Abstract
Human herpesvirus 8 (HHV-8) is the etiologic agent of all Kaposi's sarcoma (KS), the outcome of which is associated with immuno-dysregulation, resulting in the abnormal production of inflammatory cytokines and chemokines. We quantified by enzyme-linked immunosorbent assay serum levels of interleukin (IL)-10, IL-17, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α from patients with KS-AIDS, classic KS, and human immunodeficiency virus (HIV) without KS. A correlation between HHV-8 molecular detection and cytokine production was also performed. We observed that IL-10 production was higher in patients with KS-AIDS when compared to those with classic KS or HIV. However, no significant differences were seen for IFN-γ, TNF-α, or IL-17 production between studied groups. When patients with KS-AIDS were analyzed according to lesion topography, IL-10 levels were higher in patients with disseminated disease than those observed in patients with only cutaneous lesions or cutaneous and digestive and/or respiratory tract lesions. Finally, patients with KS-AIDS that presented viral DNA for HHV-8 in serum showed a higher production of IL-10 when compared with those patients with a negative result for nested polymerase chain reaction for the virus. The results presented here are the first to demonstrate that there exists a stratification of patients with KS-AIDS according to lesion topography where IL-10 levels are higher in those individuals with disseminated disease than those with only localized lesions.
Introduction
Human herpesvirus 8 (HHV-8) is the etiologic agent of all Kaposi's sarcoma (KS) forms, including KS-AIDS, classic KS, endemic KS, and iatrogenic KS (3), and is also related to rare diseases such as primary effusion lymphoma and multicentric Castleman's disease (MCD) (2,20).
The pathogenesis of KS is complex, and its development is linked to immunodeficiency, as in KS-AIDS and iatrogenic KS. On the other hand, classic and endemic KS patients usually have a fully functional immune system (10).
HHV-8 establishes a persistent infection, and the virus adopts one of two forms: a latent replicative cycle that is relatively stable and immunologically silent, or a lytic cycle, necessary for the production of new viral particles and the transmission to the host. Then, once an individual is infected by HHV-8, evasion mechanisms of the virus overcome the balance between the immune system and HHV-8 persistence (4). Thus, KS outcome is more likely associated with immuno-dysregulation resulting in the abnormal production of inflammatory cytokines and chemokines (10).
Many proteins coded by HHV-8 promote a response associated to Th2 cells in several biologic assays while potentially inhibiting Th1 responses. It was demonstrated that T CD4+ and CD8+ cells isolated from KS lesions produced IL-4 rather than IFN-γ, polarizing the immune response to a Th2 profile that is less effective against intracellular pathogens (21).
The purpose of the present study was to analyze the potential participation of interleukin (IL)-10, IL-17, interferon (INF)-γ, and tumor necrosis factor (TNF)-α in the outcome of different clinical manifestations of human Kaposi's sarcoma associated or not to human immunodeficiency virus (HIV) infection. In addition, a correlation between HHV-8 molecular detection and cytokine production was also performed.
Materials and Methods
Patients
Patients participating in this study were recruited from the Infectious Disease Treatment Special Unit at the Clinics Hospital of Ribeirão Preto Medical School, University of São Paulo, Brazil. The study was approved by the local ethics committee (protocol number 12999/2006), and written informed consent was obtained from all participants. For this study, 54 patients with clinical and/or histopathologic diagnosis of KS were enrolled. Within our cohort, a group of 46 patients with KS were HIV positive (KS-AIDS), and eight patients with classic KS were enrolled. In addition, 40 subjects with positive serology for HIV without KS matched by age and gender were recruited as a control group. Information regarding HIV serology was obtained from medical records at the Clinics Hospital.
Considering that HHV-8 is the etiologic agent for KS, all patients with or without KS recruited for the study were screened for HHV-8 antibodies by immunofluorescence. All individuals in the control group were negative for HHV-8 antibodies, while patients clinically diagnosed for KS analysis were positive.
Diagnosis criteria for KS
All patients with KS-AIDS presented cutaneous and/or visceral lesions characteristics of KS as demonstrated by histological analysis, except for cases of tracheal, endobronchial, and digestive tract lesions in which the macroscopic morphological characteristics of the lesions are sufficient for diagnosis. Diagnosis of pulmonary KS was done through bronchoscopy.
Regarding the eight patients with classic KS, six presented with localized lesions on inferior members, one patient presented with a lesion on the left shoulder, and another patient, in addition to lesions on the inferior members, showed a lesion on the base of the tongue.
Immunofluorescence assay for IgG anti-HHV-8
BCBL-1 cells were cultivated in RPMI medium with 20 ng/mL of 12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma, St. Louis, MO) for 96 h. Cells were then washed twice with phosphate-buffered saline (PBS) supplemented with 3% fetal bovine serum (FBS), placed on glass slides (10 μL/well), fixed in cold acetone for 10 min, and stored at −20°C. Glass slides were incubated with human serum diluted 1:40 in PBS with 3% FBS for 30 min at 37°C, washed, and incubated with fluorescein isothiocyanate-conjugated goat anti-human IgG (Dako, Carpinteria, CA) at 1:256 dilution in Evans blue for 30 min at 37°C, washed again, and air dried. Glass slides were mounted using buffered glycerol and placed under a coverslip. Entire cell fluorescence in about 20% of the TPA-treated cells was considered positive for antibodies against lytic-phase antigens (Fig. 1).
FIG. 1.
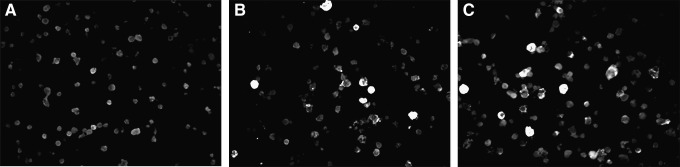
Immunofluorescence assay (IFA) to identify antibodies against antigens of the lytic-phase of human herpesvirus 8 (HHV-8). (A) Negative control of BCBL-1 cells with cytoplasm with no fluorescence label. (B) Positive control of BCBL-1 cells with cytoplasmic green fluorescence indicating reactivity of antibodies to HHV-8 lytic antigens. (C) HHV-8 positive sample from patient with KS-AIDS (400×).
Cytokine quantification by enzyme-linked immunosorbent assay
The cytokine production was measure by quantitative cytokine enzyme-linked immunosorbent assay (ELISA) using commercially available kits: IL-10-OptEIA Set, TNF-α-OptEIA Set, IFN-γ-OptEIA Set (all from BD Pharmingen, San Diego, CA), and IL-17 Quantikine Immunoassay (R&D Systems, Mineapolis, MN). The methodological procedures were followed according to the manufacturer's instructions. The results were calculated by reference to a standard curve and expressed as pg/mL.
Viral DNA extraction and nested polymerase chain reaction
Viral DNA was extracted from 200 μL of serum with the QIAamp DNA blood kit (Qiagen, Valencia, CA) according to the manufacturer's instructions, and DNA was eluted with 50 μL buffer AE. The polymerase chain reaction (PCR) mixture contained, in a total volume of 50 μL, 0.2 mM of each dNTP, 25 pmol of each sense and antisense primer (5′-AGC CGA AAG GAT TCC ACC AT-3′ and 5′-TCC GTG TTG TCT ACG TCC AG-3′) (3), 1.5 mM MgCl2, and 2.5 U Taq DNA Polymerase (Invitrogen, New York), and 5 μL DNA was extracted from each sample. PCR amplification of HHV-8 was done at 94°C for 1 min, followed by 35 cycles of 1 min at 94°C, 1 min at 58°C, and 1 min at 72°C with a final extension step (10 min at 72°C) to allow complete extension of the amplicons. The nested PCR amplification mixture contained 1 μL of the first PCR mixture and the same PCR reagents described above, except that 25 pmol of each sense and antisense internal primers (5′-TTC CAC CAT TGT GCT CGA AT-3′ and 5′-TAC GTC CAG ACG ATA TGT GC-3′) (3) were used. Again, the amplification was done at 94°C for 1 min, followed by 40 cycles of 1 min at 94°C, 1 min a 60°C, 1 min at 72°C, and a final extension of 10 min at 72°C. Ten μL of each reaction mixture were analyzed in a 2% agarose gel electrophoresis. Positive reactions yielded an amplicon of 211bp easily visualized by ethidium bromide staining.
Statistical analysis
Comparisons between patients groups were performed using two way analysis of variance (ANOVA), and the association between cytokines levels and topography of SK-AIDS lesions were tested by using one-way ANOVA. Both tests were followed by Bonferroni correction. Correlation between IL-10 serum levels and nested-PCR for HHV-8 was established using the Mann–Whitney U-test. All values were considered significantly at p<0.05. The software employed for analysis was GraphPad InStat (GraphPad Software, Inc., La Jolla, CA). Values of cytokines levels were reported as mean±standard deivation (SD) pg/mL.
Results and Discussion
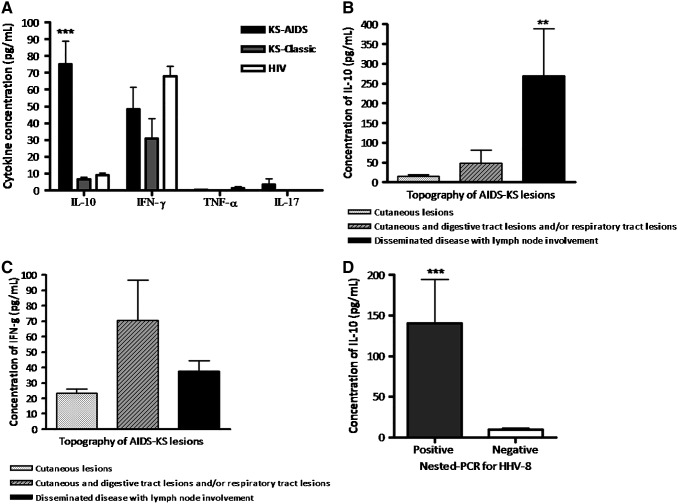
The purpose of the present study was to analyze the potential participation of IL-17, IL-10, IFN-γ, and TNF-α in the outcome of different clinical manifestations of human Kaposi's sarcoma associated or not to HIV infection. We analyzed the levels of these cytokines in serum samples from 46 patients with KS-AIDS: eight patients with classic KS, and, 40 HIV patients with negative serology for HHV-8 and no manifestations of KS as controls. In particular, our study shows that IL-10 production was higher in patients with KS-AIDS when compared to those with classic KS (M=75.10±93.11 vs. 6.54±3.92 pg/mL; p<0.001) or HIV (M=8.92±8.65 pg/mL; p<0.001). However, no significant differences (p>0.05) were seen for IFN-γ, TNF-α, or IL-17 production between the studied groups (Fig. 2A).
FIG. 2.
Measurement of cytokines levels by enzyme-linked immunosorbent assay in serum samples from patients with Kaposi's sarcoma (KS)-AIDS, classic KS, and seropositive patients for HIV with no manifestations of KS. Forty-six samples were tested for KS-AIDS, eight for classic KS, and 40 samples from HIV-positive patients with no manifestation of KS. (A) Interleukin (IL)-10, interferon (IFN)-γ, tumor necrosis factor-α, and IL-17 production in studied groups. (B) IL-10 levels according to the lesion topography in KS patients. (C) IFN-γ production according to KS lesion topography. (D) Correlation between IL-10 levels and nested polymerase chain reaction for HHV-8 in serum from KS-AIDS. **p<0.01; ***p<0.001.
Interestingly, when patients with KS-AIDS were analyzed according to lesion topography, IL-10 levels were higher in patients with disseminated disease than those observed in patients with only cutaneous lesions (269.00±337.30 vs. 14.68±16.10 pg/mL; p<0.01) or cutaneous and digestive and/or respiratory tract lesions (48.54±154.8 pg/mL; p<0.05; Fig. 2B).
Thus, our results suggest that this increase in serum IL-10 probably favors a progression of the disease once it suppresses cytokines production by Th1 lymphocytes, reducing the cell-mediated response to the virus. Moreover, in B-lymphocytes, IL-10 induces proliferation of these cells associated with higher antibody production, resulting in a Th2 profile response. Furthermore, regarding macrophages, IL-10 decreases the production of TNF-α, IL-1, IL-2, IL-6, IL-8, and GM-CSF, consequently inhibiting their role as T-cell activators (22).
Our results are supported by the observation that IL-10 is expressed in many types of tumor cells such as those seen in lung, breast, colon, gastric, skin, pancreas, hepatocellular, kidney, and head and neck cancer (1,5–9,11,14,19). In addition, an increase in IL-10 serum concentration in most human cancer cells is frequently associated with a negative prognosis of the disease, as IL-10 systemic concentration augments in the later stages of cancer (12). Furthermore, it was shown in cell culture systems that HHV-8 infection and HHV-8 microRNA overexpression induced the preferential secretion of IL-6 and IL-10 by murine macrophages and human myelomonocytic cells. These data support a role for HHV-8 in the activation of macrophages cytokine response to HHV-8-related tumor progression (18).
On the other hand, the analysis of serum IFN-γ in KS-AIDS patients classified according to lesion topography showed no differences between the studied groups (p>0.05; Fig. 2C), as well as TNF-α and IL-17 (data not shown). In support of our observations, Pugliese et al. (17) found no significant differences regarding IFN-γ serum levels between HIV-1 positive/HHV-8 negative and HIV-1 positive/HHV-8 positive subjects.
As HHV-8 is the etiologic agent for KS, all patients with or without KS recruited for the study were screened for HHV-8 antibodies by immunofluorescence. In addition, the detection rate of HHV-8 through nested-PCR was 50% (23/46) and 25% (2/8) in serum samples of patients with KS-AIDS and classic KS respectively. Interestingly, patients with KS-AIDS that presented viral DNA for HHV-8 in serum showed a higher production of IL-10 when compared with those patients with negative result for nested-PCR for the virus (140.7±259.2 vs. 9.52±10.16 pg/mL; p=0.0004; Fig. 2D).
As mentioned before, HHV-8 is related to MCD, a disease characterized by high serum levels of C reactive protein and high HHV-8 viral load in peripheral blood mononuclear cells. What is more interesting, as observed in our patients with KS, patients with MCD present high plasma IL-6 and IL-10 levels, and the strong correlation between both cytokines with HHV-8 viral load suggest that both IL-6 and IL-10 may be involved in the pathogenesis of this virus-associated lymphoproliferative disorder (13,16). Furthermore, a similar pattern of cytokine expression was seen in patients with HHV-8 inflammatory cytokines syndrome (KICS). Patients with this syndrome exhibit elevated HHV-8 viral load, and vIL-6, IL-6, and IL-10 comparable to those seen in individuals with MCD (15).
Despite the increasing number of studies carried out to investigate the role of HHV-8 in KS pathogenesis, limited and controversial information exists about the contribution of cytokines, in particular IL-10, to the clinical outcome of this disease. Furthermore, the results presented here are the first to demonstrate that a stratification of patients with KS-AIDS exists according to lesion topography where IL-10 levels are higher in those individuals with disseminated disease than those with only localized lesions. This observation sheds light over the possible role of this cytokine in the pathogenesis and outcome of KS due to HHV-8 infection, and contributes to a better understanding of the immunologic response to this virus in a KS context, which could be used for the development of alternative therapies.
Acknowledgments
P.R.L.M. was the recipient of a fellowship from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) (Grant #142858/2006-4).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Beckebaum S, Zhang X, Chen X, et al. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res 2004;10:7260–7269 [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. New Engl J Med 1995;332:1186–1191 [DOI] [PubMed] [Google Scholar]
- 3.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA-sequences in AIDS-associated Kaposi's sarcoma. Science 1994;266:1865–1869 [DOI] [PubMed] [Google Scholar]
- 4.Coscoy L. Immune evasion by Kaposi's sarcoma-associated herpesvirus. Nat Rev Immunol 2007;7:391–401 [DOI] [PubMed] [Google Scholar]
- 5.Ebrahimi B, Tucker SL, Li DH, et al. Cytokines in pancreatic carcinoma—correlation with phenotypic characteristics and prognosis. Cancer 2004;101:2727–2736 [DOI] [PubMed] [Google Scholar]
- 6.Galizia G, Orditura M, Romano C, et al. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol 2002;102:169–178 [DOI] [PubMed] [Google Scholar]
- 7.Hatanaka H, Abe Y, Kamiya T, et al. Clinical implications of interleukin (IL)-10 induced by non-small-cell lung cancer. Ann Oncol 2000;11:815–819 [DOI] [PubMed] [Google Scholar]
- 8.Ikeguchi M, Hatada T, Yamamoto M, et al. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer 2009;12:95–100 [DOI] [PubMed] [Google Scholar]
- 9.Jebreel A, Mistry D, Loke D, et al. Investigation of interleukin 10, 12 and 18 levels in patients with head and neck cancer. J Laryngol Otol 2007;121:246–252 [DOI] [PubMed] [Google Scholar]
- 10.Jensen KK, and Lira SA. Chemokines and Kaposi's sarcoma. Semin Cancer Biol 2004;14:187–194 [DOI] [PubMed] [Google Scholar]
- 11.Kozłowski L, Zakrzewska I, Tokajuk P, and Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst 2003;48:82–84 [PubMed] [Google Scholar]
- 12.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 2013;14: e218–228 [DOI] [PubMed] [Google Scholar]
- 13.Oksenhendler E, Carcelain G, Aoki Y, et al. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric Castleman disease in HIV-infected patients. Blood 2000;96:2069–2073 [PubMed] [Google Scholar]
- 14.Onishi T, Ohishi Y, Imagawa K, Murata K. An assessment of the immunological environment based on intratumoral cytokine production in renal cell carcinoma. BJU Int 1999;83:488–492 [DOI] [PubMed] [Google Scholar]
- 15.Polizzotto MN, Uldrick TS, Hu D, and Yarchoan R. Clinical manifestations of Kaposi sarcoma herpesvirus lytic activation: multicentric Castleman disease (KSHV-MCD) and the KSHV inflammatory cytokine syndrome. Front Microbiol 2012;3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polizzotto MN, Uldrick TS, Wang V, et al. Human and viral interleukin-6 and other cytokines in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood 2013;122:4189–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pugliese A, Torre D, Saini A, et al. Cytokine detection in HIV-1/HHV-8 co-infected subjects. Cell Biochem Funct 2002;20:191–194 [DOI] [PubMed] [Google Scholar]
- 18.Qin Z, Kearney P, Plaisance K, and Parsons CH. Pivotal advance: Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded microRNA specifically induce IL-6 and IL-10 secretion by macrophages and monocytes. J Leukoc Biol 2010;87:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato T, McCue P, Masuoka K, et al. Interleukin 10 production by human melanoma. Clin Cancer Res 1996;2:1383–1390 [PubMed] [Google Scholar]
- 20.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi's sarcoma-associated herpesvirus-like DNA-sequences in multicentric Castleman's disease. Blood 1995;86:1276–1280 [PubMed] [Google Scholar]
- 21.Sozzani S, Luini W, Bianchi G, et al. The viral chemokine macrophage inflammatory protein-II is a selective Th2 chemoattractant. Blood 1998;92:4036–4039 [PubMed] [Google Scholar]
- 22.Weiss E, Mamelak AJ, La Morgia S, et al. The role of interleukin 10 in the pathogenesis and potential treatment of skin diseases. J Am Ac Dermatol 2004;50:657–675 [DOI] [PubMed] [Google Scholar]