Abstract
Interspecific arms races between cuckoos and their hosts have produced remarkable examples of mimicry, with parasite eggs evolving to match host egg appearance and so evade removal by hosts. Certain bronze-cuckoo species, however, lay eggs that are cryptic rather than mimetic. These eggs are coated in a low luminance pigment that camouflages them within the dark interiors of hosts' nests. We investigated whether cuckoo egg crypsis is likely to have arisen from the same coevolutionary processes known to favour egg mimicry. We added high and low luminance-painted eggs to the nests of large-billed gerygones (Gerygone magnirostris), a host of the little bronze-cuckoo (Chalcites minutillus). Gerygones rarely rejected either egg type, and did not reject natural cuckoo eggs. Cuckoos, by contrast, regularly removed an egg from clutches before laying their own and were five times more likely to remove a high luminance model than its low luminance counterpart. Given that we found one-third of all parasitized nests were exploited by multiple cuckoos, our results suggest that competition between cuckoos has been the key selective agent for egg crypsis. In such intraspecific arms races, crypsis may be favoured over mimicry because it can reduce the risk of egg removal to levels below chance.
Keywords: crypsis, evolutionary arms race, brood parasitism, multiple parasitism, bronze-cuckoo
1. Introduction
Mimicry and crypsis have evolved in diverse taxa across the animal kingdom as a means to evade detection or identification [1]. Some of the most striking and best-documented examples of mimicry are observed in the eggs of obligate brood parasitic birds [2–4]. Parasites such as cuckoos and cowbirds lay their eggs in the nests of other species, which then incur the full costs of parental care on the parasite's behalf. Egg mimicry arises, because many hosts defend themselves against parasitism by recognizing and removing odd-looking eggs from the nest, thereby triggering a coevolutionary arms race in which parasites' eggs evolve to ever-more-closely resemble those of their hosts [5,6].
Egg crypsis is also known from brood parasitic birds [7,8] but, in contrast to egg mimicry, the evolutionary process by which it arises are poorly understood. Several species of Australasian bronze-cuckoos (Chalcites sp.) lay dark-coloured eggs that bear no visual resemblance to the white speckled eggs of their common hosts (Gerygone and Acanthiza spp.). These cuckoos' eggs appear to be unique among birds in three ways: they are an unusual olive to brown colour, they are extremely thickly coated in pigment, and the pigment is deposited in the outer cuticle rather than in the shell itself [8]. The thick, dark pigment on the eggshell dramatically reduces the luminance of the egg and visual modelling indicates that, through the eye of a bird, this renders the egg indistinguishable from the nest lining within the dark interior of their host's domed nests [8]. Phylogenetic analysis of the bronze-cuckoo clade shows that dark egg pigmentation is a derived trait, associated with exclusive parasitism of dome-nesting species, which is consistent with adaptive crypsis [8].
Two hypotheses for the evolution of egg crypsis in bronze-cuckoos are commonly proposed, which are not mutually exclusive. First, egg crypsis may have been favoured for the same reasons as egg mimicry in other parasites. That is, dark eggs may be difficult for hosts to detect and consequently to reject, and thus represent the outcome of an interspecific arms race between host and parasite. In this case, we are left with the puzzle of why crypsis, rather than mimicry, has evolved in this system, particularly given that at least one closely related cuckoo (Horsfield's bronze-cuckoo, Chalcites basalis) has evolved egg mimicry [9].
The second possibility draws on the observation that in bronze-cuckoo systems, host nests are often parasitized by more than one cuckoo [8,10]. Bronze-cuckoos remove an existing egg from the host's clutch at the time that they lay their own [10], so the egg of any first-to-lay cuckoo is vulnerable to removal by subsequent females that parasitize the nest. Indeed, because cuckoo nestlings evict all other eggs or chicks, it would be highly advantageous for female cuckoos to selectively remove any previously laid cuckoo eggs that they encounter [6]. This would, in turn, select for egg adaptations that reduce the risk of such removal. Crypsis then could be the outcome of an intraspecific arms race, in which cuckoo eggs are selected to evade detection by conspecifics competing for host nests [11]. In this case, there is a clear benefit of crypsis over mimicry, because the risk of removal of a mimetic egg would be equal to that of other eggs in the nest, whereas a cryptic egg would have a risk of removal lower than chance [12].
In this study, we tested both of the above hypotheses for little bronze-cuckoos (Chalcites minutillus) parasitizing their primary host [12], the large-billed gerygone (Gerygone magnirostris) in northeastern Australia. Large-billed gerygones are not reported to reject cuckoo eggs [13], but it is unknown whether this is, because the eggs are cryptic, or because these hosts lack defensive egg rejection altogether [14]. Multiple parasitism of large-billed gerygone nests occurs regularly, and genetic analysis has confirmed that this is due to laying by multiple females rather than maladaptive repeat laying by a single female [8]. We assessed the responses of both host and cuckoos to experimental clutches containing a natural host egg and two model eggs: one painted to be more cryptic (i.e. lower luminance), and the other to be less cryptic (i.e. higher luminance) than the host egg. If crypsis succeeds in reducing the visibility of eggs to either hosts or cuckoos, and each relies on vision to recognize eggs in the nest, then we expected the removal of our ‘cryptic’ model to occur at lower than chance rates.
2. Methods
(a). Model eggs
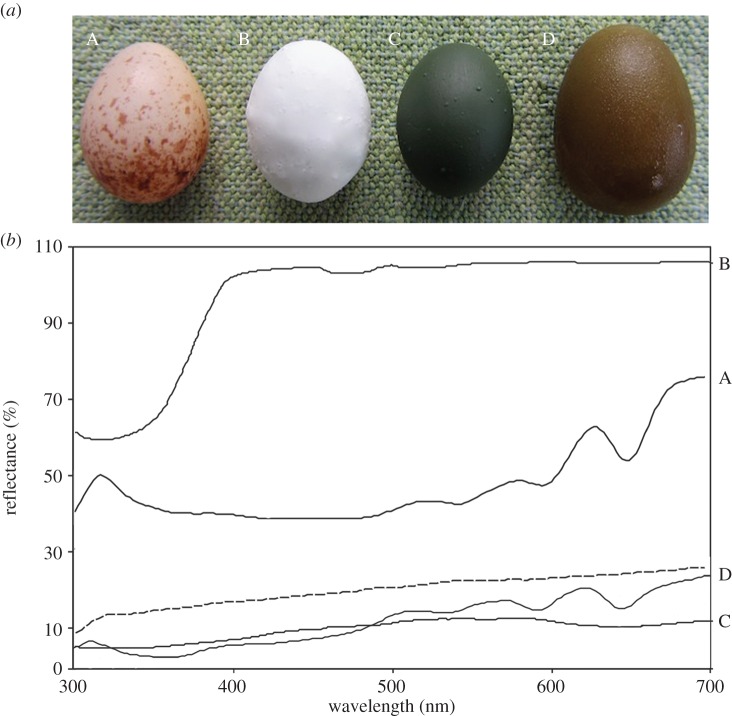
We used eggs of the domestic zebra finch (Taeniopygia guttata) painted with non-toxic paint (Reel Wings Bird Vision UV Decoy Range) as model eggs (figure 1a). Zebra finch eggs (15 × 11 mm) are slightly smaller on average than large-billed gerygone eggs (17 × 12 mm) though still within the natural range of variation [15]. The use of real eggs rather than plastic or clay eggs has two advantages. First, small-billed hosts are sometimes unable to grasp a whole egg in their bill and instead reject eggs by puncturing and then gripping the broken shell, so experiments using hard artificial eggs may underestimate natural egg rejection rates [16]. Second, the use of real eggs ensured that no harm came to cuckoos, which typically eat the eggs they remove [10]. We created two model types: (i) high luminance, or ‘non-cryptic’, eggs were painted bright white, and (ii) low luminance, or ‘cryptic’, eggs were painted dark olive. The reflectance of painted eggs was measured using an Ocean Optics (Dunedin, FL) EL200 Jaz spectrometer with a pulsed xenon light source and UV–vis QR400-7-SR reflectance probe. Figure 1b shows these reflectance spectra, alongside those of natural eggs and nest interiors measured during an earlier study (see [8]). Our high luminance models were brighter than gerygone eggs, and thus expected to be at least as visible as them inside the nests' dark interior. Furthermore, these models were sufficiently distinct from gerygone eggs that hosts might be expected to reject them if they routinely rejected ‘non-cryptic’ foreign eggs. Our low luminance models were less bright than gerygone eggs (and indeed, similar in brightness to cuckoo eggs), and thus expected to be less visible than gerygone eggs inside nests.
Figure 1.
(a) Example photographs and (b) mean reflectance spectra of eggs of the large-billed gerygone (‘A’, n = 17), high luminance model eggs (‘B’, n = 10) and low luminance (‘C’, n = 10) model eggs as used in our experiment, and eggs of the little bronze-cuckoo (‘D’, n = 25). Spectra are shown relative to that of gerygone nest interiors, indicated by the dotted line (n = 13). Sample sizes are those used to calculate mean spectra.
(b). Experimental method
We searched for large-billed gerygone nests along creeklines and billabong edges in and around Cairns, northeastern Australia (16°55′ S, 145°46′ E) during September–December 2013. Nests are untidy domed chambers made from grass, moss and spiders' egg-sacks, typically positioned overhanging water such that the nest resembles flood debris. Gerygones, at this site, typically lay a clutch of three eggs (median = 3, mean ± s.e. = 2.7 ± 0.7, range = 1–4, n = 63), with one egg laid every second day. Eggs are pinkish-white, covered with small pinkish-red freckles, and smaller than cuckoo eggs (cuckoo eggs: approx. 19 × 13 mm; figure 1a [12]). Incubation commences after clutch completion and lasts for 15–21 days [12].
A single experimental protocol was used to test for both host and cuckoo responses to model eggs. Nests that were located in construction were monitored until the appearance in the nest of the first gerygone egg, at that point, we added one high luminance and one low luminance model egg to the nest to create an experimental clutch of three eggs (figure 2a). We thereafter checked nests prior to 9.00 every second day. This time was subsequent to gerygone egg-laying, which occurs at dawn, but preceded any cuckoo egg-laying that day, which occurs mid-morning. If a nest check revealed that no eggs were missing, we removed that morning's freshly laid gerygone egg and stored it in a cool dark place. In this way, we maintained the size and composition of the experimental clutch until either an egg went missing from the nest or incubation began (i.e. 4–7 days, mean ± s.e. = 5.6 ± 0.3, n = 16). These events signalled the end of the experiment, at which point we removed model eggs and returned any gerygone eggs. If a nest check revealed an egg was missing and the nest had been parasitized, we considered the egg had been removed by the cuckoo. If an egg was missing, but the nest was unparasitized, we considered that the gerygones had rejected the egg. We then scored: (i) the proportion of nests at which hosts and cuckoos removed an egg or eggs during the experimental period, and (ii) the proportion of each egg type removed. Nests were further monitored once a week during incubation and chick rearing until chicks fledged or the nest was predated. Clutch manipulations did not adversely affect nest survival relative to nests that were already in incubation when located and thus not manipulated (14 of 56 versus nine of 32 nests, respectively, Fisher's exact test p = 0.8).
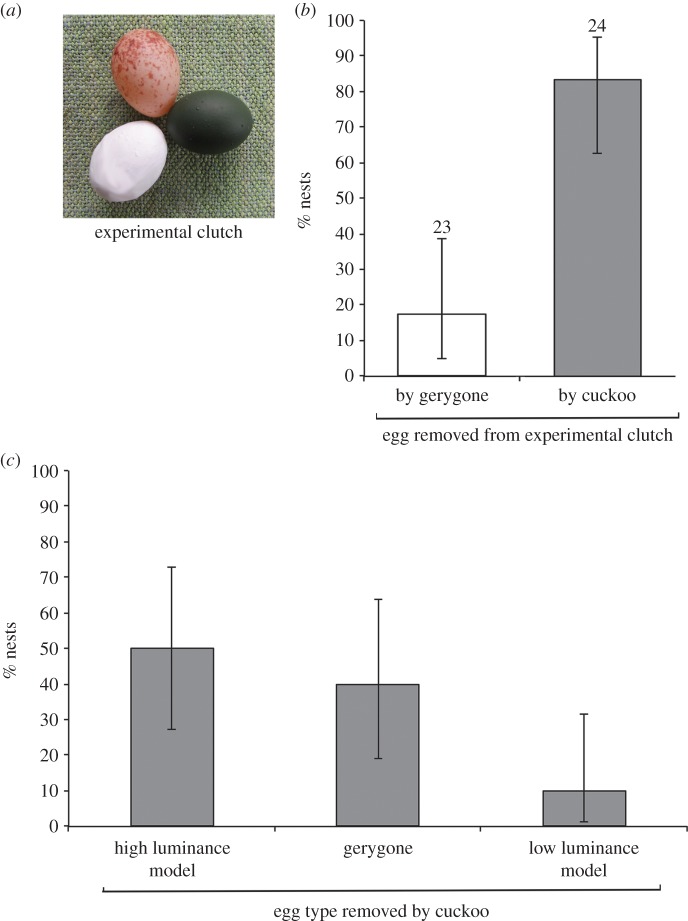
Figure 2.
(a) Experimental clutches comprised one gerygone egg, one high luminance model and one low luminance model; (b) the proportion of nests containing experimental clutches in which an egg or eggs were removed by gerygones (white bars, unparasitized nests) and by cuckoos (grey bars, parasitized nests), and (c) the proportion of each egg type that was removed by cuckoos (n = 20 nests). In one nest, multiple parasitism meant that it was unknown whether a cuckoo had removed a high luminance model or a gerygone egg. For the purposes of this graph, this nest has been added to the gerygone egg column. Numbers above bars indicate sample sizes and error bars represent 95% CI.
Assigning egg removal to either host or cuckoo based on a nest's parasitism status presented a risk of bias. This is because cuckoos sometimes do not remove an egg at the time of parasitism (11% of parasitism, see Results). Thus, if a particular cuckoo neglects to remove an egg when it lays, but the host at that nest happens to reject one egg on the day of parasitism, then the missing egg will be mistakenly assigned to the cuckoo. This risk of bias, however, is likely to be small in our dataset for two reasons. First, if hosts commonly rejected eggs on the same day that parasitism occurred, we would expect to see parasitized nests with two or more missing eggs, but this was rarely observed (one in 27 nests, 3.7%). Second, the incidence of parasitism without egg removal from experimental clutches was similar to that observed from natural clutches, which is consistent with egg removals at experimental clutches being correctly assigned (17% versus 11%, see Results).
(c). Statistical analyses
We used chi-squared tests and binomial tests (SPSS v.19) to compare the removal rates of high and low luminance model eggs with those expected under chance. The inclusion of a natural gerygone egg in the experimental clutch served two purposes. First, when assessing cuckoos responses, it controlled for the possibility that cuckoos selected an egg for removal based on a visual template of their host's egg in that case we expected gerygone eggs to be removed more frequently than either model egg type. Second, it ensured that if hosts rejected all foreign eggs (i.e. both models), nests would not be empty of eggs and thus abandoned. We excluded from our analysis nests that were predated prior to either parasitism or incubation. Confidence intervals (CIs) of proportions were calculated using the exact method [17].
When a gerygone nest failed, a new nest would appear nearby (less than 20 m), and we considered this nest to have been built by the same gerygone pair. Where we created experimental clutches in successive nesting attempts of the same pair, only the first nest from each pair was used in our analysis of host responses. In the case of cuckoo responses, it was not possible to confirm that every response we scored represented a unique cuckoo, but the independence of data was probably high. Parasitized nests were scored at 13 different creeks located 1.6–26 km apart. Based on the home ranges of other bronze-cuckoos, movement of females between our study creeks was therefore unlikely [18]. Those creeks where we scored more than one cuckoo response all had multiple cuckoos active (at least two to three females per creek), evidenced by the multiple parasitism of host nests ([8] see the electronic supplementary material, table S1). Also, high variation in the shape and colour of same-creek cuckoo eggs in our dataset was not suggestive of repeat sampling of the same cuckoo per creek, assuming that each female lays eggs of a fixed appearance (electronic supplementary material table S1).
(d). Additional field data
We also report on four aspects of cuckoo parasitism of gerygones that are relevant to the hypotheses being tested.
(1) The rate of parasitism and multiple parasitism. This was calculated based only on those nests that survived until at least 14 days after the start of host-laying (n = 60), to ensure that cases of late parasitism were included.
(2) The rates of clutch abandonment following parasitism. Some hosts respond to parasitism by abandoning clutches rather than rejecting eggs [19]. We considered that parasitism did not trigger abandonment if the nest remained active for at least one week after parasitism. Rates of abandonment were compared with those at experimental clutches that were not parasitized (Fisher's exact test).
(3) Egg removal by cuckoos in the cases of natural multiple parasitism. When a cuckoo egg appeared in an experimental nest, it was marked with permanent marker and photographed (see the electronic supplementary material, table S1). If the nest was parasitized again, we scored whether the second cuckoo did or did not remove the first cuckoo's egg. Clutch composition at the time of subsequent parasitism varied and in some cases could not be precisely determined. To establish the expected number of nests at which cuckoo eggs were removed if all eggs in a clutch were equally likely to be removed, we summed the expected values for each clutch size (following Langmore & Kilner [20]). We then compared this value with the observed number of nests at which cuckoo eggs were removed using a binomial test. For uncertain clutch sizes, we used intermediate values (e.g. if clutch size was either 3 or 4 we calculated the expected value for a clutch of 3.5 eggs [6]).
(4) The duration of cuckoo egg-laying visits. In other parasitic species, egg-laying and clutch reduction have been shown to occur extremely rapidly [21–24]. We aimed to determine whether visits were similarly rapid in little-bronze cuckoos, because the time available to cuckoos at the nest may influence the cues they use to select eggs for removal. We filmed the entrance of a subset of experimental nests (n = 8) using motion-sensor TrailCams (SG565F, eight megapixels) activated for 24 h. Cuckoo parasitism was captured at three of these nests. Unfortunately, because nest entrances typically pointed outwards over a creek, cameras had to be positioned at considerable distance on the opposite bank and this resulted in low picture resolution. Also, cameras had a 0.5 s delay between detecting motion and starting to record, which meant that in two cases, cuckoos had already reached the nest entrance when recording began.
3. Results
(a). Multiple parasitism rates
The incidence of little bronze-cuckoo parasitism of gerygone nests was 63% (38 of 60), and the incidence of multiple parasitism was 18% (11 of 60). Thus, 29% of all parasitized nests (11 of 38), received either two (n = 9) or three (n = 2) cuckoo eggs.
(b). Egg rejection by hosts
We assessed gerygones' responses to experimental clutches from the unparasitized nests of 23 breeding pairs. At the majority of nests, gerygones did not reject any eggs (19 of 23; 82.6%; figure 2b).
Those nests at which rejection did occur (four of 23; 17.4%) were too few for rejection rates between egg types to be compared, although each egg type was rejected at least once: the low luminance model (n = 2), the gerygone egg (n = 1) or both the high luminance model and gerygone egg (n = 1). Two of the nests overhung mud rather than water, and the rejected eggs were found intact beneath the nest, indicating the gerygones did not puncture the eggs in order to reject them.
Gerygones did not reject natural cuckoo eggs from parasitized nests (zero of 34 nests), nor did they abandon parasitized nests at rates significantly above that seen in unparasitized nests (three of 34 nests, 9%, and two of 32 nests, 6%, respectively; Fisher's exact test, p = 1).
(c). Egg removal by cuckoos
Cuckoos removed an egg prior to parasitism at the majority of nests containing experimental clutches (20 of 24 nests, 83.3%; figure 2). This incidence was not significantly different to that observed at nests that contained only natural eggs at the time of parasitism (16 of 18 nests, 89%; Fisher's exact test, p = 0.69), suggesting that the presence of model eggs did not influence whether or not cuckoos removed an egg.
Figure 2c shows the proportion of nests at which cuckoos removed each egg type from experimental clutches (n = 20 nests). At one multiply parasitized nest, we could not determine whether the first-to-arrive cuckoo had removed the gerygone egg or the high luminance model, so this nest was scored only as the removal of a ‘non-cryptic’ egg. An omnibus test indicated that cuckoos' egg selection differed from chance at levels nearing significance (χ22 = 5.2, p = 0.07, n = 19). When we consider only those nests in which a model egg was selected for removal, low luminance models were removed significantly less than expected by chance (binomial test: p = 0.04, n = 12). Similarly, if we categorize eggs as either cryptic (i.e. low luminance models) or non-cryptic (high luminance models and gerygone eggs), then cryptic eggs were removed at significantly lower-than-chance rates (χ21 = 4.9, p = 0.03, n = 20). The two non-cryptic egg types were removed similarly often (gerygone eggs versus high luminance models, binomial test: p = 0.63, n = 17).
Cameras captured three instances of parasitism by cuckoos (electronic supplementary material, video S1). Cuckoos spent on average just 13 s at the nest (s.e. = 1.5 s, range: 10–15 s). Parasitism occurred mid-morning (mean ± s.e. = 6.2 ± 0.6 h after sunrise), and all cuckoos were mobbed during their visit by both gerygone parents.
We also assessed 13 cases of parasitism at nests that already contained one or more cuckoos eggs. Egg removal occurred in at least 11 of these cases, and did not vary detectably from chance in the egg type selected for removal (binomial test: p = 0.13, n = 11). Clutch compositions of each nest varied and are given in table 1.
Table 1.
The clutch size and composition at large-billed gerygone nests already parasitized by little-bronze cuckoos at the time of subsequent parasitism, and the egg that was removed by the latter cuckoo. (In most cases, nests had been restored to natural clutch composition after use in our experiment (n = 10, see main text), but some nests were multiply parasitized whilst still containing one or more model eggs (n = 3). At four nests, we were unable to ascertain which of two to three candidate clutch variations was present at the time of second parasitism, and egg removals for these nests are listed accordingly.)
| no. | egg removed | clutch size | clutch composition (egg types) |
|---|---|---|---|
| 1 | cuckoo | 3 | one cuckoo + two gerygone |
| 2 | cuckoo | 2 | one cuckoo + one gerygone |
| 3 | gerygone or none | 4 or 3 | one cuckoo + one gerygone + both models, or one cuckoo + both models |
| 4 | none | 3 | two cuckoo + one gerygone |
| 5 | gerygone or high luminance model | 4 | one cuckoo + one gerygone + both models, or one cuckoo + two gerygone + low luminance model |
| 6 | cuckoo | 3 | one cuckoo + two gerygone |
| 7 | cuckoo | 3 | one cuckoo + two gerygone |
| 8 | gerygone | 3 | one cuckoo + two gerygone |
| 9 | cuckoo | 3 | one cuckoo + two gerygone |
| 10 | gerygone | 3 | one cuckoo + two gerygone |
| 11 | gerygone | 4 or 3, or 2 | one cuckoo + three gerygone or one cuckoo + two gerygone or one cuckoo + one gerygone |
| 12 | cuckoo | 3 | one cuckoo + two gerygone |
| 13 | gerygone or high luminance model |
3 | one cuckoo + both models or one cuckoo + one gerygone + low luminance model |
4. Discussion
(a). Cryptic cuckoo eggs: who are they hiding from?
The eggs of the little bronze-cuckoo are coated in dark pigment, making them low in luminance and cryptic inside the dark interiors of their host's domed nests [8]. We show that model eggs with low luminance are less likely to be removed by cuckoos during parasitism than either high luminance model eggs or the eggs of their common host, the large-billed gerygone. We also confirm that multiple parasitism of gerygone nests is commonplace, with around one-third of all parasitized nests receiving two or more cuckoo eggs, and that cuckoos remove an egg and lay their own egg very rapidly. These results support the idea that low luminance reduces the risk that a cuckoo's egg is detected and removed by subsequent cuckoos targeting the same nest, and thus support intraspecific competition as a selective force favouring cryptic cuckoo eggs.
Whether natural cuckoo eggs enjoy removal rates by other cuckoos as low as those observed for our cryptic models remain unclear. We were able to score egg removal during parasitism at only a limited number of nests containing cuckoo eggs, and failed to detect differences from the rates expected if cuckoo and non-cuckoo eggs were proportionally removed. In any case, however, it is the reduction in risk relative to a non-cryptic cuckoo egg that ultimately determines the benefit of crypsis. For example, it may be that low luminance serves to counteract cuckoo eggs' large size, with the risk of detection by a competing cuckoo determined by some interaction of an egg's size and relative conspicuousness. The ancestral non-cryptic cuckoo egg on which selection acted may well have been less luminous than our immaculate white model [8], but the similar removal rates of high luminance models and natural gerygone eggs suggest that above a certain luminance threshold, eggs experience similar removal risks.
Competition between parasites has also been implicated in one case of egg mimicry in a brood parasitic bird, the greater honeyguide (Indicator indicator [25]). In most cases of egg mimicry by parasites, however, it is the rejection of foreign eggs by hosts that drives change in the parasite egg [3,4,6]. We did not find support for a similar role of this host defence in the evolution of bronze-cuckoo egg crypsis [7,10,11], as gerygones rarely rejected our model eggs, irrespective of whether they were higher or lower luminance than their own eggs. Nor did gerygones abandon clutches with model eggs. Grey warblers (Gerygone igata) in New Zealand, a host of shining bronze-cuckoos (Chrysococcyx lucidus) are similarly reported to routinely accept eggs that differ in luminance to their own (R. Thorogood 2011, unpublished data) Nevertheless, two other scenarios in which host defences could favour cuckoo egg crypsis remain to be tested, neither of which are mutually exclusive with cuckoos as a selective agent. First, gerygones might commonly reject non-cryptic foreign eggs, but only when they are provided with multiple cues of parasitism. We assessed gerygone responses to foreign eggs at unparasitized nests only (i.e. responses to the single cue of a foreign egg), but some rejector hosts of brood parasites have been shown to lower their threshold for rejection based on the sight of a parasite near the nest [26–29], and may even use social information from neighbouring host pairs [30]. Second, gerygones may recognize foreign eggs, but choose to act on this recognition only later in the nesting cycle. Large-billed gerygones sometimes reject little bronze-cuckoo chicks from their nests [13], despite nestling cuckoos being good mimics of gerygone young [31]. Delaying rejection until the chick stage may occur, because there are physical constraints on gerygones removing eggs larger than their own ([32], but see [33]) or because retaining cuckoo eggs in the nest has the beneficial effect of diluting the risk of further gerygone egg loss should the nest suffer subsequent parasitism [14,34].
(b). Multiple parasitism and intraspecific arms races
There is growing evidence that the multiple parasitism of host nests is an important consideration for host–parasite interactions in a variety of avian brood parasite systems [21,25,33–37]. Arms races between brood parasites and their hosts have been well documented as potent drivers of parasite adaptations [38], but intraspecific arms races within parasites, arising wherever females are forced to compete for the same host nests, can be a similarly fierce selective agent on precisely the same traits [39]. Thus, parasite egg morphology, chick competitiveness and incubation times—all of which will affect parasite fitness relative to other parasites in the same nest—could often be shaped by the selective pressures of two simultaneous arms races, as cuckoos try to best both their hosts and their competition.
Arms races between and within species may each influence the outcome of the other. For example, in the case of the little bronze-cuckoo, selection for cryptic eggs arising from intraspecific competition could have limited the ability of hosts to recognize cuckoo eggs [11], in turn, favouring chick rejection as a host's primary defence and triggering the evolution of parasite chick mimicry [31]. Another example may lie in the thick-shelled eggs of parasitic cowbirds [40,41]. Most cowbirds reduce clutches by puncturing holes in existing eggs, and also engage in frequent multiple parasitism [42]. Selection on cowbird eggs to resist puncture from other cowbirds would hinder hosts from evolving puncture-rejection of cowbird eggs [40,43], in turn favouring novel egg rejection methods (e.g. kick-ejection [33]) or alternative defences.
What is the expected outcome of an intraspecific arms race favouring egg crypsis in little bronze-cuckoos? The same female cuckoos whose eggs are selected to evade detection from competitors when they are first-to-lay must also be selected for increasingly good recognition of competitor's eggs when they are second-to-lay. On the one hand, selection here might be assumed to be symmetric [39], because, in both cases, the survival of the cuckoo egg depends upon successful evasion or detection. It seems probable, however, that escalating evolution for egg recognition is in fact constrained by other demands of parasitic egg-laying. We filmed three cases of cuckoo parasitism, and in all cases, the cuckoo spent fewer than 15 s at the nest. During her short visit, she must both select an egg for removal and lay an egg, all while clinging awkwardly half-out of the nest and enduring the attack of gerygone hosts. Egg recognition by cuckoos therefore has a time constraint that egg recognition by hosts does not. This would apply also to selection for non-visual recognition, such as recognition via tactile cues as proposed for some cowbird hosts [44] and honeyguides [25]. Thus, the issue of timing, combined with the limitations of avian vision in low light, may prevent the evolution of more sophisticated egg discrimination in little bronze-cuckoos and so curb further escalation of an arms race between competing females.
Supplementary Material
Acknowledgements
We kindly thank Simon Griffith for supplying us with zebra finch eggs. Golo Maurer and the Cairns Birding Group provided initial tips on where to find large-billed gerygones in Cairns. We also thank Cairns Regional Council for access to the Botanic Gardens and other council property, and the various residents who permitted us to access nests on private land. We are grateful to Keita Tanaka, Nozomu Sato, Rose Thorogood and our anonymous reviewers for their helpful comments on this manuscript.
All nest manipulations were conducted under licence from the Australian National University Animal Experimentation Ethics Committee and the Queensland National Parks and Wildlife Service (permit nos. WITK13209213 and WITK13209413).
Funding statement
This study was supported by an Association for the Study of Animal Behaviour Research Grant to R.G. and an ARC Discovery Grant to N.E.L.
References
- 1.Ruxton GD, Sherratt TN, Speed MP. 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals, and mimicry. New York, NY: Oxford University Press. [Google Scholar]
- 2.Stoddard MC, Stevens M. 2011. Avian vision and the evolution of egg color mimicry in the common cuckoo. Evolution 65, 2004–2013. ( 10.1111/j.1558-5646.2011.01262.x) [DOI] [PubMed] [Google Scholar]
- 3.Spottiswoode CN, Stevens M. 2011. How to evade a coevolving brood parasite: egg discrimination versus egg variability as host defences. Proc. R. Soc. B 278, 3566–3573. ( 10.1098/rspb.2011.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starling M, Heinsohn R, Cockburn A, Langmore NE. 2006. Cryptic gentes revealed in pallid cuckoos Cuculus pallidus using reflectance spectrophotometry. Proc. R. Soc. B 273, 1929–1934. ( 10.1098/rspb.2006.3490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothstein SI. 1990. A model system for coevolution: avian brood parasitism. Annu. Rev. Ecol. Syst. 21, 481–508. ( 10.1146/annurev.es.21.110190.002405) [DOI] [Google Scholar]
- 6.Davies NB, Brooke MDL. 1988. Cuckoos versus reed warblers: adaptations and counteradaptations. Anim. Behav. 36, 262–284. ( 10.1016/S0003-3472(88)80269-0) [DOI] [Google Scholar]
- 7.Marchant S. 1972. Evolution of the genus Chrysococcyx. Ibis 114, 219–233. ( 10.1111/j.1474-919X.1972.tb02604.x) [DOI] [Google Scholar]
- 8.Langmore NE, Stevens M, Maurer G, Kilner RM. 2009. Are dark cuckoo eggs cryptic in host nests? Anim. Behav. 78, 461–468. ( 10.1016/j.anbehav.2009.06.003) [DOI] [Google Scholar]
- 9.Langmore NE, Hunt S, Kilner RM. 2003. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422, 157–160. ( 10.1038/nature01460) [DOI] [PubMed] [Google Scholar]
- 10.Brooker MG, Brooker LC. 1989. Cuckoo hosts in Australia. Aust. Zool. Rev. 2, 1–67. [Google Scholar]
- 11.Brooker LC, Brooker MG, Brooker AMH. 1990. An alternative population genetics model for the evolution of egg mimesis and egg crypsis in cuckoos. J. Theor. Biol. 146, 122–143. ( 10.1016/S0022-5193(05)80048-7) [DOI] [Google Scholar]
- 12.Higgins PJ, Peter JM. (eds). 2002. Handbook of Australian, New Zealand and Antarctic birds. Melbourne, Australia: Oxford University Press. [Google Scholar]
- 13.Sato NJ, Tokue K, Noske RA, Mikami OK, Ueda K. 2010. Evicting cuckoo nestlings from the nest: a new anti-parasitism behaviour. Biol. Lett. 6, 67–69. ( 10.1098/rsbl.2009.0540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato NJ, Mikami OK, Ueda K. 2010. The egg dilution effect hypothesis: a condition under which parasitic nestling ejection behaviour will evolve. Ornithol. Sci. 9, 115–121. ( 10.2326/osj.9.115) [DOI] [Google Scholar]
- 15.Barrett G, Silcocks A, Barry S, Cunningham R, Poulter R. 2003. The new atlas of Australian birds. Melbourne, Australia: Royal Australasian Ornithologists Union. [Google Scholar]
- 16.Martin-Vivaldi M, Soler M, Møller AP. 2002. Unrealistically high costs of rejecting artificial model eggs in cuckoo Cuculus canorus hosts. J. Avian Biol. 33, 295–301. ( 10.1034/j.1600-048X.2002.330311.x) [DOI] [Google Scholar]
- 17.Zar JH. 1999. Biostatistical analysis, 4th edn London, UK: Prentice-Hall International Ltd. [Google Scholar]
- 18.Langmore NE, Adcock GJ, Kilner RM. 2007. The spatial organization and mating system of Horsfield's bronze-cuckoos, Chalcites basalis. Anim. Behav. 74, 403–412. ( 10.1016/j.anbehav.2006.09.019) [DOI] [Google Scholar]
- 19.Hosoi SA, Rothstein SI. 2000. Nest desertion and cowbird parasitism: evidence for evolved responses and evolutionary lag. Anim. Behav. 59, 823–840. ( 10.1006/anbe.1999.1370) [DOI] [PubMed] [Google Scholar]
- 20.Langmore NE, Kilner R. 2009. Why do Horsfield's bronze-cuckoo Chalcites basalis eggs mimic those of their hosts? Behav. Ecol. Sociobiol. 63, 1127–1131. ( 10.1007/s00265-009-0759-9) [DOI] [Google Scholar]
- 21.Gloag R, Fiorini VD, Reboreda JC, Kacelnik A. 2013. The wages of violence: mobbing by mockingbirds as a frontline defence against brood-parasitic cowbirds. Anim. Behav. 86, 1023–1029. ( 10.1016/j.anbehav.2013.09.007) [DOI] [Google Scholar]
- 22.Seel DC. 1973. Egg-laying by the cuckoo. Brit. Birds 66, 528–535. [Google Scholar]
- 23.Sealy SG, Neudorf DLH, Hill DP. 1995. Rapid laying by brown-headed cowbirds Molothrus ater and other parasitic birds. Ibis 137, 76–84. ( 10.1111/j.1474-919X.1995.tb03222.x) [DOI] [Google Scholar]
- 24.Brooker MG, Brooker LC, Rowley I. 1988. Egg deposition by the bronze-cuckoos Chrysococcyx basalis and Ch. lucidus. Emu 88, 107–109. ( 10.1071/MU9880107) [DOI] [Google Scholar]
- 25.Spottiswoode CN. 2013. A brood parasite selects for its own egg traits. Biol. Lett. 9, 20130573 ( 10.1098/rsbl.2013.0573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moksnes A, Røskaft E, Korsnes L. 1993. Rejection of cuckoo (Cuculus canorus) eggs by meadow pipits (Anthus pratensis). Behav. Ecol. 4, 120–127. ( 10.1093/beheco/4.2.120) [DOI] [Google Scholar]
- 27.Bártol I, Karcza Z, Moskát C, Røskaft E, Kisbenedek T. 2002. Responses of great reed warblers Acrocephalus arundinaceus to experimental brood parasitism: the effects of a cuckoo Cuculus canorus dummy and egg mimicry. J. Avian Biol. 33, 420–425. ( 10.1034/j.1600-048X.2002.02945.x) [DOI] [Google Scholar]
- 28.Guigueno MF, Sealy SG. 2011. Aggression towards egg-removing cowbird elicits clutch abandonment in parasitized yellow warblers, Dendroica petechia. Anim. Behav. 81, 211–218. ( 10.1016/j.anbehav.2010.10.005) [DOI] [Google Scholar]
- 29.Langmore NE, Cockburn A, Russell AF, Kilner RM. 2009. Flexible cuckoo chick-rejection rules in the superb fairy-wren. Behav. Ecol. 20, 978–984. ( 10.1093/beheco/arp086) [DOI] [Google Scholar]
- 30.Davies NB, Welbergen JA. 2009. Social transmission of a host defense against cuckoo parasitism. Science 324, 1318–1320. ( 10.1126/science.1172227) [DOI] [PubMed] [Google Scholar]
- 31.Langmore NE, Stevens M, Maurer G, Heinsohn R, Hall ML, Peters A, Kilner RM. 2011. Visual mimicry of host nestlings by cuckoos. Proc. R. Soc. B 278, 2455–2463. ( 10.1098/rspb.2010.2391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonov A, Stokke BG, Moksnes A, Røskaft E. 2009. Evidence for egg discrimination preceding failed rejection attempts in a small cuckoo host. Biol. Lett. 5, 169–171. ( 10.1098/rsbl.2008.0645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Mársico MC, Gloag R, Ursino CA, Reboreda JC. 2013. A novel method of rejection of brood parasitic eggs reduces parasitism intensity in a cowbird host. Biol. Lett. 9, 20130076 ( 10.1098/rsbl.2013.0076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gloag R, Fiorini VD, Reboreda JC, Kacelnik A. 2012. Brood parasite eggs enhance egg survivorship in a multiply parasitized host. Proc. R. Soc. B 279, 1831–1839. ( 10.1098/rspb.2011.2047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moskát C, Hauber ME, Avilés JM, Ban M, Hargitai R, Honza M. 2009. Increased host tolerance of multiple cuckoo eggs leads to higher fledging success of the brood parasite. Anim. Behav. 77, 1281–1290. ( 10.1016/j.anbehav.2009.01.030) [DOI] [Google Scholar]
- 36.Stevens M, Troscianko J, Spottiswoode CN. 2013. Repeated targeting of the same hosts by a brood parasite compromises host egg rejection. Nat. Commun. 4, 2475 ( 10.1038/ncomms3475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rivers JW, Young S, Gonzalez EG, Horton B, Lock J, Fleischer RC. 2012. High levels of relatedness between brown-headed cowbird (Molothrus ater) nestmates in a heavily parasitized host community. Auk 129, 623–631. ( 10.1525/auk.2012.11236) [DOI] [Google Scholar]
- 38.Davies NB. 2000. Cuckoos, cowbirds and other cheats. London, UK: Princeton University Press. [Google Scholar]
- 39.Dawkins R, Krebs JR. 1979. Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511. ( 10.1098/rspb.1979.0081) [DOI] [PubMed] [Google Scholar]
- 40.Picman J. 1989. Mechanism of increased puncture resistance of eggs of brown-headed cowbirds. Auk 106, 577–583. [Google Scholar]
- 41.Spaw CD, Rohwer S. 1987. A comparative study of eggshell thickness in cowbirds and other passerines. Condor 89, 307–318. ( 10.2307/1368483) [DOI] [Google Scholar]
- 42.Ortega CP. 1998. Cowbirds and other parasites. Tuscon, AZ: The University of Arizona Press. [Google Scholar]
- 43.Massoni V, Reboreda JC. 2002. A neglected cost of brood parasitism: egg punctures by shiny cowbirds during inspection of potential host nests. Condor 104, 407–412. ( 10.1650/0010-5422(2002)104[0407:ANCOBP]2.0.CO;2) [DOI] [Google Scholar]
- 44.Mason P, Rothstein SI. 1986. Coevolution and avian brood parasitism: cowbird Molothrus bonariensis eggs show evolutionary response to host discrimination. Evolution 40, 1207–1214. ( 10.2307/2408948) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.