Abstract
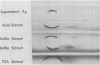
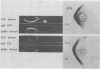
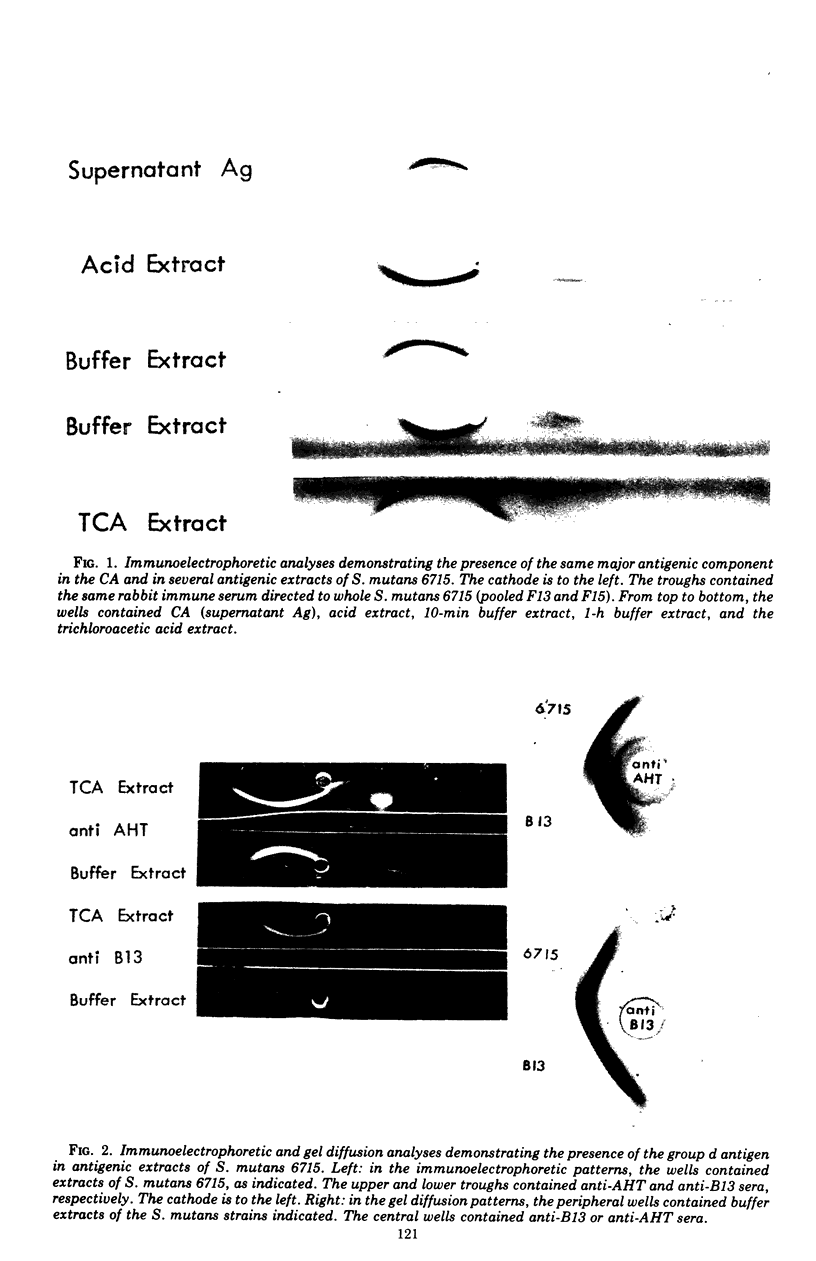
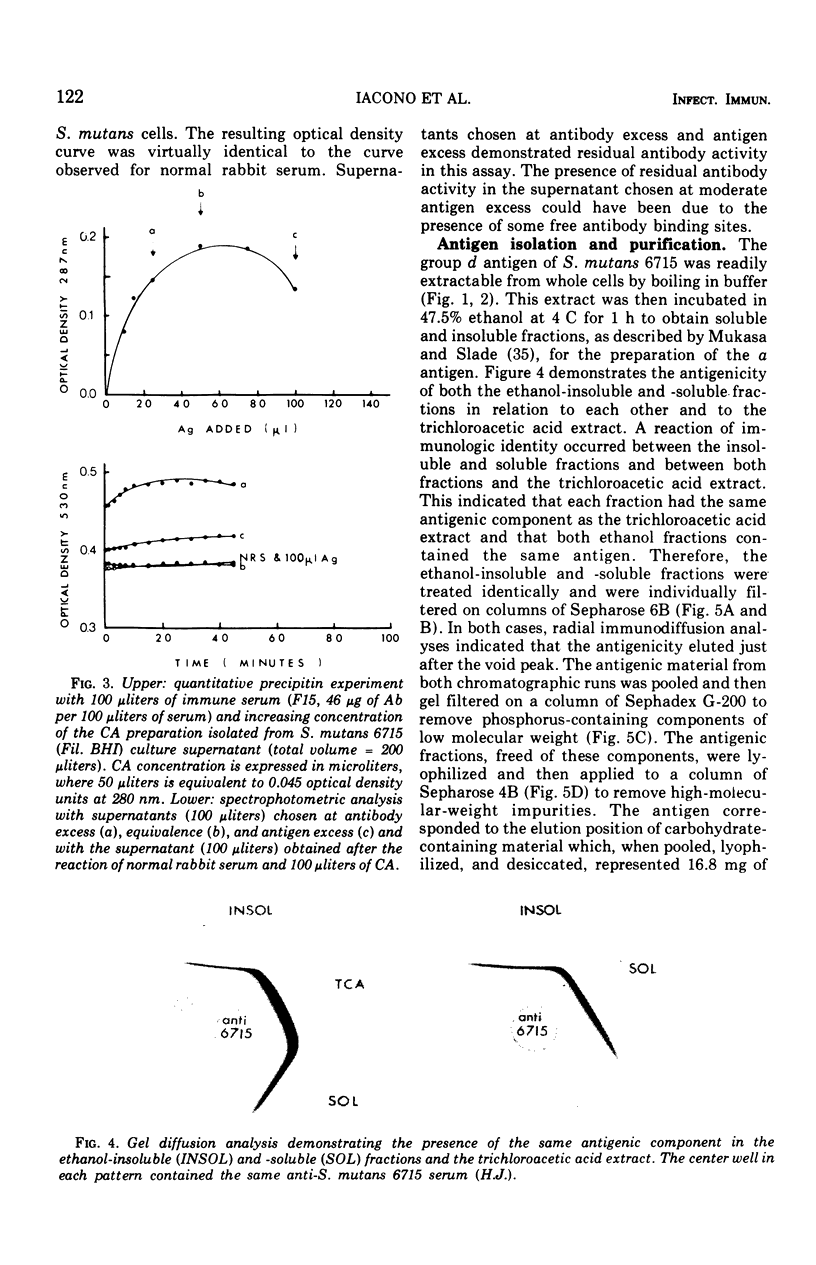
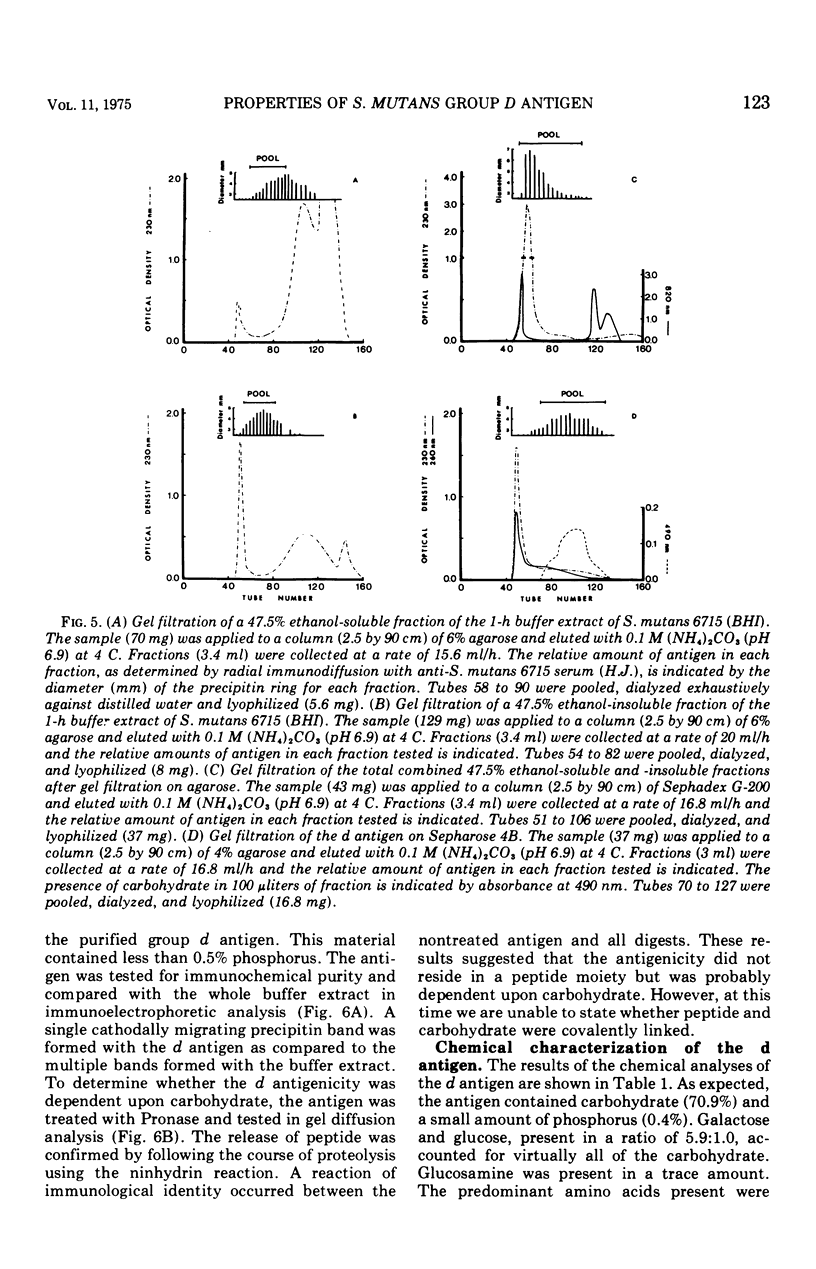
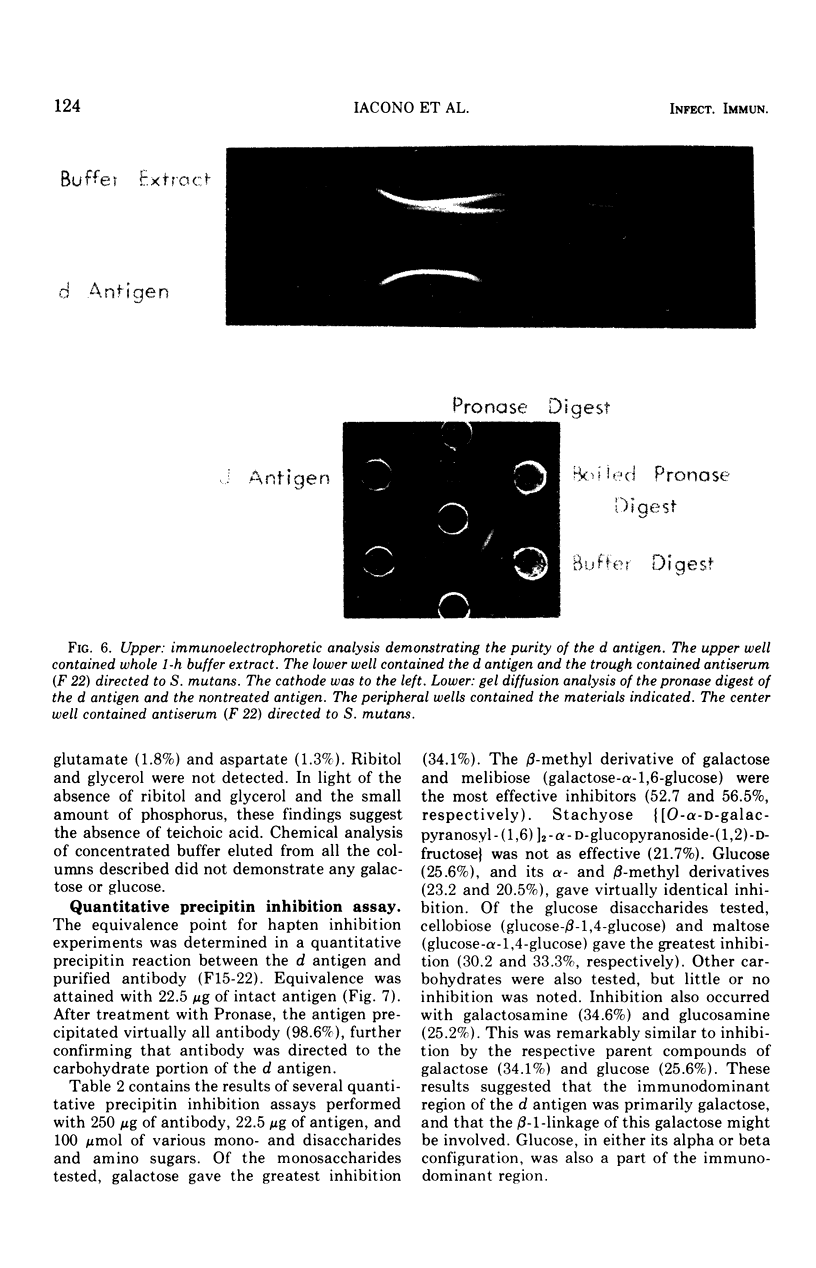
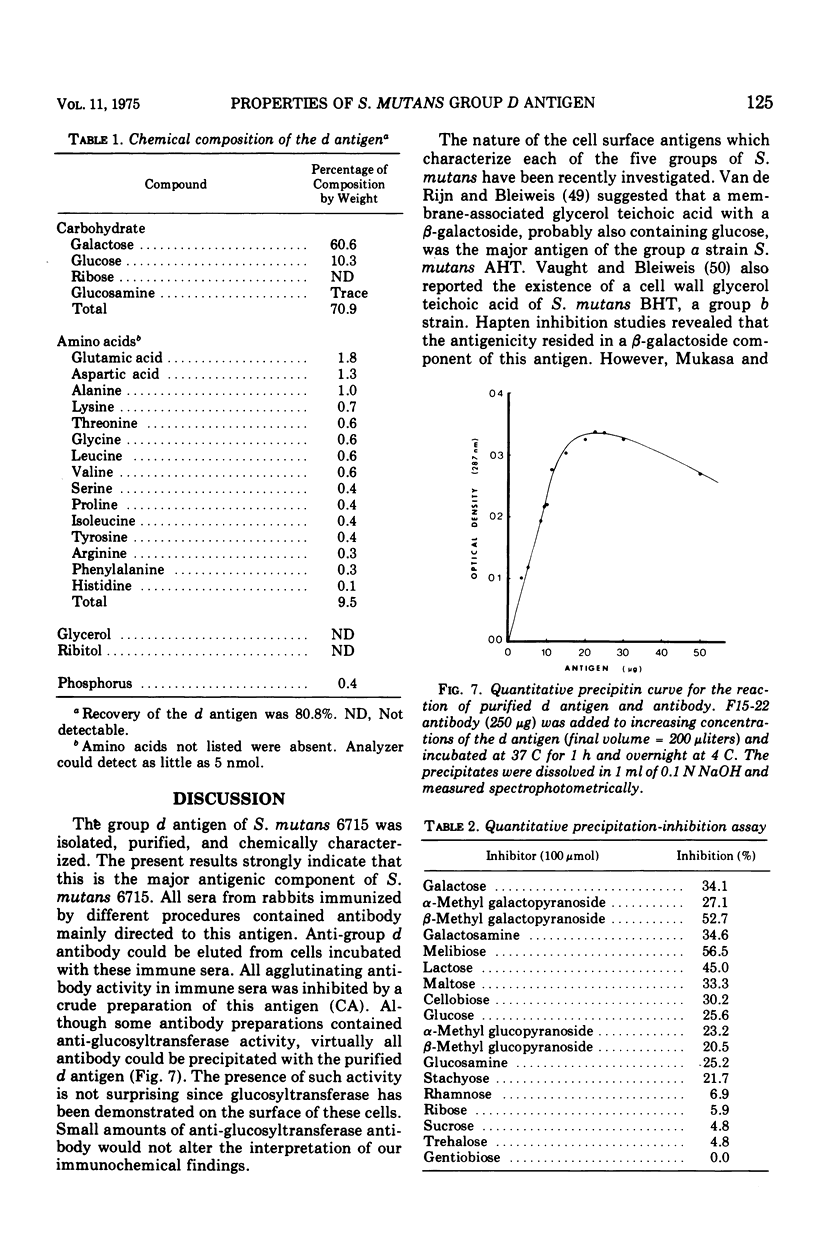
The group d antigen of Streptococcus mutans 6515 was isolated from a buffer (pH 7.3)-boiled extract of whole cells and analyzed immunochemically. Rabbits immunized in three different fashions with whole S. mutans 6715 each responded to the same antigenic cell surface component. This presumptive major antigen was found in culture supernatant, sonically treated supernatant, acid and buffer extracts of whole cells, and trichloroacetic acid extract of cell membranes. A crude preparation of this antigen could completely inhibit antibody-mediated cell (S. mutans 6715) agglutination in a spectrophotometric analysis. The antigen was purified from buffer-boiled extracts by gel filtration on columns of Sepharose 4B. The antigen did not migrate to the anode on electrophoresis nor did it contain appreciable quantities of phosphorus, glycerol, or ribitol. This suggested that the d antigenicity did not reside in a teichoic acid. The d antigen contained galactose and glucose as the sole saccharides, in a ratio of 5.9:1.0. Protein (9.5%) appeared to be a portion of the antigen, although Pronase-digested antigen retained the same electrophoretic mobility and could precipitate virtually all (98.6%) purified antibody directed to the intact antigen. The data obtained from hapten innvolved. Glucose also contributed to the immunodominant region. Antibody directed to the d antigen may be of importance in the inhibition of adherence phenomena manifested by S. mutans organisms of the d group.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- Bratthall D., Carlsson J. Oral streptococci and commercial grouping sera. Odontol Revy. 1968;19(2):205–209. [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Bratthall D. Serological studies on Streptococcus mutans. Odontol Revy Suppl. 1972;23:1–20. [PubMed] [Google Scholar]
- Burgess T. E., Edwards J. R. Chemical characterization of a cell wall antigen from Streptococcus mutans FA1. Infect Immun. 1973 Sep;8(3):491–493. doi: 10.1128/iai.8.3.491-493.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coykendall A. L. Genetic heterogeneity in Streptococcus mutans. J Bacteriol. 1971 Apr;106(1):192–196. doi: 10.1128/jb.106.1.192-196.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. B., Melville T. H. The classification of some oral streptococci of human or rat origin. Arch Oral Biol. 1971 Aug;16(8):845–853. doi: 10.1016/0003-9969(71)90174-9. [DOI] [PubMed] [Google Scholar]
- Edwardsson S. Characteristics of caries-inducing human streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968 Jun;13(6):637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- Evans R. T., Genco R. J. Inhibition of glucosyltransferase activity by antisera to known serotypes of Streptococcus mutans. Infect Immun. 1973 Feb;7(2):237–241. doi: 10.1128/iai.7.2.237-241.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHER F. G., NEBEL H. J. Nachweis und Bestimmung von Glucosamin und Galaktosamin auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1955 Sep 21;302(1):10–19. [PubMed] [Google Scholar]
- FITZGERALD R. J., KEYES P. H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J Am Dent Assoc. 1960 Jul;61:9–19. doi: 10.14219/jada.archive.1960.0138. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Grant W. D., Wicken A. J. Muramic acid phosphate and the linkage of teichoic acid to peptidoglycan in Bacillus stearothermophilus cell walls. Biochem Biophys Res Commun. 1968 Jul 26;32(2):122–128. doi: 10.1016/0006-291x(68)90356-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Guggenheim B. Streptococci of dental plaques. Caries Res. 1968;2(2):147–163. doi: 10.1159/000259553. [DOI] [PubMed] [Google Scholar]
- Jordan H. V. Bacteriological aspects of experimental dental caries. Ann N Y Acad Sci. 1965 Sep 30;131(2):905–912. doi: 10.1111/j.1749-6632.1965.tb34856.x. [DOI] [PubMed] [Google Scholar]
- KRAUSE R. M. SYMPOSIUM ON RELATIONSHIP OF STRUCTURE OF MICROORGANISMS TO THEIR IMMUNOLOGICAL PROPERTIES. IV. ANTIGENIC AND BIOCHEMICAL COMPOSITION OF HEMOLYTIC STREPTOCOCCAL CELL WALLS. Bacteriol Rev. 1963 Dec;27:369–380. doi: 10.1128/br.27.4.369-380.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linzer R., Slade H. D. Purification and characterization of Streptococcus mutans group d cell wall polysaccharide antigen. Infect Immun. 1974 Aug;10(2):361–368. doi: 10.1128/iai.10.2.361-368.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Matsuno T., Slade H. D. Composition and properties of a group A streptococcal teichoic acid. J Bacteriol. 1970 Jun;102(3):747–752. doi: 10.1128/jb.102.3.747-752.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCARTY M., LANCEFIELD R. C. Variation in the group-specific carbohydrate of group A streptococci. I. Immunochemical studies on the carbohydrates of variant strains. J Exp Med. 1955 Jul 1;102(1):11–28. doi: 10.1084/jem.102.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Extraction, purification, and chemical and immunological properties of the Streptococcus mutans group "a" polysaccharide cell wall antigen. Infect Immun. 1973 Aug;8(2):190–198. doi: 10.1128/iai.8.2.190-198.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Structure and immunological specificity of the Streptococcus mutans group b cell wall antigen. Infect Immun. 1973 Apr;7(4):578–585. doi: 10.1128/iai.7.4.578-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. A., Bleiweis A. S., Small P. A., Jr Adherence inhibition of Streptococcus mutans: an assay reflecting a possible role of antibody in dental caries prophylaxis. Infect Immun. 1972 Apr;5(4):419–427. doi: 10.1128/iai.5.4.419-427.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRYTZ B., JABLON J. M. Studies on the type-specific M-fraction of the hemolytic Streptococcus, group A. J Bacteriol. 1955 May;69(5):529–535. doi: 10.1128/jb.69.5.529-535.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- Soprey P., Slade H. D. Immunochemistry of the streptococcal group R cell wall polysaccharide antigen. Infect Immun. 1972 Jan;5(1):91–97. doi: 10.1128/iai.5.1.91-97.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Talbman M. A., Smith D. J. Effects of local immunization with Streptococcus mutans on induction of salivary immunoglobulin A antibody and experimental dental caries in rats. Infect Immun. 1974 Jun;9(6):1079–1091. doi: 10.1128/iai.9.6.1079-1091.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Krichevsky M. I., Keyes P. H. The metabolic fate of glucose catabolized by a washed stationary phase caries-conducive streptococcus. Caries Res. 1969;3(2):167–177. doi: 10.1159/000259580. [DOI] [PubMed] [Google Scholar]
- Taubman M. A., Genco R. J. Induction and properties of rabbit secretory A antibody directed to group A streptococcal carbohydrate. Immunochemistry. 1971 Dec;8(12):1137–1155. doi: 10.1016/0019-2791(71)90392-2. [DOI] [PubMed] [Google Scholar]
- Van de Rijn I., Bleiweis A. S. Antigens of Streptococcus mutans. I. Characterization of a serotype-specific determinant from Streptococcus mutans. Infect Immun. 1973 May;7(5):795–804. doi: 10.1128/iai.7.5.795-804.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaught R. M., Bleiweis A. S. Antigens of Streptococcus mutans. II. Characterization of an antigen resembling a glycerol teichoic acid in walls of strain BHT. Infect Immun. 1974 Jan;9(1):60–67. doi: 10.1128/iai.9.1.60-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLERS J. M., MICHEL M. F., SYSMA M. J., WINKLER K. C. CHEMICAL ANALYSIS AND INHIBITION REACTIONS OF THE GROUP AND TYPE ANTIGENS OF GROUP F STREPTOCOCCI. J Gen Microbiol. 1964 Jul;36:95–105. doi: 10.1099/00221287-36-1-95. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. A serological comparison of the membrane teichoic acids from lactobacilli of different serological groups. J Gen Microbiol. 1971 Aug;67(2):251–254. doi: 10.1099/00221287-67-2-251. [DOI] [PubMed] [Google Scholar]
- ZINNER D. D., JABLON J. M., ARAN A. P., SASLAW M. S. EXPERIMENTAL CARIES INDUCED IN ANIMALS BY STREPTOCOCCI OF HUMAN ORIGIN. Proc Soc Exp Biol Med. 1965 Mar;118:766–770. doi: 10.3181/00379727-118-29964. [DOI] [PubMed] [Google Scholar]
- de Stoppelaar J. D., König K. G., Plasschaert A. J., van der Hoeven J. S. Decreased cariogenicity of a mutant of Streptococcus mutans. Arch Oral Biol. 1971 Aug;16(8):971–975. doi: 10.1016/0003-9969(71)90186-5. [DOI] [PubMed] [Google Scholar]