high carbohydrate diet that makes them more attractive to females: implications for the study of condition dependence. Evolution 65, 1594–1606. ( 10.1111/j.1558-5646.2011.01233.x) [DOI] [PubMed] [Google Scholar]
- 13.Elser JJ, et al. 2000. Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578–580. ( 10.1038/35046058) [DOI] [PubMed] [Google Scholar]
- 14.Elser JJ, et al. 2003. Growth rate–stoichiometry couplings in diverse biota. Ecol. Lett. 6, 936–943. ( 10.1046/j.1461-0248.2003.00518.x) [DOI] [Google Scholar]
- 15.Anderson TR, Pond DW. 2000. Stoichiometric theory extended to micronutrients: comparison of the roles of essential fatty acids, carbon, and nitrogen in the nutrition of marine copepods. Limnol. Oceanogr. 45, 1162–1167. ( 10.4319/lo.2000.45.5.1162) [DOI] [Google Scholar]
- 16.Bertram SM, Schade JD, Elser JJ. 2006. Signalling and phosphorus: correlations between mate signalling effort and body elemental composition in crickets. Anim. Behav. 72, 899–907. ( 10.1016/j.anbehav.2006.02.012) [DOI] [Google Scholar]
- 17.Schade JD, Kyle M, Hobbie SE, Fagan WF, Elser JJ. 2003. Stoichiometric tracking of soil nutrients by a desert insect herbivore. Ecol. Lett. 6, 96–101. ( 10.1046/j.1461-0248.2003.00409.x) [DOI] [Google Scholar]
- 18.Criddle N. 1925. Field crickets in Manitoba. Can. Entomol. 57, 79–84. ( 10.4039/Ent5779-4) [DOI] [Google Scholar]
- 19.Gangwere SK. 1961. A monograph on food selection in Orthoptera. Trans. Am. Entomol. Soc. 87, 67–230. [Google Scholar]
- 20.Carmona DM, Menalled FD, Landis DA. 1999. Gryllus pennsylvanicus (Orthoptera: Gryllidae): laboratory weed seed predation and within field activity-density. J. Econ. Entomol. 92, 825–829. [Google Scholar]
- 21.Bertram SM, Bowen M, Kyle M, Schade JD. 2008. Extensive natural intraspecific variation in stoichiometric (C : N : P) composition in two terrestrial insect species. J. Insect Sci. 8, 1–7. ( 10.1673/031.008.2601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joern A, Behmer ST. 1997. Importance of dietary nitrogen and carbohydrates to survival, growth, and reproduction in adults of the grasshopper Ageneotettix deorum (Orthoptera: Acrididae). Oecologia 112, 201–208. ( 10.1007/s004420050301) [DOI] [PubMed] [Google Scholar]
- 23.Woods HA, Fagan WF, Elser JJ, Harrison JF. 2004. Allometric and phylogenetic variation in insect phosphorus. Funct. Ecol. 18, 103–109. ( 10.1111/j.1365-2435.2004.00823.x) [DOI] [Google Scholar]
- 24.Raubenheimer D, Jones SA. 2006. Nutritional imbalance in an extreme generalist omnivore: tolerance and recovery through complementary food selection. Anim. Behav. 71, 1253–1262. ( 10.1016/j.anbehav.2005.07.024) [DOI] [Google Scholar]
- 25.Alexander RD. 1961. Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae). Behaviour 17, 130–223. ( 10.1163/156853961X00042) [DOI] [Google Scholar]
- 26.Holzer B, Jacot A, Brinkhof MWG. 2003. Condition-dependent signaling affects male sexual attractiveness in field crickets, Gryllus campestris. Behav. Ecol. 14, 353–359. ( 10.1093/beheco/14.3.353) [DOI] [Google Scholar]
- 27.Bertram SM, Whattam EM, Visanuvimol L, Bennett R, Lauzon C. 2009. Phosphorus availability influences cricket mate attraction displays. Anim. Behav. 77, 525–530. ( 10.1016/j.anbehav.2008.11.012) [DOI] [Google Scholar]
- 28.Wagner WE, Reiser MG. 2000. The importance of calling song and courtship song in female mate choice in the variable field cricket. Anim. Behav. 59, 1219–1226. ( 10.1006/anbe.1999.1428) [DOI] [PubMed] [Google Scholar]
- 29.Kodric-Brown A, Brown JH. 1987. Anisogamy, sexual selection, and the evolution and maintenance of sex. Evol. Ecol. 1, 95–105. ( 10.1007/BF02067393) [DOI] [Google Scholar]
- 30.Wheeler D. 1996. The role of nourishment in oogenesis. Annu. Rev. Entomol. 41, 407–431. ( 10.1146/annurev.en.41.010196.002203) [DOI] [PubMed] [Google Scholar]
- 31.Perkins MC, Woods HA, Harrison JF, Elser JJ. 2004. Dietary phosphorus affects the growth of larval Manduca sexta. Arch. Insect Biochem. Physiol. 55, 153–168. ( 10.1002/arch.10133) [DOI] [PubMed] [Google Scholar]
- 32.Simpson SJ, Abisgold JD. 1985. Compensation by locusts for changes in dietary nutrients: behavioural mechanisms. Physiol. Entomol. 10, 443–452. ( 10.1111/j.1365-3032.1985.tb00066.x) [DOI] [Google Scholar]
- 33.Fitzsimmons LP, Bertram SM. 2011. The calling songs of male spring field crickets (Gryllus veletis) change as males age. Behaviour 148, 1045–1065. ( 10.1163/000579511X588812) [DOI] [Google Scholar]
- 34.Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution 37, 1210–1226. ( 10.2307/2408842) [DOI] [PubMed] [Google Scholar]
- 35.Engqvist L. 2005. The mistreatment of covariate interaction terms in linear model analyses of behavioural and evolutionary ecology studies. Anim. Behav. 70, 967–971. ( 10.1016/j.anbehav.2005.01.016) [DOI] [Google Scholar]
- 36.Urabe J, Sterner RW. 2001. Contrasting effects of different types of resource depletion on life-history traits in Daphnia. Funct. Ecol. 15, 165–174. ( 10.1046/j.1365-2435.2001.00511.x) [DOI] [Google Scholar]
- 37.Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL, Bussière LF. 2004. High-quality male field crickets invest heavily in sexual display but die young. Nature 432, 1024–1027. ( 10.1038/nature03084) [DOI] [PubMed] [Google Scholar]
- 38.Prestwich KN, Walker TJ. 1981. Energetics of singing in crickets: effect of temperature in three trilling species (Orthoptera: Gryllidae). J. Comp. Physiol. B 143, 199–212. [Google Scholar]
- 39.Hoback WW, Wagner WE. 1997. The energetic cost of calling in the variable field cricket, Gryllus lineaticeps. Physiol. Entomol. 22, 286–290. ( 10.1111/j.1365-3032.1997.tb01170.x) [DOI] [Google Scholar]
- 40.Bateman PW, Gilson LN, Ferguson JWH. 2001. Male size and sequential mate preference in the cricket Gryllus bimaculatus. Anim. Behav. 61, 631–637. ( 10.1006/anbe.2000.1617) [DOI] [Google Scholar]
- 41.Zuk M. 1988. Parasite load, body size, and age of wild-caught male field crickets (Orthoptera: Gryllidae): effects on sexual selection. Evolution 42, 969–976. ( 10.2307/2408912) [DOI] [PubMed] [Google Scholar]
- 42.Popov AV, Shuvalov VF. 1977. Phonotactic behavior of crickets. J. Comp. Physiol. A 119, 111–126. ( 10.1007/BF00655876) [DOI] [Google Scholar]
- 43.Scheuber H, Jacot A, Brinkhof MWG. 2003. Condition dependence of a multicomponent sexual signal in the field cricket Gryllus campestris. Anim. Behav. 65, 721–727. ( 10.1006/anbe.2003.2083) [DOI] [Google Scholar]
- 44.Cotton S, Fowler K, Pomiankowski A. 2004. Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc. R. Soc. Lond. B 271, 771–783. ( 10.1098/rspb.2004.2688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner WE. 1996. Convergent song preferences between female field crickets and acoustically orienting parasitoid flies. Behav. Ecol. 7, 279–285. ( 10.1093/beheco/7.3.279) [DOI] [Google Scholar]
- 46.Visanuvimol L, Bertram SM. 2010. Dietary phosphorus availability influences female cricket lifetime reproductive effort. Ecol. Entomol. 35, 386–395. ( 10.1111/j.1365-2311.2010.01195.x) [DOI] [Google Scholar]
- 47.Jones SA, Raubenheimer D. 2001. Nutritional regulation in nymphs of the German cockroach, Blattella germanica. J. Insect Physiol. 47, 1169–1180. ( 10.1016/S0022-1910(01)00098-1) [DOI] [PubMed] [Google Scholar]
- 48.Simpson SJ, Raubenheimer D. 1993. The central role of the haemolymph in the regulation of nutrient intake in insects. Physiol. Entomol. 18, 395–403. ( 10.1111/j.1365-3032.1993.tb00613.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data for experiments 1–3 are accessible at Dryad: doi:10.5061/dryad.5m8p3.