Abstract
Many bacterial endosymbionts of insects are capable of manipulating their host’s reproduction for their own benefit. The most common strategy of manipulation is cytoplasmic incompatibility (CI), in which embryonic mortality results from matings between uninfected females and infected males. In contrast, embryos develop normally in infected females, whether or not their mate is infected, and infected progeny are produced. In this way, the proportion of infected females increases in the insect population, thereby promoting the spread of the maternally-inherited bacteria. But what happens when multiple endosymbionts inhabit the same host? The parasitoid wasp Encarsia inaron is naturally infected with two unrelated endosymbionts, Cardinium and Wolbachia, both of which have been documented to cause CI in other insects. Doubly-infected wasps show the CI phenotype. We differentially cured E. inaron of each endosymbiont, and crossed hosts of different infection status to determine whether either or both bacteria caused the observed CI phenotype in this parasitoid, and whether the two symbionts interacted within their common host. We found that Wolbachia caused CI in E. inaron, but Cardinium did not. We did not find evidence that Cardinium was able to modify or rescue Wolbachia-induced CI, nor did we find that Cardinium caused progeny sex ratio distortion, leaving the role of Cardinium in E. inaron a mystery.
Keywords: bacterial endosymbionts, multiple infection, reproductive parasites, reproductive manipulators, sex ratio, symbiosis
Introduction
Maternally inherited bacterial endosymbionts are extremely common in arthropods (Douglas, 1989; Duron et al, 2008; Hilgenboeker et al, 2008), and induce a variety of phenotypes in their hosts, ranging from obligate nutritional mutualism to facultative reproductive parasitism (Werren and O’Neill, 1997). Further, it is becoming increasingly evident that many arthropods are infected with multiple lineages of symbionts (e.g., Zchori-Fein and Perlman, 2004; Chiel et al, 2007; Weinert et al, 2007). Within multiply-infected hosts, different facultative symbionts may exhibit tissue tropism, regulate their density independently or in aggregate, and interact in ways that affect the host phenotype (Ijichi et al, 2002; Mouton et al, 2004; Kondo et al, 2005; Oliver et al, 2006). The extent to which multiple infections differ from single infections is as yet poorly understood, and ultimately, requires dissecting the role of each symbiont in isolation, as well as documenting the interactions among them. In the present study, we focus on the reproductive phenotype and interactions of a co-infection of two independent symbiont lineages that are known to promote their own spread by manipulating host reproduction: Cardinium, in the Bacteroidetes, and Wolbachia, in the α-proteobacteria.
Both Cardinium and Wolbachia bacteria have been documented to cause cytoplasmic incompatibility (CI) in their hosts (Hoffmann and Turelli, 1997; Hunter et al, 2003). CI is an interesting phenomenon because it requires interaction between bacteria in different host individuals for its manifestation. The phenotype can be best described with a “modification/rescue” model (Werren, 1997). In infected males, the sperm is modified by the symbiont. When uninfected females mate with these infected males, the most common result is embryonic mortality following fertilization. In contrast, the symbiont present in infected females acts to “rescue” the sabotaged sperm, allowing the host to produce infected progeny. Consequently, infected females produce more offspring than uninfected females, causing the proportion of infected females to increase in the host population over time, and thereby promoting the propagation of the maternally-inherited bacteria (Caspari and Watson, 1959; Turelli, 1994; Werren, 1997). Symbionts have also been shown to promote the production of female offspring via parthenogenesis, male-killing, feminization, or other manipulation of offspring sex ratio (O’Neill et al, 1997), but CI appears to be the most common reproductive manipulation in arthropods (Stouthamer et al, 1999).
The precise mechanisms of CI remain to be elucidated, although recent studies of Wolbachia-induced CI have made substantial progress in understanding both the initial sperm modification and the post-zygotic rescue mechanism (e.g., Tram and Sullivan, 2002; Xi et al, 2008). It is clear, however, that there is diversity among Wolbachia strains in both the modification and rescue function. Through crossing experiments with singly- and multiply-infected hosts, it has been shown that various Wolbachia strains evoke CI at different intensities (e.g., Bordenstein and Werren, 2007), are sometimes but not always able to rescue one another (e.g., Mercot and Poinsot, 1998), and that a single strain may have multiple, distinct rescue functions (Zabalou et al, 2008), allowing rescue of alternative modification strains in the population. Co-infection by multiple CI-inducing Wolbachia strains is also a relatively common phenomenon (e.g., Kondo et al, 2002; Mouton et al, 2005), allowing multiply-infected females to rescue sperm from males with any subset of their symbionts. Maintenance of co-infection by multiple CI-inducing symbionts is therefore often evolutionarily favored (Frank, 1998; Vautrin et al, 2007).
CI was once thought to be a unique phenotype of Wolbachia (Weeks et al, 2002), but Cardinium was the second bacterial lineage discovered to induce CI in arthropods (Hunter et al, 2003; Gotoh et al, 2007a; Perlman et al, 2008). Virtually nothing is known about the mechanism of Cardinium-induced CI. In much the same way that multiple infection by Wolbachia strains has given insight into the mechanisms and evolution of Wolbachia-induced CI, interaction of these two distantly related bacteria in a common host may elucidate the similarities or differences in their mode of action. Hosts harboring both Cardinium and Wolbachia are relatively common (e.g., Weeks et al, 2003; Gotoh et al, 2007b; Duron et al, 2008), but in virtually all cases, the effect of either symbiont is completely unknown. One exception is Encarsia inaron (Hymenoptera: Aphelinidae), a parasitic wasp that was introduced from the Middle East and Europe to North America to control the ash whitefly (Siphoninus phillyreae) (Pickett and Pitcairn, 1999). Wasps collected in Tucson, Arizona were shown to have both Cardinium and Wolbachia endosymbionts, and to have a CI phenotype (Perlman et al, 2006). Like all Hymenoptera, E. inaron is haplodiploid and males develop from unfertilized eggs. CI in haplodiploid systems affects the diploid incipient females, and can result in embryonic lethality, (the “female mortality” type) or conversion of fertilized embryos to haploid males (the “male conversion” type) (Vavre et al, 2000). In doubly-infected E. inaron, the CI phenotype appeared to be the female mortality type. However, the role of each symbiont remained obscure; initial antibiotic curing experiments removed both symbionts (Perlman et al, 2006).
In the present study we examined the CI phenotype in E. inaron to determine the relative contributions of Cardinium and Wolbachia, and to examine potential interactions occurring between the symbionts. Specifically, we sought to 1) determine whether both or one of the bacteria caused the CI phenotype, 2) test whether the bacterial lineages interacted in the expression and rescue of CI, and 3) determine whether the bacteria had any other effects on the sex ratio of E. inaron.
Materials and Methods
Cultures
The doubly-infected Encarsia inaron culture (“Both”) originated from pupae collected in Tucson, Arizona in 2002 (Perlman et al, 2006). Encarsia inaron is a solitary endoparasitoid of whiteflies, and is propagated on sweet potato whitefly (Bemisia tabaci) in our laboratory as described in Perlman et al, (2006). In this species, male and female eggs are laid in first to third instar whitefly nymphs. Single adult wasps emerge approximately two weeks later.
To generate differentially infected cultures, we treated adult wasps with antibiotics. The “Cured” culture received rifampicin-infused honey (50 mg/ml) for 48 hr in three successive generations (Perlman et al, 2006). Curing was verified by polymerase chain reaction (PCR, see below). The “Cardinium” (Card.) and “Wolbachia” (Wol.) lines were generated by treating female wasps with a low dose (1.0 mg/ml) of either ampicillin or doxycycline for 48 hr. Cardinium appears to be more susceptible to ampicillin than Wolbachia, at least in cell culture, (Stouthamer, 1991; Morimoto et al, 2006), and both symbionts are susceptible to doxycycline. At sufficiently low doses, however, the antibiotics did not cure the wasps, but destabilized the infection such that bacterial transmission to offspring was not always complete. Individual treated females were allowed to oviposit in whitefly nymphs on 35 mm leaf disk arenas (Hunter et al, 2003). The resulting progeny were isolated as pupae, to prevent mating between siblings of potentially different infection status. Female offspring were mated to cured males in bulk, individually given an opportunity to oviposit on leaf disk arenas, and then sacrificed to determine infection status via PCR (see below). Most F1 individuals were either cured or still infected with both symbionts, but a small proportion had only one symbiont or the other. Progeny of the singly-infected F1 females were retained, and the individual propagation and PCR screening procedure was repeated for another generation to ensure stable transmission of the remaining symbiont. In the end, the Cardinium and Wolbachia lines were each initiated with F3 individuals descended from at least eight different antibiotic-treated females.
Verification of symbiont infection
We used diagnostic PCR to assess infection status during the initiation of the laboratory cultures, as well as for verification that experimental wasps contained the expected symbionts. Previous work by Perlman et al (2006) found no other symbionts in E. inaron except Cardinium and Wolbachia. DNA was extracted by grinding individual wasps in 3 μL of 20 mg/ml proteinase K, incubating the samples at 37 °C in 50 μl 10% w/v Chelex (Sigma-Aldrich, St. Louis, MO) in purified water for one hour with periodic vortexing, followed by 8 min at 96 °C for enzyme denaturation (T. Groot, personal communication). Extracted samples were stored at −20 °C. For Cardinium amplification, we used 10 μL reactions (4.9 μL purified water, 1 μL Invitrogen 10× buffer, 0.8 μL of 10 mM dNTPs, 0.2 μL of 50 mM MgCl2, 0.5 μL each of 5 pmol μL−1 forward and reverse primer, 0.1 μL of 5 U μL−1 Invitrogen Taq polymerase and 2 μL DNA sample). We used Cardinium-specific primers (Ch-F 5′ - TACTGTAAGAATAAGCACCGGC - 3′, Ch-R 5′ - GTGGATCACTTAACGCTTTCG - 3′) that amplify a 394 bp product (Zchori-Fein and Perlman, 2004). Each PCR was run for one cycle of 94 °C for 2 min, 30 cycles of 94 °C for 30 sec, 51 °C for 30 sec, 72 °C for 30 sec and a final extension of 5 min at 72 °C. Wolbachia amplification was similar, except the volume of 50 mM MgCl2 was increased to 0.8 μL, the volume of purified water was decreased to 4.3 μL, and Wolbachia-specific ftsZ primers were used that amplify a 775 bp product (ftsZunif 5′ - GG(CT)AA(AG)GGTGC(AG)GCAGAAGA - 3′, ftsZunir 5′ - ATC(AG)AT(AG)CCAGTTGCAAG - 3′) (Lo et al, 2002). The PCR program was one cycle of 94 °C for 2 min, 30 cycles of 94 °C for 30 sec, 56 °C for 30 sec, 72 °C for 45 sec and a final extension of 6 min at 72 °C. All PCRs were accompanied by positive and negative DNA controls. Encarsia pergandiella wasps served as positive controls for Cardinium, and Encarsia formosa wasps served as positive controls for Wolbachia. To visualize the reaction products, we added 1.6 μL 20X SYBR Green to each reaction, and visualized them on a 1.125% agarose gel in a UV transilluminator.
Experimental crosses
To test for CI among the differentially infected E. inaron cultures, we conducted a full factorial experiment, in which males of each of the four cultures were crossed with females of each culture, resulting in 16 treatments. We isolated pupae from each culture into individual 1.2 ml vials that contained a small droplet of honey, and plugged the vials with cotton. Upon emergence, wasps within each culture were sexed by visual inspection, and randomly assigned to mate with the opposite sex from one of the four cultures. Approximately 30 males and 30 females were assigned to each of the 16 treatments, and allowed to mate for 4 d in 3.8 L mating jars with free access to water and honey. Ten to 15 female wasps were selected at random from each mating jar, and individually placed on cowpea (Vigna ungulata) leaf disks on 1% agar medium in a 35 mm petri dish. Each leaf disk had ~50 first to third instar B. tabaci hosts for oviposition (range = 30–75 whiteflies per disk). The petri dishes were covered with modified screen-top lids, and placed in an environmental chamber at 27 °C, 60 %RH, 16:8 h photoperiod. After 24 h, the wasps were transferred to a second leaf disk and allowed to oviposit for an additional 24 h to maximize progeny production per wasp. The wasps were then frozen at −20 °C for later analysis. The leaf disks were maintained on agar for two wk for parasitoid development, and the parasitoid progeny that emerged were quantified and sexed. If a mother produced no female offspring, it was possible that she was either affected by CI or unmated (because haplodiploid wasps can produce haploid male offspring without mating). To distinguish between these possibilities, we dissected out the spermatheca from each female that produced only male offspring, cleared the spermatheca in a lactophenol solution (1 part carbolic acid, 1 part lactic acid, 2 parts glycerine, 1 part distilled water), and examined it at 200–400X magnification for the presence of sperm. Unmated females were excluded from the dataset. To confirm that each mother had the expected symbionts, we extracted the DNA either from her or one of her offspring (if the mother had been dissected), followed by diagnostic PCR. We also tested the infection status of a sample of males from each mating jar.
In addition to the experiment described above, we performed three other preliminary experiments that involved only partial sets of crosses. We occasionally draw on these in the following sections for comparison with results from the full experiment. These earlier experiments were conducted in the same fashion as described above, but, for brevity, are not described in detail here.
Statistics and contrasts
We used logistic regression (Arc v. 1.06) to compare offspring sex distribution among the treatments. We used Williams’ correction (Williams, 1982) to correct for moderate overdispersion in the data. When the overall model was significant, rather than multiple comparisons of all treatment pairs (120 pairs) we tested the significance of specific contrasts of interest using Wald statistics.
Question 1: Which symbiont(s) cause(s) CI?
We first verified that doubly-infected E. inaron retained a CI phenotype by comparing the progeny sex ratio of doubly-infected males mated to cured females (test cross: Both ♂ × Cured ♀) relative to a control cross of cured males mated to cured females (Cured ♂ × Cured ♀) and also relative to a control cross of doubly-infected males mated to doubly-infected females (Both ♂ × Both ♀). In this way we were able to control for both female type and male type. Significantly reduced female production in the test cross relative to controls indicates CI. If CI was detected, we followed up with t-tests comparing total offspring production and male offspring production between the test cross and the cured by cured control, to determine whether CI was the “female mortality” (female embryos die) or “male replacement” (incipient female embryos develop as males) type (Vavre et al, 2000). Female mortality CI would be characterized by lower total offspring production in the test cross than control, but similar male offspring production. Male replacement CI would be characterized by similar total offspring production between the test cross and control, but male offspring production would be higher in the test cross.
To test whether Cardinium or Wolbachia causes CI, we used parallel contrasts to those described for the doubly-infected line, except using the Cardinium only line (test cross = Card. ♂ × Cured ♀, contrasted with Cured ♂ × Cured ♀ and Card. ♂ × Card. ♀ controls) or the Wolbachia only line (test cross = Wol. ♂ × Cured ♀, contrasted with Cured ♂ × Cured ♀ and Wol. ♂ × Wol. ♀ controls). If CI was detected from either symbiont, we again used t-tests of total progeny and male progeny production to distinguish between the male replacement and female mortality type.
Question 2: Do the symbionts interact in the expression and rescue of CI?
To test whether the presence of one symbiont in any way modifies the CI induced by the other symbiont, we investigated several contrasts, each designed to address a specific type of interaction (Table 1). Table 1 includes the contrasts used to test for the effect Cardinium might have on Wolbachia-induced CI; we did not conduct the analogous set of contrasts to test for the effect Wolbachia might have on Cardinium-induced CI because we didn’t detect Cardinium-induced CI.
Table 1.
Potential cytoplasmic incompatibility interactions between Cardinium and Wolbachia in Encarsia inaron.
| Potential interaction | Test cross | Control cross |
|---|---|---|
| A) Cardinium “rescues” Wolbachia- induced CI | Wol. ♂ × Card. ♀ | Wol. ♂ × Cured ♀ |
| B) Cardinium in the male modifies the strength of Wolbachia-induced CI | Both ♂ × Cured ♀ | Wol. ♂ × Cured ♀ |
| C) Cardinium in the male modifies the strength of Wolbachia-induced CI when Cardinium is also in the female | Both ♂ × Card. ♀ | Wol. ♂ × Card. ♀ |
| D) Cardinium in both male and female modifies the strength of Wolbachia- induced CI | Both ♂ × Card. ♀ | Wol. ♂ × Cured ♀ |
| E) Cardinium in a Wolbachia-bearing female affects the ability to rescue CI | Wol. ♂ × Both ♀ | Wol. ♂ × Wol. ♀ |
| F) Cardinium in a Wolbachia-bearing male affects CI rescue in a Wolbachia-bearing female. | Both ♂ × Wol. ♀ | Wol. ♂ × Wol. ♀ |
Question 3: Does either symbiont manipulate progeny sex ratio?
Symbionts may also promote their own spread by directly manipulating the progeny sex ratio in favor of female offspring. To test this possibility, we compared the progeny sex ratio of each infected female type mated to cured males relative to that of cured females mated to cured males (Cured ♂ × Both ♀, Cured ♂× Card. ♀, and Cured ♂× Wol. ♀ contrasted with Cured ♂× Cured ♀). In this way we were able to eliminate the potential influence of symbiont-altered sperm on progeny production.
Results
Question 1: Which symbiont(s) cause(s) CI?
Consistent with Perlman et al (2006), we found evidence for CI in doubly-infected E. inaron. Cured females mated to doubly-infected males (the test cross) had a male bias in their offspring (2 ♂: 1 ♀; Figure 1). In contrast, cured females mated to cured males had a female-biased sex ratio in their offspring (1 ♂: 1.7 ♀). This significant difference (Wald = 3.521, P < 0.001) indicates that low female offspring production in the predicted CI cross was not due to male bias in cured females. Likewise we found that doubly-infected females mated to doubly-infected males also had a female bias in their offspring (1 ♂: 1.5 ♀). This ratio was again significantly different than what was observed in the test cross (Wald = 2.698, P = 0.007), indicating that sperm from doubly-infected males is not intrinsically low quality; it is only when mated to cured females that incompatibility occurs, just as one would expect in symbiont-induced CI.
Figure 1.
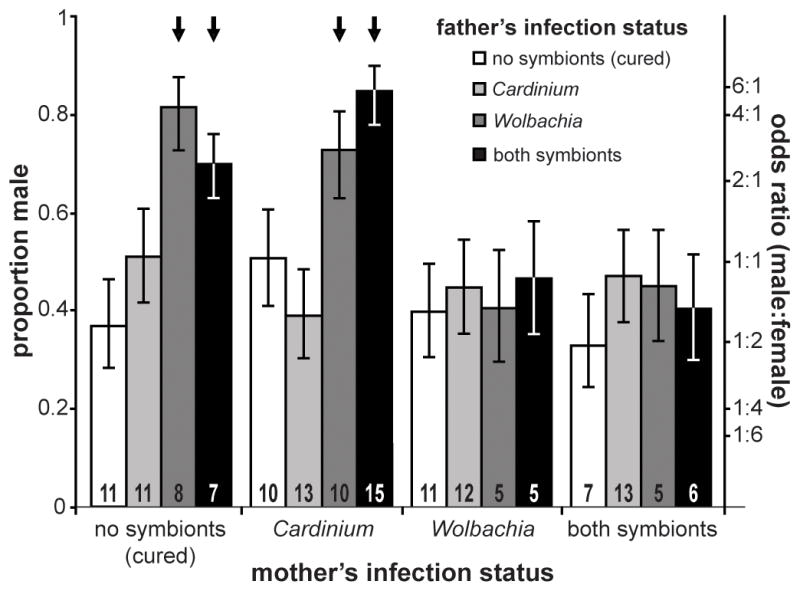
Back-transformed logistic regression estimates ± SE of proportion male progeny and corresponding odds ratios produced by all 16 crosses. Numbers at the base of each column represent the sample size of mothers for that cross. Crosses where CI was detected are indicated by bold arrows above a column.
On the basis of this experiment alone, it was not clear whether CI was of the male replacement or female mortality type. We found that total offspring production was not significantly different between the test cross (22.3 ± 3.0) and the cured × cured control (29.0 ± 2.8; t = 1.589, d.f. = 15, P = 0.133), but we also found no difference in male production between the two crosses (test cross = 12.1 ± 1.0, control = 9.3 ± 1.5; t = 1.483, d.f. = 15, P = 0.159). However, we also found a small proportion of uninfected males in the test cross: 2/20 males checked were uninfected, rather than doubly-infected. The origin of these uninfected males is unclear, but may have resulted from incomplete vertical transmission. Note that this was the only case where the parasitoids did not have the expected infection status: all experimental females, and all other males sampled were of the expected infection status. When we inspected the data, we found that three females in the test cross showed no evidence of CI, and may have mated with the uninfected males. These females had the highest total offspring production; the other females, which had male biased offspring, also had reduced total offspring production (15.5 ± 1.7), suggesting female mortality CI consistent with the findings of Perlman et al (2006) and other results from our laboratory (JAW unpublished data).
We found strong evidence that Wolbachia induces CI in E. inaron. Cured females mated to Wolbachia-infected males had a very strong male bias in their offspring (4.4 ♂: 1 ♀; Figure 1), strongly contrasting with the female bias of cured females mated to cured males (1 ♂: 1.7 ♀; Wald = 4.547, P < 0.001), or Wolbachia-infected females mated to Wolbachia-infected males (1 ♂: 1.46 ♀; Wald = 3.565, P < 0.001). Total offspring production was halved in the test cross relative to the control (test cross = 14.9 ± 1.5, control = 29.0 ± 2.8; t = 4.178, d.f. = 16, P = 0.001), whereas male production did not differ significantly (test cross = 10.5 ± 1.3, control = 9.3 ± 1.5; t = 0.605, d.f. = 16, P = 0.554), indicating that Wolbachia induced the female mortality type of CI.
We did not find evidence for Cardinium-induced CI in this experiment, but the results are equivocal. The test cross of cured females mated to Cardinium-infected males produced nearly equal proportions of male and female offspring (1.05 ♂: 1 ♀; Figure 1). This value was not significantly more male-biased than the offspring of cured females mated to cured males (1 ♂: 1.7 ♀, Wald = 1.809, P = 0.071) or Cardinium-infected females mated to Cardinium-infected males (1 ♂: 1.6 ♀, Wald = 1.606, P = 0.108), but in both contrasts there was a trend toward significance. In two previous experiments, however, we found no evidence of Cardinium-induced CI. In each previous experiment, the test cross showed female-biased offspring production (expt. 1 = 1 ♂: 1.4 ♀ n = 11; expt. 2 = 1 ♂: 1.6 ♀, n = 33) that did not differ significantly from the cured × cured treatment (expt. 1 Wald = 0.518, P = 0.605; expt. 2 Wald = 0.159, P = 0.874) or the Card. × Card. treatment (expt. 1 Wald = 0.987, P = 0.324; expt. 2 Wald = 0.922, P = 0.357). The preponderance of evidence thus suggests that Cardinium does not cause CI in E. inaron.
Question 2: Do the symbionts interact in the expression and rescue of CI?
We did not find evidence that Cardinium modifies Wolbachia-induced CI. For potential interaction A (Table 1), we found that Cardinium-infected females cannot “rescue” Wolbachia modified sperm. Cardinium-infected females mated to Wolbachia-infected males produce 2.7 ♂: 1 ♀ offspring (Figure 1), which is statistically equivalent to the 4.4 ♂: 1 ♀ produced by cured females mated to Wolbachia-infected males (Wald = 1.003, P = 0.316).
The additional presence of Cardinium in a male does not alter the strength of Wolbachia-induced CI. For interaction B (Table 1), we found that cured females produced male-biased offspring whether mated to doubly-infected males (2.3 ♂: 1♀) or Wolbachia-infected males (4.4 ♂: 1♀; Wald = 1.267, P = 0.205). Likewise, for potential interaction C (Table 1), we found that Cardinium-infected females produced male-biased offspring whether mated to doubly-infected males (5.7 ♂: 1♀) or Wolbachia-infected males (2.7 ♂: 1 ♀; Wald = 1.697, P = 0.090). There is an apparent trend toward reduced male bias in Cardinium-infected females mated with Wolbachia-infected males, but note that a previous experiment had found a very strong male bias in this treatment (6.7 ♂: 1 ♀, n = 10 mothers), supporting the lack of significance in this contrast.
The presence of Cardinium in both male and female of a cross does not appear to change CI expression. For potential interaction D (Table 1), we found that the sex ratio produced by Cardinium-infected females mated to doubly-infected males (5.7 ♂: 1 ♀) is similar to that of cured females mated to Wolbachia-infected males (4.4 ♂: 1 ♀; Wald = 0.542, P = 0.588).
Similarly, the additional presence of Cardinium in either Wolbachia-infected males or females does not affect the ability of Wolbachia-infected females to rescue Wolbachia-induced CI. For potential interaction E (Table 1) we found that doubly-infected females mated to Wolbachia-infected males produced 1 ♂: 1.2 ♀, which did not differ significantly from the 1 ♂: 1.5 ♀ produced by Wolbachia-infected females mated to Wolbachia-infected males (Wald = 0.368, P = 0.713). Also, for potential interaction F (Table 1), we found that Wolbachia-infected females mated to doubly-infected males produced 1 ♂: 1.1 ♀, which did not differ significantly from the sex ratio produced by Wolbachia-infected females mated to Wolbachia-infected males (Wald = 0.497, P = 0.619), indicating that the additional presence of Cardinium in the male does not interfere with sperm modification in such a way that it cannot be rescued.
Question 3: Does either symbiont manipulate progeny sex ratio?
We found no significant differences in the offspring sex ratios among females of the different lines mated to cured males, although there was a trend toward reduced female bias in the Cardinium line. Cardinium-infected females mated to cured males produced 1 ♂:1 ♀, whereas cured females mated to cured males produced 1 ♂:1.7 ♀ (Wald = 1.692, P = 0.091; Figure 1). Females carrying Wolbachia alone produced female-biased offspring and did not differ from cured females (1 ♂:1.5 ♀, Wald = 0.364, P = 0.716). Likewise, doubly-infected females mated to cured males produced female biased offspring and did not differ from cured females (1 ♂: 2 ♀; Wald = 0.457, P = 0.646).
Discussion
In doubly-infected E. inaron, Wolbachia, and not Cardinium causes cytoplasmic incompatibility of the female mortality type. CI is the most prevalent phenotype induced by Wolbachia (Stouthamer et al, 1999), and multiple infections of Wolbachia and other symbionts are common, but to our knowledge, this is the first record of differential curing being used to dissect the relative contributions of Wolbachia and another symbiont to CI modification and rescue in a doubly-infected host. It is also interesting that it is Wolbachia and not Cardinium that causes CI in E. inaron. Wolbachia is prevalent in this family of parasitoids, the Aphelinidae in the Chalcidoidea (e.g., Weeks et al, 2003) but to date has been associated only with the induction of parthenogenesis (Gottlieb et al, 1998). The only other documented instance of CI in the Aphelinidae, in Encarsia pergandiella, is caused by Cardinium (Hunter et al, 2003).
Presuming that CI induction by Cardinium follows a modification/rescue model similar to that proposed for Wolbachia (Werren, 1997), our results suggest that Cardinium in E. inaron has a mod− phenotype. However, host background has also been shown to be important in the expression of CI (Veneti et al, 2003), and it is possible that E. inaron has evolved a phenotype that is not permissive of Cardinium-induced CI, yet permissive of Wolbachia-induced CI. Horizontal transfer experiments of E. inaron Cardinium into other host backgrounds would therefore be necessary to absolutely verify a mod− phenotype. Similarly, even though our experiment found that Cardinium in E. inaron is resc− with respect to Wolbachia-induced CI, it is possibly resc+ for Cardinium-induced CI. Such a dichotomy would be particularly likely if the mechanisms for Cardinium- and Wolbachia-induced CI are very different. It would be most informative to investigate CI mechanisms and interactions in a system in which both Cardinium and Wolbachia cause CI within the same host. Unfortunately, all doubly-infected arthropods that have been investigated to date have either had CI caused by only one symbiont (present study, Ros and Breeuwer, submitted) or a CI phenotype was not present (Gotoh et al, 2007a).
Since Cardinium doesn’t appear to cause or influence CI in E. inaron, it remains to be determined what, if anything, Cardinium does. We did not find that Cardinium promotes its own existence in E. inaron by encouraging a female bias in progeny: if anything, the Cardinium line showed a trend toward fewer female progeny than the other lines (but the effect was not significant). It is possible that Cardinium provides E. inaron with some sort of fitness benefit, such as increased fecundity (Weeks and Stouthamer, 2004). Alternatively, Cardinium could be effectively neutral within E. inaron, but is maintained in the population via perfect maternal transmission (Hoffmann et al, 1996). It is also possible that maintenance of Cardinium within E. inaron is directly attributable to co-infection with Wolbachia. Perfect co-transmission with Wolbachia would confer the same CI transmission advantage to Cardinium as its CI-inducing partner. Recent theoretical studies have suggested that parameters for symbiont invasion and maintenance within a population can be altered by co-infection (Vautrin et al, 2007). Finally, studies of co-infecting symbiont taxa have found complementarity between their genomes (e.g., McCutcheon and Moran, 2007) suggesting that species may lose redundant portions of their genomes. Symbiont interactions appear to be more dynamic over relatively short periods of time than previously appreciated (Riegler and O’Neill, 2007; Weeks et al, 2007), and it is possible that Cardinium has lost an historical ability to cause CI because co-infection and co-transmission with Wolbachia rendered it unnecessary. To test such speculations, however, more will need to be known about the history of the Cardinium/Wolbachia association within E. inaron. Intriguingly, other populations of E. inaron appear to harbor only Cardinium (JAW, unpublished data), raising questions about the origin of the Wolbachia infection, and whether Cardinium causes CI when it is alone in the host.
Acknowledgments
We would like to thank A. Kozuch, J. Garcia, H. Kim, and T. Montgomery for technical assistance, and E. Chiel, J. Deas, C. Gibson, K. Oliver, and three anonymous reviewers for helpful comments on previous versions of the manuscript. This research was supported by the Center for Insect Science through NIH Training Grant #2 K12 Gm00708-06, the Canadian Institute for Advanced Research, and an NSF DEB grant (DEB-0542961) to MSH and SJP.
References
- Bordenstein SR, Werren JH. Bidirectional incompatibility among divergent Wolbachia and incompatibility level differences among closely related Wolbachia in Nasonia. Heredity. 2007;99:278–287. doi: 10.1038/sj.hdy.6800994. [DOI] [PubMed] [Google Scholar]
- Caspari E, Watson GS. On the evolutionary importance of cytoplasmic sterility in mosquitoes. Evolution. 1959;13:568–570. [Google Scholar]
- Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, Inbar M, Ghanim M. Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull Entomol Res. 2007;97:407–413. doi: 10.1017/S0007485307005159. [DOI] [PubMed] [Google Scholar]
- Douglas AE. Mycetocyte symbiosis in insects. Biol Rev Cambridge Phil Soc. 1989;64:409–434. doi: 10.1111/j.1469-185x.1989.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Duron O, Hurst GDD, Hornett EA, Josling JA, Engelstadter J. High incidence of the maternally inherited bacterium Cardinium in spiders. Mol Ecol. 2008;17:1427–1437. doi: 10.1111/j.1365-294X.2008.03689.x. [DOI] [PubMed] [Google Scholar]
- Frank SA. Dynamics of cytoplasmic incompatibility with multiple Wolbachia infections. J Theor Biol. 1998;192:213–218. doi: 10.1006/jtbi.1998.0652. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Noda H, Ito S. Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity. 2007a;98:13–20. doi: 10.1038/sj.hdy.6800881. [DOI] [PubMed] [Google Scholar]
- Gotoh T, Sugasawa J, Noda H, Kitashima Y. Wolbachia-induced cytoplasmic incompatibility in Japanese populations of Tetranychus urticae (Acari : Tetranychidae) Expl Appl Acarol. 2007b;42:1–16. doi: 10.1007/s10493-007-9072-3. [DOI] [PubMed] [Google Scholar]
- Gottlieb Y, Zchori-Fein E, Faktor O, Rosen D. Phylogenetic analysis of parthenogenesis-inducing Wolbachia in the genus Aphytis (Hymenoptera : Aphelinidae) Insect Mol Biol. 1998;7:393–396. doi: 10.1046/j.1365-2583.1998.740393.x. [DOI] [PubMed] [Google Scholar]
- Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH. How many species are infected with Wolbachia? - a statistical analysis of current data. FEMS Microbiol Let. 2008;281:215–220. doi: 10.1111/j.1574-6968.2008.01110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Clancy D, Duncan J. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity. 1996;76:1–8. doi: 10.1038/hdy.1996.1. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Turelli M. Cytoplasmic incompatibility in insects. In: O’Neill SL, Hoffmann AA, Werren JH, editors. Influential passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford University Press; New York: 1997. pp. 42–80. [Google Scholar]
- Hunter MS, Perlman SJ, Kelly SE. A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc R Soc Lond Series B. 2003;270:2185–2190. doi: 10.1098/rspb.2003.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi N, Kondo N, Matsumoto R, Shimada M, Ishikawa H, Fukatsu T. Internal spatiotemporal population dynamics of infection with three Wolbachia strains in the adzuki bean beetle, Callosobruchus chinensis (Coleoptera : Bruchidae) Appl Environl Microbiol. 2002;68:4074–4080. doi: 10.1128/AEM.68.8.4074-4080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo N, Ijichi N, Shimada M, Fukatsu T. Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera : Bruchidae) Mol Ecol. 2002;11 :167–180. doi: 10.1046/j.0962-1083.2001.01432.x. [DOI] [PubMed] [Google Scholar]
- Kondo N, Shimada M, Fukatsu T. Infection density of Wolbachia endosymbiont affected by co-infection and host genotype. Biol Lett. 2005;1:488–491. doi: 10.1098/rsbl.2005.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo N, Casiraghi M, Salati E, Bazzocchi C, Bandi C. How many Wolbachia supergroups exist? Mol Biol Evol. 2002;19:341–346. doi: 10.1093/oxfordjournals.molbev.a004087. [DOI] [PubMed] [Google Scholar]
- McCutcheon JP, Moran NA. Parallel genomic evolution and metabolic interdependence in an ancient symbiosis. Proc Natl Acad Sci USA. 2007;104:19392–19397. doi: 10.1073/pnas.0708855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercot H, Poinsot D. Rescuing Wolbachia have been overlooked and discovered on Mount Kilimanjaro. Nature. 1998;391:853–853. doi: 10.1038/36021. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Kurtti TJ, Noda H. In vitro cultivation and antibiotic susceptibility of a Cytophaga-like intracellular symbiote isolated from the tick Ixodes scapularis. Curr Microbiol. 2006;52:324–329. doi: 10.1007/s00284-005-0349-7. [DOI] [PubMed] [Google Scholar]
- Mouton L, Dedeine F, Henri H, Bouletreau M, Profizi N, Vavre F. Virulence, multiple infections and regulation of symbiotic populations in the Wolbachia-Asobara tabida symbiosis. Genetics. 2004;168:181–189. doi: 10.1534/genetics.104.026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton L, Henri H, Bouletreau M, Vavre F. Multiple infections and diversity of cytoplasmic incompatibility in a haplodiploid species. Heredity. 2005;94 :187–192. doi: 10.1038/sj.hdy.6800596. [DOI] [PubMed] [Google Scholar]
- O’Neill SL, Hoffmann AA, Werren JH, editors. Influential Passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford University Press; New York: 1997. p. 214. [Google Scholar]
- Oliver KM, Moran NA, Hunter MS. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc R Soc Lond Series B. 2006;273:1273–1280. doi: 10.1098/rspb.2005.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SJ, Kelly SE, Zchori-Fein E, Hunter MS. Cytoplasmic incompatibility and multiple symbiont infection in the ash whitefly parasitoid, Encarsia inaron. Biol Cont. 2006;39:474–480. [Google Scholar]
- Perlman SJ, Kelly SE, Hunter MS. Population biology of cytoplasmic incompatibility: maintenance and spread of Cardinium symbionts in a parasitic wasp. Genetics. 2008;178:1003–1011. doi: 10.1534/genetics.107.083071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett CH, Pitcairn MJ. Classical biological control of ash whitefly: factors contributing to its success in California. Biocontrol. 1999;44:143–158. [Google Scholar]
- Riegler M, O’Neill SL. Evolutionary dynamics of insect symbiont associations. Trends Ecol Evol. 2007;22:625–627. doi: 10.1016/j.tree.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Ros VID, Breeuwer JAJ. The effects of, and interactions between, Cardinium and Wolbachia in the doubly infected spider mite Bryobia sarothamni. doi: 10.1038/hdy.2009.4. (submitted to Heredity) [DOI] [PubMed] [Google Scholar]
- Stouthamer R. Effectiveness of several antibiotics in reverting thelytoky to arrhenotoky in Trichogramma spp. Colloques de l’INRA. 1991;56:119–122. [Google Scholar]
- Stouthamer R, Breeuwer JAJ, Hurst GDD. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu Rev Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- Tram U, Sullivan W. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science. 2002;296:1124–1126. doi: 10.1126/science.1070536. [DOI] [PubMed] [Google Scholar]
- Turelli M. Evolution of incompatibility-inducing microbes and their hosts. Evolution. 1994;48:1500–1513. doi: 10.1111/j.1558-5646.1994.tb02192.x. [DOI] [PubMed] [Google Scholar]
- Vautrin E, Charles S, Genieys S, Vavre F. Evolution and invasion dynamics of multiple infections with Wolbachia investigated using matrix based models. J Theor Biol. 2007;245:197–209. doi: 10.1016/j.jtbi.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Vavre F, Fleury F, Varaldi J, Fouillet P, Bouletreau M. Evidence for female mortality in Wolbachia-mediated cytoplasmic incompatibility in haplodiploid insects: Epidemiologic and evolutionary consequences. Evolution. 2000;54:191–200. doi: 10.1111/j.0014-3820.2000.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Veneti Z, Clark ME, Zabalou S, Karr TL, Savakis C, Bourtzis K. Cytoplasmic incompatibility and sperm cyst infection in different Drosophila-Wolbachia associations. Genetics. 2003;164:545–552. doi: 10.1093/genetics/164.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks AR, Reynolds KT, Hoffmann AA, Mann H. Wolbachia dynamics and host effects: what has (and has not) been demonstrated? Trends Ecol Evol. 2002;17:257–262. [Google Scholar]
- Weeks AR, Stouthamer R. Increased fecundity associated with infection by a Cytophaga-like intracellular bacterium in the predatory mite, Metaseiulus occidentalis. Proc R Soc Lond Series B. 2004;271:S193–S195. doi: 10.1098/rsbl.2003.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks AR, Velten R, Stouthamer R. Incidence of a new sex-ratio-distorting endosymbiotic bacterium among arthropods. Proc R Soc Lond Series B. 2003;270:1857–1865. doi: 10.1098/rspb.2003.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA. From parasite to mutualist: Rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biology. 2007;5:997–1005. doi: 10.1371/journal.pbio.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert LA, Tinsley MC, Temperley M, Jiggins FM. Are we underestimating the diversity and incidence of insect bacterial symbionts? A case study in ladybird beetles. Biol Lett. 2007;3:678–681. doi: 10.1098/rsbl.2007.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH. Biology of Wolbachia. Annu Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- Werren JH, O’Neill SL. The evolution of heritable symbionts. In: O’Neill SL, Hoffmann AA, Werren JH, editors. Influential passengers: Inherited Microorganisms and Arthropod Reproduction. Oxford University Press; New York: 1997. pp. 1–41. [Google Scholar]
- Williams DA. Extra-binomial variation in logistic linear models. Appl Stat. 1982;31 :144–148. [Google Scholar]
- Xi ZY, Gavotte L, Xie Y, Dobson SL. Genome-wide analysis of the interaction between the endosymbiotic bacterium Wolbachia and its Drosophila host. BMC Genomics. 2008;9:Article 1. doi: 10.1186/1471-2164-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalou S, Apostolaki A, Pattas S, Veneti Z, Paraskevopoulos C, Livadaras I, Markakis G, Brissac T, Mercot H, Bourtzis K. Multiple rescue factors within a Wolbachia strain. Genetics. 2008;178:2145–2160. doi: 10.1534/genetics.107.086488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zchori-Fein E, Perlman SJ. Distribution of the bacterial symbiont Cardinium in arthropods. Mol Ecol. 2004;13:2009–2016. doi: 10.1111/j.1365-294X.2004.02203.x. [DOI] [PubMed] [Google Scholar]


