Abstract
Background: Tumor necrosis factor-alpha (TNF-α) appears to be linked with hyperandrogenism (HA), increased insulin resistance (IR), and obesity (Ob), which were common features noted with polycystic ovarian syndrome (PCOS). Our aim was to study the role of TNF-α in the pathogenesis of IR and Ob in PCOS, as well as a C850T (rs1799724) polymorphism in the promoter region of the TNF-α gene, in a group of 204 PCOS patients and 204 age-matched healthy controls. Results: Significant differences were observed between PCOS patients and controls. All the PCOS had elevated body mass index, waist circumference, waist-to-hip ratio, fasting insulin, homeostatic model assessment (HOMA) score, and serum TNF-α when compared with controls (p<0.05). Genotype distribution for the C-850T polymorphism was observed with the frequency of the variant T allele being 0% in the PCOS group and 9% in the control group (p=0.0032). Conclusions: In conclusion, our present results suggest that the TNF-α system might contribute to the pathogenesis of HA, Ob, and IR in PCOS independent of a polymorphism of the TNF-α C850T (rs1799724) in our population.
Background
Polycystic ovarian syndrome (PCOS) is one of the most common endocrine dysfunctions in women of reproductive age with a prevalence of approximately 5–10% worldwide (Azziz et al., 2006, 2009; Dasgupta and Mohan Reddy, 2008) The principle features of PCOS are insulin resistance (IR), hyperandrogenism (HA), obesity (Ob), oligo/anovulation, and polycystic ovaries (PCO). Many candidate genes have been proposed as important contributors to PCOS (Legro, 1995; Urbanek et al., 1999) but none have yet achieved acceptance as a major cause for this important clinical condition. Hyperexpression of tumor necrosis factor-alpha (TNF-α) in muscle and adipose tissues is implicated in the development of IR in humans, by decreasing the tyrosine kinase activity of the insulin receptor (Hotamisligil, 1999; Escobar- Morreale et al., 2001; Hart et al., 2004). TNF-α promotes IR, causes HA, and is involved in follicular development and, hence, it has been implicated in the pathophysiology of PCOS. The TNF-α gene is located on 6p21.3, spans approximately 3 kb, and has four exons. -G308A, and -C850T polymorphisms in the promoter region of TNF-α have been associated with chronic inflammatory diseases such as ulcerative colitis, rheumatoid arthritis, and Crohn's disease (Yun et al., 2011).
Therefore, the aim of this study was to study various clinical manifestations of HA, body mass index (BMI), IR, serum TNF-α levels, and C850T (rs1799724) polymorphism in the promoter region of the TNF-α gene in PCOS patients and age-matched healthy controls of our study population.
Materials and Methods
Subjects
This study was approved by the Institutional Ethics Committee, and informed written consent was obtained from all subjects. In this prospective case-control study, we included 204 consecutive PCOS patients from different Obstetrics and Gynecology centers from July 2011 to January 2013. Subjects ranged in age from 17 to 35 years and were diagnosed using the 2006 AES (Androgen Excess Society) criteria (Azziz et al., 2006, 2009; Dasgupta and Mohan Reddy, 2008); 1.HA clinical or biochemical and either; 2. Oligo/anovulation or 3. Polycystic ovarian morphology. All subjects underwent a transvaginal ultrasound or transabdominal ultrasound in the follicular phase to evaluate ovary morphology and any lesions in the pelvic area.
Exclusion criteria
Women excluded from the study were those with inherited disorders such as congenital adrenal hyperplasia, androgen secreting neoplasms, androgenic/anabolic drug use or abuse, Cushing's syndrome, syndromes of severe IR, thyroid dysfunction, and hyperprolactinemia. In addition, 204 controls were included in this study over the same period. They visited the health-care center in a super-speciality hospital as a part of a group checkup for work or an individual need for an annual comprehensive medical checkup with no specific health problems. Subjects ranged from 17 to 35 years and did not show hirsutism, acne, or male-type alopecia. All of them had regular menstrual cycles, and none of them satisfied any of the 2006 AES criteria. All control subjects also underwent an ultrasonographic examination, and women who had any pathologic findings such as PCO were excluded from the study.
Sampling
Two milliliters of peripheral blood and serum were collected from all the patients and controls along with clinical data, personal history, and family history.
Biochemical and hormonal findings: fasting plasma glucose (enzymatic colorimetric method), fasting insulin, serum TNF-α, total and free testosterone, androstenedione, and dehydroxy epiandrostenedione were estimated by ELISA using DRG kits.
Laboratory controls were used to check the accuracy and precision of the analyzer, reagents, and assay results.
Isolation of DNA and genotype analysis
Genomic DNA was isolated from the peripheral blood of subjects according to the method routinely used in our laboratory (Govindan et al., 2007; Movva et al., 2007). The DNA was stored at −20°C until it was processed. Genotyping for the TNF-α polymorphism (rs1799724) was performed by a polymerase chain reaction (PCR) with specific published primers (Pazarbasi et al., 2007) (Table 1), synthesized from Bioserve Biotechnology Ltd. (Hyderabad, India), followed by restriction fragment length polymorphism analysis. A three-step PCR was performed using an XP thermal cycler; briefly, the PCR conditions included an initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, extension at 68°C for 45 s, and a final extension at 68°C for 5 min. The 131 bp amplified PCR product was digested with Hind II (MBI Fermentas, Hannover, MD), in a total volume of 20 μL for 2 h at 37°C, and analyzed on a 12% polyacrylamide gel after electrophoresis and staining with silver nitrate. In the case of the C allele at position-850, Hind II digestion produces 106 and 25 bp fragments. In contrast, the 131 bp fragment remains undigested when the T allele is located at this position. Restriction enzyme-digested PCR products (Table 2 and Fig. 1) were imaged and analyzed by documentation in a UVI Tech gel documentation system (UVI Tech Ltd., Cambridge, United kingdom).
Table 1.
Comparison of Anthropometric and Biochemical Characteristics in Polycystic Ovarian Syndrome Patients and Controls with Their Mean and Standard Deviations
| S.No. | Parameter | Patients (n=204) | Controls (n=204) | p-Value |
|---|---|---|---|---|
| 1 | Age (years) | 28±3.6 | 28±5.1 | 1.0000 |
| 2a | BMI (kg/m2) | 27.12±4.93 | 23.4±3.2 | <0.0001 |
| 3a | WC (inches) | 37±4.3 | 30.36±3.3 | <0.0001 |
| 4 | HC (inches) | 39.4±4.1 | 38.11±3.7 | 0.0008 |
| 5a | WHR | 0.93±0.04 | 0.79 0±05 | <0.0001 |
| 6 | Fasting glucose (mg/dL) | 88±8.6 | 86.85±7.1 | 0.0678 |
| 7a | Fasting insulin (μU/mL) | 16.94±7.26 | 6.66±3.19 | <0.0001 |
| 8a | HOMA score | 3.73±3.8 | 1.44±0.75 | <0.0001 |
| 9a | Serum TNF-α (pg/mL) | 13.24±10 | 5.5±3.8 | <0.0001 |
| 10a | Total Testosterone (ng/mL) | 5.8±4.319 | 1.32±1.05 | <0.0001 |
| 11a | Free Testosterone (ng/mL) | 8.39±6.69 | 2.6±1.4 | <0.0001 |
| 12a | Androstenedione (ng/mL) | 2.41±1.5 | 1.046±0.68 | <0.0001 |
| 13a | DHEA (ng/mL) | 6.22±5.66 | 1.9±0.99 | <0.0001 |
Significant values (p is <0.05).
BMI, body mass index; DHEA, dehydroxyepiandrostenedione; HC, hip circumference, HOMA score, homeostatic model assessment score; TNF-α, tumor necrosis factor alpha; WC, waist circumference; WHR, waist/hip ratio.
Table 2.
Tumor Necrosis Factor Alpha Gene–Primer Sequence
| TNF-α promoter region | Primers | PCR product | TT | 131 bp | |
|---|---|---|---|---|---|
| rs 1799724 | Forward | AAGTCGAGTATGGGGACCCCCCGTTAA | CT | 131 bp | |
| 106 bp | |||||
| 25 bp | |||||
| HIND II | Reverse | CCCCAGTGTGTGGCCATATCTTCTT | CC | 106 bp | |
| 25 bp | |||||
FIG. 1.
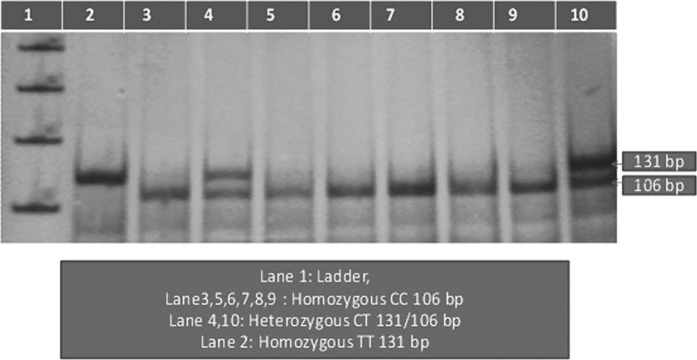
12% polyacrylamide gel electrophoresis (-C850T polymorphism of tumor necrosis factor-α).
Data and statistical analysis
BMI=weight/height2 (kg/m2). The homeostatic model assessment for IR (HOMA-IR) was calculated by using the formula: fasting serum insulin (μU/mL)×fasting plasma glucose (mg/dL)/405 (Chae et al., 2008). Statistical analysis was performed using “Medcalc” statistical software (MSS), the United States. Chi-square test (χ2), odds ratio (OR), and 95% confidence interval (CI) were performed to assess the association between the groups. A p-value of <0.05 was considered statistically significant.
The Hardy–Weinberg distribution of genotypes in the PCOS and control groups was assessed using a web-tool [χ2 test] Hardy–Weinberg equilibrium calculator (Santiago Rodriguez et al., 2009) and were found to be in equilibrium in both patient and control groups.
Results
Clinical findings
Among 204 patients, the percentages of various clinical HA features such as central Ob, 95%; hirsutism, 92%; acne, 88%; alopecia, 65%; male pattern of hair loss, 18%; and acanthosis nigricans, 7% were noted. The other anthropometric measurements such as weight, height, BMI, waist circumference (WC), hip circumference, and waist/hip ratio (WHR) of PCOS patients were compared with those of controls (Table 1). Significant differences were noted with BMI, WC, and WHR.
Biochemical findings
Significant increases in fasting insulin, HOMA score, serum TNF-α, and male hormones were observed with PCOS patients when compared with controls (Table 1).
Genetic analysis
A total of 204 PCOS patients and 204 age-matched healthy control women were genotyped for the C-850T polymorphism in the TNF-α gene promoter, and genotype and allele frequencies were shown in Table 3. The distribution of T allele was slightly more with controls when compared with patients (p<0.0001, odds ratio 0.1755; 95% CI 0.1117 to 0.2759). The odds ratio for PCOS associated with the pooled TT and CT genotypes was 0.1643 (95% CI 1.002 to 0.268) (Table 4). The genotypes were found to be in Hardy–Weinberg equilibrium in both patient and control groups.
Table 3.
Genotypes and Alleles of TNF-α Polymorphism Identified in the Study
| TNF-α/HIND II (C to T change) | CC | CT | TT | C allele | T allele |
|---|---|---|---|---|---|
| Patients | 178 (87.25%) | 26 (12.75%) | — | 0.94 | 0.06 |
| Controls | 108 (53%) | 78 (38%) | 18 (9%) | 0.72 | 0.28 |
χ2=55.4, p<0.0001.
Odds ratio for alleles: 5.69, 95% CI 3.6242 to 8.9, p<0.0001.
95% CI, 95% confidence interval.
Table 4.
Statistical Analysis of Genotypes of TNF-α Polymorphism Identified in the Study
| Genotype | PCOS | Controls | Odds ratio, (95% CI) | p-Value |
|---|---|---|---|---|
| CC vs. CT+TT | 178/26 | 108/96 | 6.085 (3.70 to 9.98) | <0.0001 |
| CT vs. CC+TT | 26/178 | 78/126 | 0.236 (0.143 to 0.388) | <0.0001 |
| TT vs. CT+CC | 01/205 | 19/187 | 0.048 (0.006 to 0.0367) | 0.0032a |
| TT+CT vs. CC | 26/178 | 96/108 | 0.1643 (0.1002 to 0.268) | <0.0001 |
| CC+CT vs. TT | 205/01 | 187/19 | 20.82 (2.762 to 157) | 0.0032a |
After Yates correction.
Discussion
The principal features of PCOS are insulin resistance (IR), hyperandrogenism (HA), obesity (Ob), oligo/anovulation, and PCO (Azziz et al., 2006, 2009; Dasgupta and Mohan Reddy, 2008). Many candidate genes have been proposed as important contributors to PCOS (Legro, 1995; Urbanek et al., 1999). TNF-α is a cytokine secreted by adipose tissue that plays a key role in mediating IR (Hotamisligil, 1999). TNF-α promotes IR, causes HA, and is involved in follicular development and, hence, it has been implicated in the pathophysiology of PCOS. Hyperexpression of TNF-α in muscle and adipose tissues is implicated in the development of IR in humans, by decreasing the tyrosine kinase activity of the insulin receptor (Hotamisligil, 1999; Escobar-Morreale et al., 2001; Hart et al., 2004). Hence, we studied the clinical and biochemical manifestations of HA of PCOS and its association with -C850T (rs1799724) polymorphism in the promoter region of TNF-α in our study population.
This study showed 92% of hirsutism, 88% of acne, and 66% of androgenic alopecia as clinical manifestations of HA in our PCOS patients, which was more than a meta-analysis, which showed 65–75% hirsutism, 15–25% acne, and 10–40% of androgenic alopecia (Azziz et al., 2006, 2009). The higher prevalence of hirsutism in our population is similar to the observation made by Wijeyratne et al. (2002), among Southern Asians and it can be explained partly due to strict adherence to AES-2006 criteria (Azziz et al., 2006, 2009; Dasgupta and Mohan Reddy, 2008) in recruiting the study population.
Obesity and insulin resistance are frequent findings in hyperandrogenic women (Dunaif, 1997). Ob affects approximately 50% PCOS patients (Azziz et al., 2004)[17]; similarly, in our study population, 70% of PCOS patients were obese (BMI≥25 kg/m2), as per the Asia-Pacific definition of Ob (Chen et al., 2010). This high prevalence can be attributed to food habits and lifestyles of Indian women.
The HOMA score is a good indicator of IR. In our study, the HOMA score was significantly higher in PCOS compared with controls, similar to the findings of Chae et al. (2008). The prevalence of IR is greater in obese than in nonobese patients; as per the meta-analysis, between 50% and 70% of women with PCOS have demonstrable IR and hyperinsulinism (Azziz et al., 2006, 2009). Similarly, we have observed 59% of PCOS women with IR and hyperinsulinism in our study.
Serum levels of TNF-α were shown to increase in patients with PCOS (Hotamisligil, 1999; Escobar-Morreale et al., 2001; Hart et al., 2004); similarly, in our study, we found increased serum TNF-α in PCOS patients compared with healthy controls. As noted by Escobar-Morreale et al. (2001), serum TNF-α levels increased mainly because of Ob in both controls and hyperandrogenic women; similarly, we noted 70% Ob in our hyperandrogenemic PCOS cases.
Several polymorphisms in the promoter region of the TNF-α gene have been described. -G308A, and -C850T polymorphisms of TNF-α have been associated with chronic inflammatory diseases such as ulcerative colitis, rheumatoid arthritis, and Crohn's disease (Yun et al., 2011). In this study, no association was demonstrated between alleles at the C850T polymorphism located in the promoter region of the TNF-α gene and PCOS, similar to a study conducted by Korhonen et al. (2002) in Finnish patients. However, when we compared our results with the Korhonen study (Table 5), the frequency of the T allele is higher in the controls than in patients, and the variant (TT) genotype frequency is nil with patients and 8.8% in case of controls. This difference in the genotype frequency between our study and that of Korhonen et al. (2002) can be explained by ethnic variation. Previously, Milner et al. (1999) and Escobar-Morreale et al. (2001) failed to show an association of -308 polymorphism of TNF-α with PCOS, but Escobar-Morreale et al. (2001) concluded that -308G/A polymorphism in the promoter of TNF-α gene modulates ovarian function, resulting in increased serum androgen levels in the carriers of -308 A variant. In our study, though we have not studied the -308G/A polymorphism, we found elevated androgen levels in PCOS patients compared with controls.
Table 5.
Comparison of the Genotype and Allele Frequencies of the TNF-α Gene Promoter C-850T Polymorphism Among Women with Polycystic Ovarian Syndrome and Healthy Age-Matched Controls
| TNF-α C-850T (%) | |||||
|---|---|---|---|---|---|
| Genotype frequencies | Allele frequencies | ||||
| CC | CT | TT | C | T | |
| Korhonen et al. (2002) | |||||
| Patients (n=87) | 74 (85.1%) | 11 (12.6%) | 2 (2.3%) | 159 (91.4%) | 15 (8.6%) |
| Controls (n=115) | 96 (83.5%) | 16 (13.9%) | 3 (2.6%) | 208 (90.4%) | 22 (9.6%) |
| Our results | |||||
| Patients (n=204) | 178 (87.25%) | 26 (12.75%) | 0 (0%) | 382 (93.6%) | 26 (6.4%) |
| Controls (n=204) | 108 (52.94%) | 78 (38.23%) | 18 (8.82%) | 294 (72%) | 114 (28%) |
Conclusions
To conclude, an increased BMI, HOMA score, serum TNF-α, and androgen levels in our PCOS patients compared with age-matched healthy controls suggest that the TNF-α system might contribute to the pathogenesis of HA, Ob, and IR in PCOS independent of the polymorphism of the TNF-α C850T (rs1799724) in our population.
Acknowledgments
Grant Number: SR/WOS-A/LS-91/2011, Women Scientist Scheme-A, Department of Science and Technology, Ministry of Science and Technology, Government of India.
Management of KAMS&RC, VMRC for providing support to carry out the research.
Authors' Contributions
Sujatha Thathapudi: study design, literature search, lab work, data acquisition, data analysis, statistical analysis, and manuscript preparation; Qurratulain Hasan: study design, definition of intellectual content, data analysis, manuscript editing, and manuscript review; Jayashankar Erukkambattu: literature search, data analysis, statistical analysis, and manuscript preparation; Uma A: data analysis and statistical analysis; Anuradha K: Clinical studies, data acquisition; Vijayalakshmi Kodati: study design, manuscript review.
Author Disclosure Statement
No competing financial interests exist.
References
- Azziz R, Carmina E, Dewailly D, et al. (2006) Position statement: Criteria for Defining PCOS as a Predominantly Hyperandrogenic Syndrome: An Androgen Excess Society Guideline. J Clin Endocrinol Metab 91:4237–4245 [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, et al. (2009) The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91:456–488 [DOI] [PubMed] [Google Scholar]
- Azziz R, Sanchez LA, Knochenhauer ES, et al. (2004) Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab 89:453–462 [DOI] [PubMed] [Google Scholar]
- Chae SJ, Kim JJ, Choi YM, et al. (2008) Clinical and biochemical characteristics of polycystic ovary syndrome in Korean women. Hum Reprod 23:1924–1931 [DOI] [PubMed] [Google Scholar]
- Chen X, Ni R, Mo Y, et al. (2010) Appropriate BMI levels for PCOS patients in Southern China. Hum Reprod 25:295–302 [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Mohan Reddy B. (2008) Present status of understanding on the genetic etiology of polycystic ovary syndrome. J Postgrad Med 54:115–120 [DOI] [PubMed] [Google Scholar]
- Dunaif A. (1997) Insulin resistance and the polycystic ovary syndrome: Mechanism and implication for pathogenesis. Endocr Rev 18:774–780 [DOI] [PubMed] [Google Scholar]
- Escobar–Morreale HF, Calvo RM, Sancho J, San Milan JL. (2001) TNF-α and hyperandrogenism: a clinical and biochemical, and molecular genetic study. J Clin Endocrinol Metab 86:3761–3767 [DOI] [PubMed] [Google Scholar]
- Govindan S, Ahamad SN, Vedicherla B, et al. (2007) Association of progesterone receptor gene polymorphism (PROGINS) with endometriosis, uterine fibroids and breast cancer. Cancer Biomark 3:73–78 [DOI] [PubMed] [Google Scholar]
- Hart R, Hickey M, Franks S. (2004) Definition, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Prac Res Obstet Gynecol 18:671–683 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. (1999) The role of TNF-α and TNF receptor in obesity and insulin resistance. J Intern Med 245:621–625 [DOI] [PubMed] [Google Scholar]
- Korhonen S, Romppanen EL, Hiltunen M, et al. (2002) Lack of association between -C850T polymorphism of the gene encoding tumor necrosis factor-alpha and polycystic ovary syndrome. Gynecol Endocrinol 16:271–274 [DOI] [PubMed] [Google Scholar]
- Legro RS. (1995) The genetics of polycystic ovary syndrome. Am J Med 98Suppl 1A:165. [DOI] [PubMed] [Google Scholar]
- Milner CR, Craig JE, Hussey ND, Norman RJ. (1999) No association between the -308 polymorphism in the tumor necrosis factor alpha promoter region and polycystic ovaries. Mol Hum Reprod 5:5–9 [DOI] [PubMed] [Google Scholar]
- Movva S, Alluri R, Komandur S, et al. (2007) Relationship of angiotensin- converting gene polymorphism with nephropathy associated with type 2 diabetes mellitus in Asian Indians. J Diabetes Complications 21:237–241 [DOI] [PubMed] [Google Scholar]
- Pazarbasi A, Kasap M, Guzel AI, et al. (2007) Polymorphisms in the tumor necrosis factor alpha gene in Turkish women with pre-eclampsia and eclampsia. Acta Med Okayama 61:153–160 [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Gaunt TR, Day IN. (2009) Hardy–Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol 169:505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek M, Lergo RS, Driscoll DA, et al. (1999) Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A 96:8573–8578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijeyratne CN, Balen AH, Barth JH, Belchetz PE. (2002) Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome (PCOS) among South Asians and Caucasians: Is there a difference? Clin Endocrinol(Oxf) 57:343–350 [DOI] [PubMed] [Google Scholar]
- Yun JH, Choi JW, Lee KJ, et al. (2011) The Promoter -1031 (T/C) polymorphism in tumor necrosis factor-alpha associated with polycystic ovary syndrome. Reprod Biol Endocrinol 9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]


