Abstract
Each year funding agencies and academic institutions spend millions of dollars and euros on biobanking. All funding providers assume that after initial investments biobanks should be able to operate sustainably. However the topic of sustainability is challenging for the discipline of biobanking for several major reasons: the diversity in the biobanking landscape, the different purposes of biobanks, the fact that biobanks are dissimilar to other research infrastructures and the absence of universally understood or applicable value metrics for funders and other stakeholders. In this article our aim is to delineate a framework to allow more effective discussion and action around approaches for improving biobank sustainability. The term sustainability is often used to mean fiscally self-sustaining, but this restricted definition is not sufficient for biobanking. Instead we propose that biobank sustainability should be considered within a framework of three dimensions – financial, operational, and social. In each dimension, areas of focus or elements are identified that may allow different types of biobanks to distinguish and evaluate the relevance, likelihood, and impact of each element, as well as the risks to the biobank of failure to address them. Examples of practical solutions, tools and strategies to address biobank sustainability are also discussed.
Introduction
Human biospecimens provide a critical resource that underpins research directed towards better health outcomes. The topic of biobank sustainability is therefore also critical for health research. But recent advances in health research (including social health research, health service research and public health1,2 and advances in technology) have increased demands and created important issues for biobanking. Specifically, researchers have increasing needs in terms of biospecimen numbers and annotation, biobanks increasingly recognize the need to implement standardized processes to attain higher quality standards, funders seek performance metrics and assurances for their investments, donors require transparency and accountability for their samples, and the public has concerns around privacy, particularly in relation to their genetic information. Against this backdrop, biobanks have always struggled to achieve secure funding. Research funding agencies, institutions, and private and philanthropic organizations have often assumed the initial start up and infrastructure costs and some initial operational costs of individual biobanks. But there has been an underlying belief that biobanks at some point should be capable of becoming “self-sustaining.” This may be achievable in the context of planning a large national infrastructure with a 15- to 20-year life cycle period.3 But for most existing types of biobanks this has not proved possible4 and the challenge of initiating and then sustaining biobanks over time has only increased in the face of the ongoing world-wide financial depression since 2008.4,5
Framing the Topic of Sustainability
The growing importance and challenges of sustainability for biobanks is reflected in the increasing attention devoted specifically to this topic at recent annual meetings of the International Society for Biological and Environmental Repositories (ISBER)6 and the European, Middle Eastern, and African Society for Biopreservation and Biobanking (ESBB).7 However, productive discussion amongst the many stakeholders to identify solutions has been elusive for the following four important reasons:
1. Biobanks are significantly different from other health research infrastructures
Biobanking has sometimes been compared to other important tools and infrastructure in health research. Examples are multiuser facilities for advanced microscopy, cell culture and animal model facilities, databases and related systems. Yet biobanks differ in the significant degree to which they must address the costs of personnel and operational costs (such as the complexity of annotating data required) and the duration over which biospecimens must be stored and the entire biobank sustained before they achieve maximum value (e.g., for biobanks that are designed to support studies examining biomarkers of outcome to cancer therapies the time taken to accumulate sufficient patient outcome data can be over 5–10 years). Biobanks also differ by the nature of the complex activity of biobanking that spans ethical/legal/social, health care, and research domains. Lastly, many infrastructures including biobanks have undergone changes to accommodate and support the dramatic growth and new scales of science during the past decade,8 but the demands of such changes on biobanks are arguably greater. For example transnational research collaborations have introduced new demands on biobanks9 such as to address variations in legal, privacy, and ethics environments in addition to internal operational standards within their realm of control.
Human biospecimens are a limited resource. They are also not subject to short-term depreciation like many other research tools; that is many collections become more valuable over long periods of time. Moreover, unlike cell lines, animal models, and extracted nucleic acid products and the data derived from their analysis, biospecimens themselves cannot be duplicated and are essentially irreplaceable. Some have argued that biobanks should convert their biospecimens into products and data at the outset, as this is ultimately the mechanism to realize the value of the biospecimen asset. However, the evolution of science with new methods to analyze biospecimens provides many lessons to support the alternative view that the intact biospecimen is a unique and more valuable asset. For example, the emergence of micro RNA research has shown that routine processing to extract only proteins, mRNA and DNA, would have restricted a whole new field of discovery. Until very recently many tumor biobanks might have processed blood plasma and serum specimens entirely for protein products to support the growing area of protein biomarker discovery based on proteomics, but would then have been unable to support discovery research with circulating tumor DNA.10 Finding the right balance between immediate processing and keeping the intact specimen, may be critical to maximizing the value of a biobank's collection.
2. Biobanks are not a single homogeneous entity
Biobanks may be thought of as being research tools akin to physical tools in a toolbox. Just as such physical tools encompass many different types and variations within categories, so biobanks also arise in many types, sizes and designs as tools for a variety of purposes as defined by research questions and pertinent to the focus of a particular biobank. When differences between types of biobanks have been considered, it has been mostly in terms of physical features or in terms of general areas of research supported. The former include features such as predominant biospecimen type and/or custodian (e.g., FFPE blocks in pathology archives). The latter include terms such as tumor banks or epidemiology banks that are often equated incorrectly to a focus on only one type of biospecimen (e.g., frozen tumor tissues or blood specimens respectively). But as discussed further in the next section, these terms do not adequately convey the range of design, scale, and/or purpose across different types of biobank.
3. The need to be sustainable varies with the focus of the biobank
Different types of biobanks have different needs for scale and duration of financial support and for broader sustainability. Amongst biobank experts there is general agreement that the term biobank applies to a broad spectrum of biological sample collections that follow recognized standard operating procedures.11 However, as we have previously argued,11,12 the diversity of biobanks is poorly appreciated by most stakeholders. Human research biobanks are often described by a limited number of descriptive terms, such as “population” or “hospital integrated” or “tumor” biobank. A more detailed classification system is needed to distinguish between the many different types of biobank and to allow a more productive discussion on sustainability that considers the impacts of different factors on different designs. Although no generally accepted classification system exists, we have previously described a framework on which a functional classification might be developed. To illustrate how different types of biobanks are influenced by different aspects of sustainability it is useful to consider just one simple functional parameter; user type. While “user type” is only one parameter that might be incorporated into an internationally accepted classification system in the future, we have found this single parameter very useful, even on its own, to distinguish different needs for education and documentation between tumor biobanks in the course of implementing a certification program.13 As previously described,12 intended use of a biobank (“user type”) can be classified as mono-, oligo-, and poly-user. These three categories define biobank collections that aim at the outset to support either a single research project (mono-user), several research projects typically within a shared institution (oligo-user) or multiple users with undetermined research projects (poly-user).
Mono-user biobanks include many different designs but are often small (<200 cases) collections that arise out of funding from a direct operating research grant to address a specific hypothesis using a prospectively collected biospecimen cohort. These types of collections are by definition self-supporting for the duration of the research, and the issue of long-term sustainability for the components of such collections that can remain after the completion of the research project is not necessarily important. Nevertheless the initial investment to establish such a biobank can be significant. With only a little more investment and smarter planning (e.g., adaptation of the informed consent process) specimens and data could be converted into an oligo- or poly- user biobank or transferred to a poly-user biobank to ‘sustain’ the collection. At the other end of the spectrum, poly-user biobanks can also be diverse and include small and large (>1000 cases) collections, but often arise out of a desire to prospectively compile a generalized collection that can then efficiently and rapidly support multiple forms of “retrospective hypothesis research.” That is they serve the function of enabling researchers to address questions that involve selecting biospecimens associated with historical annotating data (e.g., patient treatment and outcomes data) on the basis of specific criteria and enabling interrogation by a range of different research assays. Once such biobanks are operational they often also engage in support of “prospective hypothesis research” by deploying the same accrual machinery to compile specialized collections, and indeed provide an ideal mechanism to deliver on this approach. For these types of biobanks, establishing even partial self-supporting mechanisms can be more challenging because there is uncertainty in the revenue that would be provided by those utilizing the specimens. Moreover, the scale of the effort required, the initial disconnection from the prospect of short-term research productivity, and the lack of metrics of value over time make it difficult to determine a model of sustainability from the outset. Sustainability is important for all of these biobanks but an appropriate balance must be made with respect to the initial deployment of the necessary resources required with some estimates of their measured value and future prospects for use.
4. Value metrics for biobanks have not yet been defined
Some forms of research infrastructure have in the past been initially established within institutions and then dispersed to external and often commercial sources. Synthesis of oligonucleotide primers for PCR, microarray chip production, and sequencing services are all examples that spawned such local infrastructures funded initially by combinations of institutional investment, direct research grants, and user fees that then became entirely end-user supported and self-sustaining in the commercial arena. Other forms of health research infrastructure have persisted but cannot be self-sustaining through user fees or spun off entirely to the commercial arena (e.g., ethics review boards, animal care facilities). But agreement around research needs, measures of value, institutional priorities, and feasibility of alternatives, have allowed equilibrium with funding of other core assets. The discipline of biobanking needs to develop and implement value metrics that are relevant to all stakeholders and appropriate for different types of biobanks so that health research funders and institutions can debate and assess the appropriate scale of funding allocation to specific forms of biobanking. Such metrics should include both quantitative and qualitative metrics. We briefly discuss and provide an example set of the former in Table 2. Qualitative examples of scientific progress and changes in clinical impact and patient outcomes attributable to biobanks are also important. The report of the Interagency Working Group on Scientific Collections (IWGSC), “Scientific Collections: Mission-Critical Infrastructure for Federal Science Agencies”14 is an example of an important compilation to address qualitative metrics of impact of biobanks.
Table 2.
Quantitative Biobank Performance Metrics: Examples of Desired and Acquired Metrics Compiled from a Tumor Biobank
| STAKEHOLDER | |||
|---|---|---|---|
| INTERNAL | EXTERNAL | ||
| MEASURE | Biobank/ study staff | Ethics Review Board | Funders / Networks |
| Participant (Patient) Phase | |||
| Participant statistics | |||
| • Number enrolled • Percentage approached • Percentage enrolled • Percentage refused • Percentage withdrawn |
✓ | ✓ | |
| Biospecimen Phase | |||
| Samples collected | |||
| Number by: • Type (e.g. blood, tissue, urine, control) • Serial collections from same participant • Samples per preservation format (e.g. fresh, frozen, formalin fixed) |
✓ | ✓ | ✓ |
| Samples quality | |||
| • DNA • RNA • Morphology, composition |
✓ | ✓ | |
| Biobank Staff Phase | |||
| Training | |||
| • Orientation for new staff • Maintenance for existing staff |
✓ | ||
| Data Phase | |||
| Data collected | |||
| • Biospecimen data (e.g. collection time, processing info, storage temp) • Clinical data (e.g. pathology, treatment, outcome) |
✓ | ✓ | |
| Database | |||
| • Ability to query data | ✓ | ||
| Quality of data | |||
| • Accuracy of data (e.g. pathology diagnosis) | ✓ | ||
| Researcher/User Phase | |||
| Sample requests | |||
| • Number of projects requesting samples • Type of projects requesting samples • Number and type of samples requested |
✓ | ✓ | |
| Sample distribution | |||
| • Number of projects for which samples are distributed • Type of projects for which samples are distributed • Number and type of samples distributed |
✓ | ✓ | ✓ |
| Response time | |||
| • Time between request and sample distribution | ✓ | ✓ | |
| User feedback | |||
| • Avenue for users to provide information on satisfaction of service | ✓ | ||
| User trends | |||
| • Number of requests • Number of new users • Number of repeat business |
✓ | ✓ | |
| Publications | |||
| • Number that used biobank samples in published research • Number that named biobank in acknowledgement or methods section |
✓ | ✓ | |
These example metrics are a compilation of desired and/or routinely collected data from individual member banks and across the Canadian Tumor Repository Network.21 Checkmarks represent different measures that have been provided to or requested by some stakeholders.
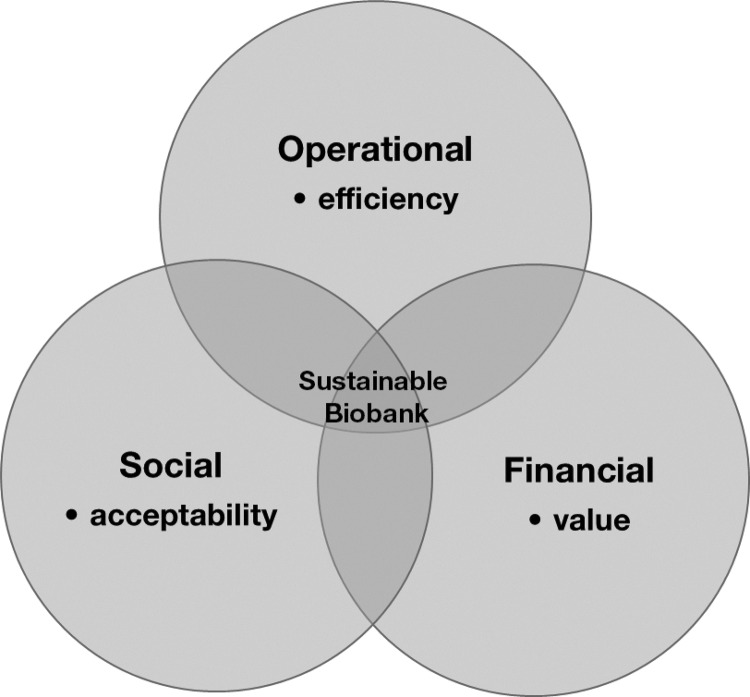
Defining Three Dimensions of Biobank Sustainability
One definition of sustainability is the “capacity to endure […] and remain diverse and productive over time”15 and as a property relating to three pillars of economy, environment, and society.16 We consider the term sustainability as applied to the activity of biobanking to refer to “financial” (i.e., analogous to economy), ‘operational’ (i.e., analogous to environment) and “social” dimensions (Figure 1). Each dimension is critical and increasingly challenging for the discipline but the discussion of sustainability is often restricted to only components of the financial dimension and is often confusing for all stakeholders. This is perhaps in part because of the complexity of biobanking, failure to adopt an informative classification system, and its diverse stakeholders. The complexity means that even when considering simple components of the biobanking process, many do not appreciate that not all components of the process are relevant to or have the same importance for some types of biobanks. For example not all biobanks require storage to hold biospecimens at all, and not all require resources to store and then annotate biospecimens with long term outcome data. The range of data needed varies depending on the type of research which is supported, e.g., only fields from a pathology report may be needed for early translational research, whereas extensive datasets about the person providing the biospecimen may be needed for epidemiological studies. As no classification system exists for biobanks it is hard for those outside the field to understand the differences between such biobanks and their costs.
FIG. 1.
Three dimensions of sustainability in relation to the activity of biobanking.
The relative importance of different dimensions of sustainability differs among stakeholders
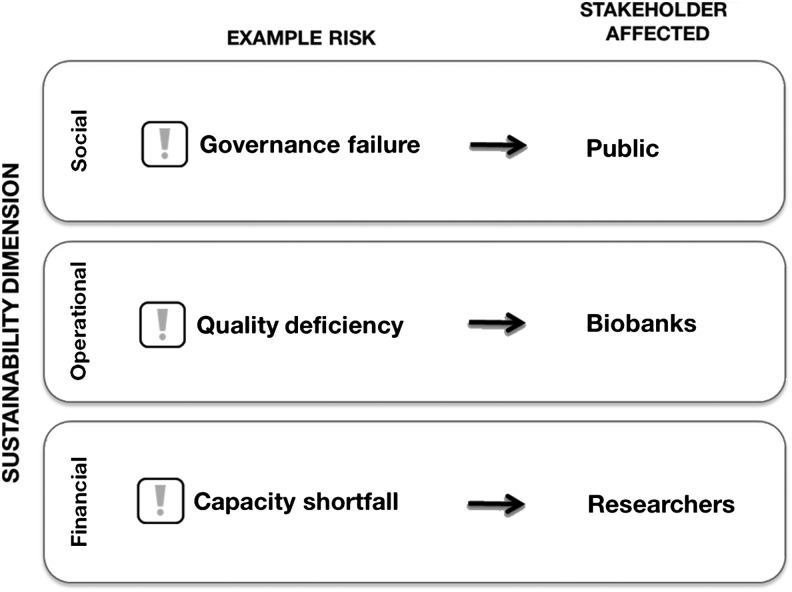
Before highlighting some of the general and specific strategies that can be implemented to enhance biobank sustainability in each dimension, it is worth briefly considering the perspectives of just some of the major stakeholders (such as the public, the biobank, the researcher, and the funders) and some of the risks to sustainability. There are of course many types of risks and the importance of these risks and their probability of occurring also varies across the range of different biobanks and the impact may be viewed differently from the perspective of a specific stakeholder (Figure 2).
FIG. 2.
Diagram to highlight that risks (e.g., governance failure, capacity shortfall, and quality deficiency) relate to different dimensions of sustainability, and that each risk will have an effect on different stakeholders.
From the public stakeholder perspective an important issue that has been raised is acceptability and trust in biobanks, and this relates to the social dimension of biobank sustainability. Public deliberations have shown that there is strong consensus around the need for good governance and standards.17 A good governance structure translates into sound and responsible best practices and standards being adopted, allowing for fair and transparent distribution of biospecimens, and this promotes trust. Failure to establish appropriate governance can occur with any biobank but the significant impact of loss of public trust can be felt by all biobanks.
From the biobank stakeholder perspective there are many issues, and since many biobankers involved in all types of biobanks are also researchers there is naturally overlap between these perspectives. But a major challenge for biobankers is how to define, assess and attain high biospecimen quality, which is linked to decisions around how to be most efficient with the deployment of the resources available to the biobank. Clearly the resources available to a biobank relate to the financial dimension, but within any existing biobank the most effective use of resources should be of paramount importance. So it can be argued that for the biobank, quality is equally an issue relevant to the operational domain. Quality depends on consistent deployment of standardized protocols and investments to collect and maintain biospecimens, and to annotate them over time. Failure in quality is a risk issue for all biobanks. It is equally a risk for the research user where inadequate or unknown quality can contribute to failure of experiments or irreproducibilty. But the issues most often raised by researchers relate to the adequacy of biobanks in terms of scale, accessibility, and responsiveness of biobanks to requests.
These same issues (i.e., an availability or capacity shortfall) are also important to funders of research and relate mostly to the financial dimension. However while these issues are relevant to all classes of biobanks, the reasons are both real and perceived, and differ by type of biobank. For some biobanks there are sometimes inherent limitations in either desire, capacity or governance structure to allow access to collections by external researchers, while for other biobanks the limited opportunities to secure funding and long term investment have made it difficult to survive let alone meet the increasing demand. And yet the impact of inadequate supply of biospecimens felt by researchers and heard by funders is also becoming a risk for the sustainability of biobanks. For example one view is that many existing biobanks are under-utilized18 and this has contributed to increased pressure from funders to provide better metrics of value and impact to justify further investments in biobanking. It is also important to recognize that estimating the full value of some types of biobank from a balance sheet of performance metrics will be as difficult as assessing the long term value and impact of investment in specific basic research programs. So despite the importance of metrics and ongoing efforts to develop and implement such metrics for biobanks, funders must recognize the societal value and acknowledge the abundant indirect evidence of critical importance and research impact of biobanks, as illustrated by the yields from research dependent on biobanks such as the cancer genome atlas projects. In the meantime it can be argued that the risk of “capacity shortfall” has begun to create a risk for the sustainability of biobanks that already have inadequate resourcing and funding available in the face of increasing demand.
General Strategies to Address Biobank Sustainability
Linking aspects of the biobanking operations through networks of biobanks to share some components is one approach to enhancing sustainability of individual biobanks
Different biobanking network models have evolved and can be viewed as fostering sustainability primarily in one or more of the three dimensions. For example, in the operational dimension by focusing on components such as consent, biospecimen accrual, and/or shared storage; in the financial dimension by focusing on components such as performance metrics, user access, and customers' needs; in the social dimension by focusing on components that promote common standards. A well established and successful example of a network in the United States that addresses the operational dimension is the Cooperative Human Tissue Network (CHTN).19 Adaptations of this model have involved creating a common efficient accrual system for biobanks.20 An example of fostering sustainability in the social dimension is the Canadian Tumour Repository Network (CTRNet)21 whose funding from the Institute of Cancer Research Canadian Institutes of Health Research (ICR-CIHR)22 is restricted to network activities (as opposed to biobanking) such as creating certification programs, standard operating procedures and policies. The Public Population Project in Genomics and Society (P3G)23 and the Biobank Resource Centre (BRC)24 are examples of resources that have harnessed collective knowledge across different networks to further develop and disseminate tools to implement sustainability strategies. The P3G is a not-for-profit consortium that works to encourage collaboration between researchers and biobankers, promotes harmonization of data, optimizes the design, setup and research activities of studies, biobanks, research databases and other similar health and social research infrastructure and facilitates the transfer of knowledge and provides training. The BRC was developed in partnership by the University Of British Columbia Office of Biobank Education and Research (OBER) and CTRNet to provide services and tools that support researchers in establishing and operating biobanks, to educate and promote certification of biobanks in order to enhance quality through adoption of best practice standards, and to publish biobank market research data.25,26 This kind of data is essential to successfully execute a biobanking business plan to facilitate shifting to customer focused biobanking. The BRC is an example of a resource that offers strategies and solutions in all sustainability dimensions.
Specific Strategies to Address Biobank Sustainability
Table 1 lists examples of specific strategies to enhance biobank sustainability, organized under the most relevant dimension and in relation to specific areas of focus. Many of these strategies have also been developed and implemented by CTRNet and more detailed information can be obtained through its BRC.
Table 1.
Dimensions of Sustainability, Areas of Focus, and Some Examples of Strategies for Biobanks to Address Each
| Financial Dimension | |
| Market strategy | • Develop a strategic plan (e.g., business, marketing, academic etc.) • Revisit and revise the plan • Foster user fee adoption |
| Stakeholder needs | • Identify different goals and motivations • Define accrual targets (e.g., biospecimen type, disease focus) |
| Brand recognition | • Communicate value for investment with all stakeholders (e.g., PI, institution, funder, etc) • Measure value and monitor impact of the biobank (e.g., BRIF) |
| Operational Dimension | |
| Input efficiency | • Patient enrollment (e.g., PTC program) • Biospecimen accrual systems (e.g., CHTN, BC BioLibrary, PTC models) |
| Internal efficiency | • Optimize processing of biospecimens & annotation (e.g., limit product extraction, create tissue microarrays to enable case selection) • Balance resources to support retrospective questions (i.e., retain relevant stock) and prospective questions (i.e., divert resources to assist specialized protocols) |
| Output efficiency | • Assess responsiveness (e.g., measure response times and survey customer satisfaction) • Offer more products (e.g., BRISQ data elements with the biospecimen) |
| Social Dimension | |
| Acceptability | • Ensure appropriate ethics review board approval for biobank and research projects using the biobank • Public/donor engagement (e.g., Deliberative forums, active roles in governance) |
| Standards | • Assurance of commitment to good practices (e.g., Accreditation or Certification) |
Financial dimension
In the financial dimension of sustainability we have defined three key areas: market strategy, customer focus and brand recognition. While there are many important activities within each of these three areas, the fundamental element should be the development and maintenance of a strategic plan. The vast majority of biobanks are small mono-user type banks as noted above, initiated to address a specific research question. These are associated with a research plan but rarely a strategic plan for the future disposition, governance or use of the collection or biobank that often remains, nor for justifying ongoing support and future funding sources. And yet these collections draw on and compete for many of the same funding resources and their custodians sometimes aspire to become oligo-user biobanks and seek ongoing support. This creates confusion of priorities for funders as many of these resources are associated with productive investigators, but data based on uneven quality biobanking, and biobanks that are not adapted to enable access to multiple users. At the other end of the spectrum, large poly-user biobanks are usually initiated with a strategic plan of some kind, but with limited access to shared opinion or market data to set scientifically and fiscally appropriate targets for stock and use. This also creates confusion of priorities for funders as many of these resources are associated with high quality biobanking but can only point to intermediate metrics of research impact (e.g., numbers and quality of immediate research publications arising from access to the biobank). The solutions lie in providing assistance to individual biobanks to develop strategic and/or business plans, a service offered by the BRC, and adaptation of appropriate performance metrics. Table 2 shows examples of performance metrics drawn from individual members of the CTRNet, some of which are collected for internal or local purposes and others are collected across the network.
Other aspects of the financial dimension are the accrual and user fee strategies. The BRC provides advice on accrual strategies, based in part on our previous work in analyzing biospecimen use in research. In this work we assessed the trends in biospecimen numbers, preservation formats, and technical applications for use, across more than two decades.25,26 Similarly, the BRC offers access to an online Biobank User Fee Calculator, to facilitate assessment of user fees.24 This calculator includes a range of default values and a setting based on the CTRNet experience and opinion, but also allows user specific values to be entered for almost all variables. It is therefore a tool that can be used by any biobank to determine its user fees based on specific local variables and operational costs but at the same time enabling comparison with and consideration of harmonization with network standards.
Operational dimension
The operational dimension is where decisions are made that influence efficiency of either input, internal, or output components of the biobanking process.
Input components often follow similar protocols and the processes are common to many biobanks operating within a center or region. These components are therefore the most amenable to some degree of merging and offer the greatest opportunities for improving efficiency for individual biobanks. It is therefore no surprise that common accrual mechanisms as exemplified by the CHTN are so successful and can be sustainable for many years. The BRC also offers a synergistic mechanism to create a common platform to facilitate the process of obtaining donor consent. We have shown that when this “Permission to Contact” platform is shared by several biobanks and clinical research projects in a center or region there can be many benefits including enhanced enrollment and reduced overall cost of consent.27,28
As noted above, there is significant and largely unrecognized diversity in types of biobanks. The small- and medium-sized mono- and oligo-user biobanks comprise the majority of biobanks, and are typically designed to address specific research questions. This means that the internal operational components are the most diverse and the perspective of these biobanks varies the most on what constitutes efficiencies and what can be practically implemented. Still, some general rules apply. In deciding on the extent of processing of biospecimens, the probability of recouping this cost from users of the biobank should be carefully considered. For example, after assuming the costs of basic preservation of intact biospecimens (e.g., freezing aliquots) some biobanks also routinely extract products such as DNA and RNA. This additional step may increase the ability to amortize the cost of collection across multiple studies and accelerate research access. However, for many biobanks and often depending on the nature of the biospecimens, an integral part of the design may be to create a collection from which cases can be selected to address “retrospective questions.” For this model of biobank our experience is that only a small proportion of cases (10%–20%) may ever be selected and eventually used for specific research studies. So for each case used in research, the user fee should encompass at least some of the costs for another 5–10 that are not used. But researchers (and their funders) are not accustomed to fees that reflect the true costs for a biobank of even the proportion of cases used. Furthermore many journals still accept data based on biospecimens of unknown quality. Similarly, extensive annotation of all cases may not be cost effective. Consideration should be given to what annotation is necessary to enable eventual case selection and what annotation can be applied after selection. For example, annotation of a breast tumor biospecimen with date of diagnosis, information about the collection and storage process, the cellular composition,29 and sufficient clinical pathology and biomarker data will allow case selection for most studies, even those where patient treatment and outcomes information are important. Initial selection within the biobank can often be made on the basis of prediction of treatment decisions and outcomes, and then the annotation of sufficient cases to meet requirements at that time will refine the final study cohort.
Output components that can be enhanced to address sustainability include optimizing the ability of potential research users to find and access the biobank and improving the response time to requests. Another way to improve output is to offer more products. This is one of the benefits for a biobank of implementing a “permission to contact” platform as a separate process in addition to operating the other components of its own existing consent mechanism.27, 28 The “permission to contact” serves the biobank itself but also provides another distinct product to researchers, either alone or in conjunction with a banked biospecimen. Similarly, adoption of the Biospecimen Reporting for Improved Study Quality (BRISQ) guidelines,29 which is required or recommended by some journals such as Nature, is made easier to accomplish by the use of an online tool offered by the BRC. While requiring additional effort by the biobank staff to enter the data relevant to a specific study cohort at the time of release to a research user, this becomes a basic reusable template for most variables that are common to most biospecimens within the biobank. Effort will then be reduced substantially at every subsequent release occasion.
Social dimension
The social dimension encompasses two main aspects. The first relates to the acceptability of the activity of biobanking. This is relevant to the public at large, people and patients who become donors, and entities that are immediately responsive to these stakeholders (e.g., public institutions, government, funders). Aside from general efforts around publicity, communication, and establishing transparency around purpose and governance, specific strategies are needed to address views on acceptability such as active solicitation of public input into operation of biobanks (e.g., deliberative forums, public workshops). Our experience has been that these events can generate information that informs useful practice changes and also establishes lines of communication that can result in public representation in the governance of the biobank.30
The second aspect of the social dimension relates to commitment to accepted standards of practice. This is relevant to all stakeholders and creates a basis for measuring value. A variety of approaches can be adopted to demonstrate and/or secure assurance of such commitments including Ethics Review Board approval, membership of networks, staff training, and external quality control measures applied to products. Many of these approaches are prerequisites for accreditation or certification programs—the former approach offers a mark of quality to which professional biobanks can aspire, while the latter approach offers recognition of adherence to common principles and standards. Both accreditation and certification therefore provide alternative mechanisms for different types of biobanks to demonstrate different forms of commitment. The focus of the BRC has been on offering a certification program12 that is suitable for and within the reach of all types of biobanks, to demonstrate a commitment to adopting common principles of best practices.
Conclusion
In summary, we believe that the topic of sustainability has been challenging for the discipline of biobanking for many reasons, but one of these is a lack of a common understanding of the several dimensions that influence sustainability of any activity. We therefore propose a framework and provide some examples of practical solutions in different areas of the activity, to guide future discussions for improving the sustainability of biobanks. We also argue that while biobanks should give careful consideration to these approaches, the viability of biobanks cannot be determined just from the financial and operational balance sheets. Thus, support for biobanks, that provide an essential fuel for the activity of basic science, should also be assessed in terms of societal values.
Acknowledgments
The authors acknowledge support and feedback from the Tumour Tissue Repository and Biobank Resource Centre team members and the Canadian Tumour Repository Network (CTRNet).
Author Disclosure Statement
The authors disclosed no conflicts of interest. S.Y. Nussbeck was supported by a fellowship within the Postdoc-Program of the German Academic Exchange Service (DAAD).
References
- 1.Medicine's new central bankers. The Economist. 2005December8; Available from: http://www.economist.com/node/5244139 Last accessed 2013August2 [Google Scholar]
- 2.Park A. 10 Ideas Changing the World Right Now. Time. 2009March12; Available from: http://www.time.com/time/specials/packages/article/0,28804,1884779_1884782_1884766,00.html Last accessed 2013August2
- 3.Vaught J, Rogers J, Myers K, Lim MD, Lockhart N, Moore H, et al. An NCI perspective on creating sustainable biospecimen resources. J Natl Cancer Inst Monogr. 2011;2011(42):1–7 [DOI] [PubMed] [Google Scholar]
- 4.Vaught J, Rogers J, Carolin T, Compton C. Biobankonomics: Developing a Sustainable Business Model Approach for the Formation of a Human Tissue Biobank. JNCI Monogr. 2011;2011(42):24–31 [DOI] [PubMed] [Google Scholar]
- 5.Where's the next Lehman? The Economist.; Available from: http://www.economist.com/news/leaders/21584975-five-years-after-maelstrom-september-2008-global-finance-safer-still-not-safe Last accessed 2013November7
- 6.ISBER. Int. Soc. Biol. Environ. Repos. Available from: http://www.isber.org Last accessed 2013November7
- 7.ESBB Biobanking Society. Eur. Middle East. Afr. Soc. Fo Biopreservation Biobanking. Available from: http://www.esbb.org/ Last accessed 2013November7
- 8.Curtis C, Shah SP, Chin S-F, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson PH, Ravid R, Eng CB, Litton J-E, Vaught J, Matusan A. What Are the Main Roadblocks to Transnational Biobank Collaboration, and How Can We Overcome Them? Biopreservation Biobanking. 2011;9(3):213–6 [DOI] [PubMed] [Google Scholar]
- 10.Dawson S-J, Tsui DWY, Murtaza M, Biggs H, Rueda OM, Chin S-F, et al. Analysis of Circulating Tumor DNA to Monitor Metastatic Breast Cancer. N Engl J Med. 2013March28;368(13):1199–209 [DOI] [PubMed] [Google Scholar]
- 11.Hewitt R, Watson PH. Defining Biobank. Biopreservation Biobanking. 2013;11(5)309–315 [DOI] [PubMed] [Google Scholar]
- 12.Matzke EAM, O'Donoghue S, Barnes RO, Daudt H, Cheah S, Suggitt A, et al. Certification for Biobanks: The Program Developed by the Canadian Tumour Repository Network (CTRNet). Biopreservation Biobanking. 2012;10(5):426–32 [DOI] [PubMed] [Google Scholar]
- 13.Watson PH, Barnes RO. A Proposed Schema for Classifying Human Research Biobanks. Biopreservation Biobanking. 2011December;9(4):327–33 [DOI] [PubMed] [Google Scholar]
- 14.Natural Science Collections Alliance » Resources. Available from: http://nscalliance.org/?page_id=12 Last accessed 2013November7
- 15.Sustainability. Wikipedia Free Encycl. 2013. Available from: http://en.wikipedia.org/w/index.php?title=Sustainability&oldid=562266292 Last accessed 2013August2
- 16.Strange T, Bayley A, Organisation for Economic Co-operation and Development. Sustainable development: linking economy, society, environment. Paris: OECD; 2008 [Google Scholar]
- 17.O'Doherty KC, Burgess MM. Engaging the Public on Biobanks: Outcomes of the BC Biobank Deliberation. Public Health Genomics. 2009;12(4):203–15 [DOI] [PubMed] [Google Scholar]
- 18.Scudellari M. Biobank managers bemoan underuse of collected samples. Nat Med. 2013;19(3):253–253 [DOI] [PubMed] [Google Scholar]
- 19.CHTN - Cooperative Human Tissue Network. Available from: http://www.chtn.nci.nih.gov/ Last 2013August2
- 20.Watson PH, Wilson-McManus JE, Barnes RO, Giesz SC, Png A, Hegele RG, et al. Evolutionary concepts in biobanking - the BC BioLibrary. J Transl Med. 2009;7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canadian Tumour Repository Network. Available from: http://www.ctrnet.ca/ Last accessed 2013August2
- 22.About ICR - CIHR. Available from: http://www.cihr-irsc.gc.ca/e/12407.html Last accessed 2013August2
- 23.Public Population Project in Genomics and Society. Available from: http://p3g.org/ Last accessed 2013August2
- 24.The Biobank Resource Centre Home. Available from: http://www.biobanking.org/brc/ Last accessed 2013August2
- 25.Hughes SE, Barnes RO, Watson PH. Biospecimen Use in Cancer Research Over Two Decades. Biopreservation Biobanking. 2010;8(2):89–97 [DOI] [PubMed] [Google Scholar]
- 26.Cole A, Cheah S, Dee S, Hughes S, Watson PH. Biospecimen Use Correlates with Emerging Techniques in Cancer Research: Impact on Planning Future Biobanks. Biopreservation Biobanking. 2012;10(6):518–25 [DOI] [PubMed] [Google Scholar]
- 27.LeBlanc J, Dee S, Braun L, Daudt H, Cheah S, Watson PH. Impact of a Permission to Contact (PTC) Platform on Biobank Enrollment and Efficiency. Biopreservation Biobanking. 2013;11(3):144–8 [DOI] [PubMed] [Google Scholar]
- 28.Cheah S, O'Donoghue S, Daudt H, Dee S, LeBlanc J, Braun L, et al. Permission to Contact' (PTC) – a strategy to enhance patient engagement in translational research. Biopreservation Biobanking. 2013;11(4):245–252 [DOI] [PubMed] [Google Scholar]
- 29.Moore HM, Kelly A, Jewell SD, McShane LM, Clark DP, Greenspan R, et al. Biospecimen Reporting for Improved Study Quality. Biopreservation Biobanking. 2011April;9(1):57–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Doherty KC, Hawkins AK, Burgess MM. Involving citizens in the ethics of biobank research: informing institutional policy through structured public deliberation. Soc Sci Med 1982. 2012November;75(9):1604–11 [DOI] [PubMed] [Google Scholar]
- 31.Mabile L, Dalgleish R, Thorisson GA, Deschênes M, Hewitt R, Carpenter J, et al. Quantifying the use of bioresources for promoting their sharing in scientific research. Gigascience. 2013;2(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]