Abstract
Recombinant virus vaccines are often less effective due to immunodominant responses against endogenous vector antigens. However, the use of small RNA virus vectors provides an opportunity to limit host exposure to endogenous virus antigens and focus immune responses on the desired vaccine antigen. Using the Daniel's strain of Theiler's murine encephalomyelitis virus, we have identified strategies to modulate responses to endogenous viral proteins by manipulating the host CD8+ T-cell repertoire prior to infection or through the use of mutations introduced into the virus genome. Both of these approaches enhance responses to vaccine antigens introduced into the picornavirus. However, the use of mutant immunodominant epitopes provides an opportunity for enhancing vaccine responses without further manipulation of the host. Using this strategy, we demonstrate that modification of the consensus MHC class I anchor residue within the virus genome can promote enhanced immunity to foreign antigens and self-antigens embedded in the virus genome.
Live virus vaccines have proven to be effective for driving CD8+ T-cell responses for therapy to treat a variety of diseases, making them appealing as vectors for antigen-specific immunotherapy (14,16,24,28,30). One of the major limitations to their efficacy, however, is the induction of immunity to vector antigens rather than recombinant target antigens (26,29). Competition between embedded and endogenous virus antigens limits the effectiveness of the vaccine response (9), decreasing their potential as antigen-specific therapy.
We have developed Theiler's murine encephalomyelitis virus (TMEV) as an attenuated live virus therapy that induces antigen-specific CD8+ T-cells against melanoma and breast cancer (21,22). As with other vectors, TMEV drives an immunodominant CD8+ T-cell response to an endogenous MHC class I peptide antigen, VP2121–130 (18). When presented in the context of H-2Db, CD8+ T-cells reactive to VP2121–130 comprise up to 70% of the CD8+ T-cells recovered from infected mice (18). Although subdominant responses have been documented, the consequences of these responses and their impact on the overall response are minimal (15), suggesting that adaptive immune responses to the VP2121–130 epitope may ultimately be exclusive for clearing and targeting virus-infected cells.
Although TMEV immunodominance provides a significant barrier to the development of this vector as a vaccine, the limited number of antigen specificities derived from this small virus makes engineering potential escape mutants less complex. We find that inhibition of this response using an H-2Db mutant that does not present this epitope to CD8+ T-cells blocks the response to the VP2121–130 peptide while enhancing vaccine responses (22). Since subverting immunodominance can lead to an enhanced response against subdominant epitopes (12,31), we asked whether the use of virus mutations identified within this TMEV epitope (2,25) could divert immunity away from the dominant epitope and increase responses to epitopes engineered within the attenuated virus, a method more amenable to clinical use.
Peptide Depletion Enhances the Proportion of Vaccine-Specific CD8+ T-Cells Using a TMEV Vector Expressing Ovalbumin Antigen
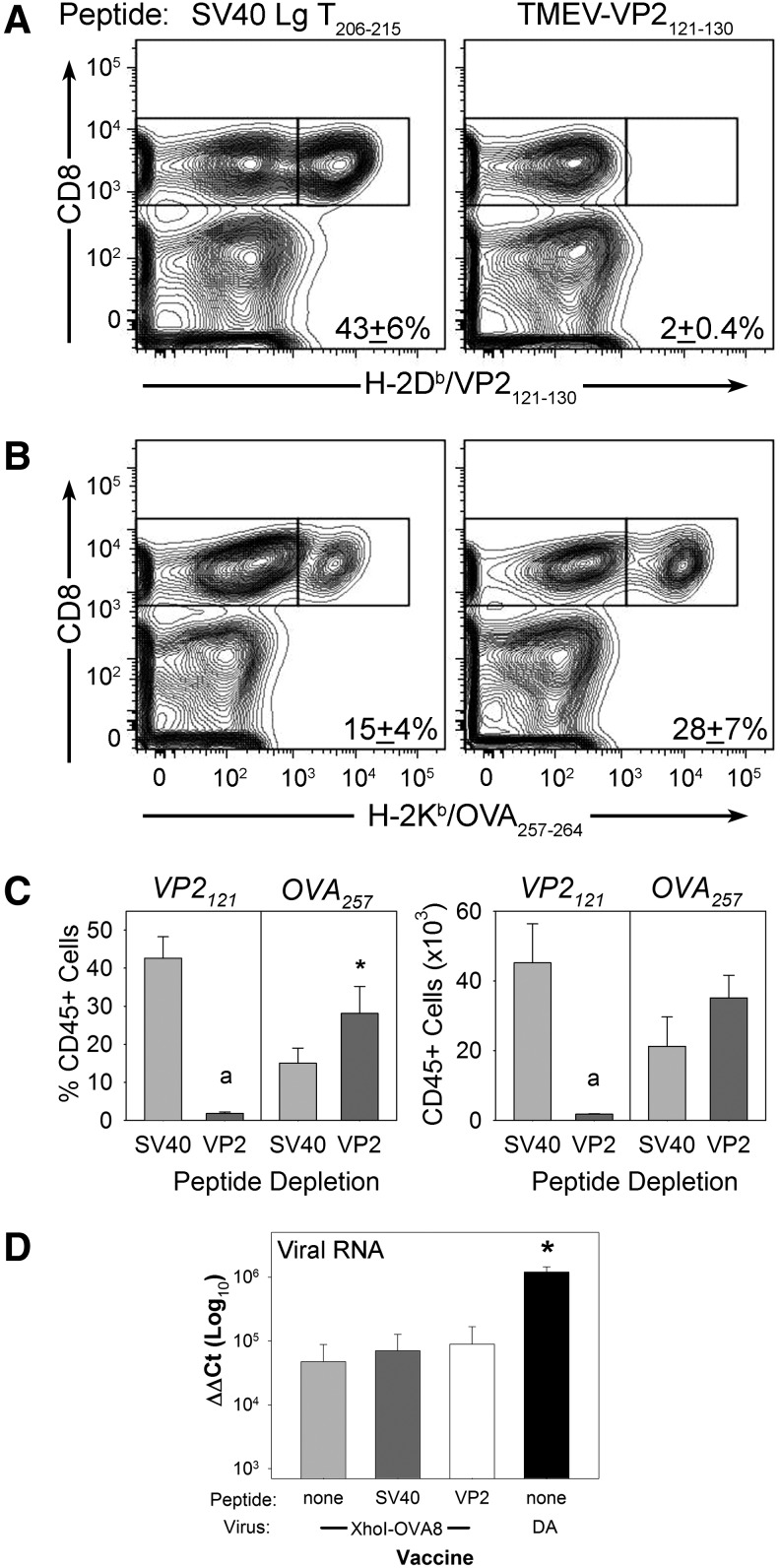
Previously, we had shown that antigen-specific depletion with VP2121–130 peptide inhibits the expansion of the CD8+ T-cell response to wild-type virus. To determine whether this approach would enhance the response to an engineered vaccine strain, we infected mice with recombinant viruses after depletion of the immunodominant response using this approach. We intravenously delivered 300 μg of VP2121–130 peptide (FHAGSLLVFM) or control peptide (HPV E749–57) to C57BL/6 mice 24 h prior to intracranial injection with 2×104 PFU of recombinant TMEV encoding the OVA257–265 model epitope (TMEV-OVA8). Infection of the brain allows the direct isolation of infiltrating T-cells at the site of infection, and provides an easily dissociated tissue that allows direct ex vivo analysis of activated cells (13). This approach has been used previously to demonstrate the efficacy of systemically delivered TMEV vaccines (21,22). Six days after infection, central nervous system infiltrating lymphocytes were analyzed by flow cytometry for the presence of virus-specific H-2Db/VP2121–130 or vaccine-targeted H-2Kb/OVA257–265 CD8+ T-cells using MHC class I tetramers. We find that VP2121 peptide delivery dramatically reduces the percentage of immunodominant virus reactive T-cells from the infected tissue site (Fig. 1A). This approach enhanced the percentage of OVA257–265-specific T-cells generated compared to the control peptide depletion (Fig. 1B). However, it did not increase the overall quantity of vaccine-specific CD8+ T-cells (Fig. 1C). Further, elimination of VP2121-specific T-cells did not affect the amount of virus RNA recovered after infection using attenuated TMEV-OVA8. However, levels of TMEV-OVA8 virus RNA were significantly reduced compared to infection with wild-type TMEV (Fig. 1D).
FIG. 1.
Qualitative enhancement of CD8+ vaccine responses using peptide inhibition of vector-specific T-cells. Central nervous system infiltrating lymphocytes were analyzed for the presence of CD8+ T-cells specific for virus antigen, as well as ovalbumin vaccine antigen after immunization with antigen encoding TMEV-OVA8. (A) H-2Db/VP2121–130-specific responses after control SV40 Lg T peptide or VP2121–130 peptide administration. (B) H-2Kb/OVA257–264-specific responses after control and VP2121–130 peptide administration. (C) Left: Percent of the CD45+ infiltrating population that are H-2Db/VP2121–130 or H-2Kb/OVA257–264 specific. Right: Number of CD45+ VP2121+or OVA257+cells (ap<0.05, rank-sum test; *p<0.05, t-test). (D) Viral central nervous system RNA levels as measured by quantitative reverse transcription polymerase chain reaction from animals infected with TMEV viruses for 6 days with or without prior peptide treatment (*p<0.05 compared to all, t-test).
A Partial Escape Mutation Enhances the Antigen-Specific CD8+ T-Cell Response to a Recombinant TMEV Vaccine
Since manipulation of the CD8+ T-cell repertoire through the induction of nonresponsiveness can modify responses to vaccine antigens, we asked whether direct manipulation of the virus genome itself could reduce immunodominance and enhance responses to recombinant vaccine antigens. Previously, we and others had identified mutations within the VP2121–130 coding sequence for TMEV, and found that immunodominance is less pronounced in infected animals using these virus mutants (2,25). Using an engineered TMEV vector system, we introduced the VP2-S125A and VP2-M130L mutations by site-directed mutagenesis into a cDNA that encodes the TMEV-OVA8 strain, a vaccine that drives H-2Kb restricted CD8+ T-cell responses to the model foreign peptide OVA257 (21). Using this approach to derive new live virus vaccines, we infected C57BL/6 mice and recovered lymphocytes from infected tissues 6 days post-infection. We found that introduction of the VP2-M130L mutation enhances the response to the model epitope OVA257. Both the percentage and number of antigen-specific CD8+ T-cells were increased using this approach (Fig. 2A–C). These mutations introduce a change that is predicted to destabilize the interaction of this peptide with the H-2Db molecule (8) according to a peptide-binding algorithm (19). However, only the M130L mutation enhances immunity to the embedded vaccine epitope, suggesting that other factors influence the vaccine response. These factors might include the generation of new epitopes (1), enhanced responses to subdominant epitopes (15,31), or modulation of virus replication and assembly (2). We found an increase in the percent of CD8+ T-cells that are not specifically recognized by the OVA257- or VP2121-specific tetramers using the VP2-S125A mutant (Fig. 2C), supporting the potential for enhanced responses to off-target viral antigens. The affect cannot be explained by levels of virus, since the virus RNA levels reached after 6 days of infection were similar among the ovalbumen expressing viruses and were decreased compared to wild-type TMEV, demonstrating that the VP2 mutant viruses are similarly attenuated (Fig. 2D). Fortuitously, the M130L mutation provides a means of enhancing responses to vaccine epitopes in a manner that also decreases the reliance on the immunodominant CD8+ T-cell responses needed for virus clearance, since integration of epitopes within the virus leader sequence provides antigen as well as a mechanism for attenuation (21,22).
FIG. 2.
Mutagenesis of the immunodominant vector epitope VP2121–130 promotes elevated CD8+ T-cell responses to an embedded vaccine antigen. CNS infiltrating lymphocytes derived from mice infected with antigen expressing vaccines containing the VP2-S125A and VP2-M130L mutations were evaluated by FACS for the presence of viral and OVA257–264-specific CD8+ T-cells. (A) H-2Db/VP2121–130-specific responses after infection with VP2-S125A-OVA8 (n=4) and VP2-M130L-OVA8 (n=4) vaccines compared to TMEV-OVA8 (n=4) and wild-type TMEV (n=3). (B) H-2Kb/OVA257–264-specific responses from the same samples in (A). (B) Quantitative assessment of the percentage and absolute numbers of CD8+cells specific for VP2121–130 and OVA257–264 (*p<0.05 compared to all others; ap<0.05 compared to M130L-OVA8 and DA-wt by ANOVA/Student/Newman–Keuls). (C) Distribution of the CD8+ T-cell response to ovalbumen expressing viruses. Other CD8+ T-cell responses represent the population of cells negative for VP2121–130 and OVA257–264 (*p<0.05 compared to all others; ANOVA/Student/Newman–Keuls). (D) Viral RNA levels from animals in (A) (*p<0.05 compared to all others; ANOVA/Student/Newman–Keuls). (E) Functional assessment of CD8+ T-cells harvested from mice challenged with ovalbumen and previously primed with vaccines containing the VP2-M130L mutation or wt-VP2. Splenocytes were gated on total CD8+ T-cells to analyze the percentage of CD44+ IFN-γ+cells after stimulation with OVA257–264 or no peptide control (*p<0.05, ANOVA/Student/Newman–Keuls).
To demonstrate the functional utility of the VP2-M130L mutations, we intraperitoneally infected B6 mice with either 2×105 PFU of wild-type TMEV-OVA8 or with VP2-M130L-OVA8 1 week prior to a second challenge with wild-type TMEV-OVA8 virus alone. Seven days after the second challenge, splenocytes were harvested for FACS analysis by intracellular IFN-γ staining (17). After re-stimulation with OVA257 peptide, the animals primed with VP2-M130L-OVA8 had an enhancement in the percentage of CD44+ IFN-γ+ CD8 T-cells after stimulation by the cognate vaccine antigen (Fig. 2E), further demonstrating the utility of this vaccine for enhancing CD8+ T-cell immunity by increasing T-cell activation and IFN-γ production. In addition, the VP2-M130L mutation provides a tool for use in heterologous boost vaccines that can promote CD8+ T-cell immunity to epitopes embedded within TMEV.
Engineering a Virus Escape Mutation into TMEV Enhances the CD8+ T-Cell Response to the Breast Cancer Antigen HER2/neu in F1 Mice
Although the enhancement of vaccine responses can be accomplished using escape mutants, the activation of therapeutic CD8+ T-cell responses to treat chronic infection or cancer provides several unique challenges (7). We have previously shown the benefit of using a HER2/neu expressing TMEV vaccine for the treatment of breast tumors in Balb/c mice (21). This vaccine expresses the H-2Kd epitope p66 derived from the oncogene rat HER2/neu (20). Since the immunodominant response to the VP2121–130 peptide is H-2Db restricted, determining whether VP2 mutants can enhance responses to the H-2Kd restricted epitope requires the use of F1 hybrid mice. Therefore, we infected Balb/C57BL/6 F1 mice (Jackson Labs, Bar Harbor, ME) with this virus for 6 days, and assessed its effectiveness by determining the quality and quantity of p66 reactive CD8+ cells by FACS. We found that the introduction of the VP2-S125A mutation into the p66 wild-type vaccine does not appreciably enhance the response to the p66 antigen. In contrast, introduction of the VP2-M130L mutation increases the percentage and number of H-2Kd/p66 reactive cells (Fig. 3A and B), demonstrating that modification of the H-2Db restricted antigen promotes the enhancement of H-2Kd restricted responses in an F1 strain expressing five unique MHC class I genes (H-2Kd, Dd, Ld, Kb, and Db).
FIG. 3.
Diverting immunodominance promotes enhanced antitumor responses to an H-2Kd restricted HER2/neu epitope. Lymphocytes isolated after infection with VP2 mutagenized TMEV p66 vaccine were analyzed for the presence of CD8+ T-cells specific for the HER2/neu peptide p66. (A) H-2Kd/p66 specific CD8+ T-cells induced with TMEV p66 vaccines encoding VP2-S125A and M130L mutations compared to a vaccine with VP2 wild-type sequence and the nonvaccine strain wild-type TMEV. (B) Assessment of the percentage and absolute number of antigen specific CD8+ T-cells induced with p66 vaccines (*p<0.05 compared to specified or ap<0.05 significant compared to all others by ANOVA/Student/Newman–Keuls).
Our studies focused on the inhibition of the response to the VP2121–130-specific CD8+ T-cell response to enhance responses to foreign or tumor antigens embedded within TMEV. This epitope has been studied extensively in mice expressing the MHC class I allele H-2Db because of its importance for protection from viral persistence and demyelinating disease (2,3,11). However, less is known about its potential for binding other MHC alleles or the potential role that non-H-2Db restricted T-cells might play in targeting this epitope. Previously, others have shown that expression of the VP2121–130 peptide in H-2q mice can modulate the extent of immune mediated pathology (6), suggesting a potential role for H-2q restricted T-cells in modulating pathology and the possibility that the responses to this peptide play a role in other MHC haplotypes. In humans, nine major MHC class I supertypes have been identified based on their structural similarity in the peptide-binding cleft (27). These studies have demonstrated a degree of degeneracy in HLA peptide binding, suggesting that the principles identified in the current report could be applied to the development of vaccines that will be beneficial across a broad spectrum of MHC haplotypes (4,5,10).
Several RNA viruses are currently being developed as recombinant vaccine vectors (23). However, the benefits of using recombinant virus vaccines have been limited by several factors, including off-target immune responses directed toward endogenous virus antigens (26,29). Many of these recombinant vaccines encode large genomes with complex antigens that compete with or inhibit desired vaccine responses (29,31). We find that a small RNA virus can drive potent CD8+ T-cell responses to recombinant antigens and that manipulation of its genome can be used to enhance the safety of this vaccine, as well as modulate its immune profile. Here, we find that genetic modification of an immunodominant MHC class I epitope can be used to promote adaptive CD8+ T-cell responses to recombinant antigens, providing a strategy to enhance the potency of this vaccine and support for the development of small RNA viruses as therapeutic vectors.
Acknowledgments
This work was supported by grants from the National Institutes of Health (5R01CA104996-08 and 5R01NS060881-06; A.J.J.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Andreansky SS, Stambas J, Thomas PG, Xie W, Webby RJ, and Doherty PC. Consequences of immunodominant epitope deletion for minor influenza virus-specific CD8+-T-cell responses. J Virol 2005;79:4329–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell MP, Renner DN, Johnson AK, and Pavelko KD. An elite controller of picornavirus infection targets an epitope that is resistant to immune escape. PLoS One 2014;9:e94332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borson ND, Paul C, Lin X, Nevala WK, Strausbauch MA, Rodriguez M, and Wettstein PJ. Brain-infiltrating cytolytic T lymphocytes specific for Theiler's virus recognize H2Db molecules complexed with a viral VP2 peptide lacking a consensus anchor residue. J Virol 1997;71:5244–5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burrows SR, Elkington RA, Miles JJ, et al. Promiscuous CTL recognition of viral epitopes on multiple human leukocyte antigens: biological validation of the proposed HLA A24 supertype. J Immunol 2003;171:1407–1412 [DOI] [PubMed] [Google Scholar]
- 5.del Guercio MF, Sidney J, Hermanson G, Perez C, Grey HM, Kubo RT, and Sette A. Binding of a peptide antigen to multiple HLA alleles allows definition of an A2-like supertype. J Immunol 1995;154:685–693 [PubMed] [Google Scholar]
- 6.Denic A, Zoecklein L, Kerkvliet J, et al. Transgenic expression of viral capsid proteins predisposes to axonal injury in a murine model of multiple sclerosis. Brain Pathol 2011;21:501–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dougan M, and Dranoff G. Immune therapy for cancer. Annu Rev Immunol 2009;27:83–117 [DOI] [PubMed] [Google Scholar]
- 8.Falk K, Rotzschke O, Stevanovic S, Jung G, and Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 1991;351:290–296 [DOI] [PubMed] [Google Scholar]
- 9.Farrington LA, Smith TA, Grey F, Hill AB, and Snyder CM. Competition for antigen at the level of the APC is a major determinant of immunodominance during memory inflation in murine cytomegalovirus infection. J Immunol 2013;190:3410–3416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischhauer K, Tanzarella S, Wallny HJ, Bordignon C, and Traversari C. Multiple HLA-A alleles can present an immunodominant peptide of the human melanoma antigen Melan-A/MART-1 to a peptide-specific HLA-A*0201+ cytotoxic T cell line. J Immunol 1996;157:787–797 [PubMed] [Google Scholar]
- 11.Johnson AJ, Njenga MK, Hansen MJ, et al. Prevalent class I-restricted T-cell response to the Theiler's virus epitope Db:VP2121-130 in the absence of endogenous CD4 help, tumor necrosis factor alpha, gamma interferon, perforin, or costimulation through CD28. J Virol 1999;73:3702–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim TS, and Perlman S. Protection against CTL escape and clinical disease in a murine model of virus persistence. J Immunol 2003;171:2006–2013 [DOI] [PubMed] [Google Scholar]
- 13.LaFrance-Corey RG, and Howe CL. Isolation of brain-infiltrating leukocytes. J Vis Exp 2011June13;(52). doi: 10.3791/2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li XF, Deng YQ, Yang HQ, et al. A chimeric dengue virus vaccine using Japanese encephalitis virus vaccine strain SA14-14-2 as backbone is immunogenic and protective against either parental virus in mice and nonhuman primates. J Virol 2013;87:13694–13705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyman MA, Lee HG, Kang BS, Kang HK, and Kim BS. Capsid-specific cytotoxic T lymphocytes recognize three distinct H-2D(b)-restricted regions of the BeAn strain of Theiler's virus and exhibit different cytokine profiles. J Virol 2002;76:3125–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mason JN, Elbahesh H, and Russell CJ. Influence of antigen insertion site and vector dose on immunogenicity and protective capacity in Sendai virus-based human parainfluenza virus type 3 vaccines. J Virol 2013;87:5959–5969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDole J, Suidan G, Boespflug E, et al. A translatable molecular approach to determining CD8 T-cell epitopes in TMEV infection. Hum Immunol 2008;69:805–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendez-Fernandez YV, Johnson AJ, Rodriguez M, and Pease LR. Clearance of Theiler's virus infection depends on the ability to generate a CD8+ T cell response against a single immunodominant viral peptide. Eur J Immunol 2003;33:2501–2510 [DOI] [PubMed] [Google Scholar]
- 19.Moutaftsi M, Peters B, Pasquetto V, et al. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol 2006;24:817–819 [DOI] [PubMed] [Google Scholar]
- 20.Nava-Parada P, Forni G, Knutson KL, Pease LR, and Celis E. Peptide vaccine given with a Toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res 2007;67:1326–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavelko KD, Bell MP, Karyampudi L, et al. The epitope integration site for vaccine antigens determines virus control while maintaining efficacy in an engineered cancer vaccine. Mol Ther 2013;21:1087–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavelko KD, Girtman MA, Mitsunaga Y, et al. Theiler's murine encephalomyelitis virus as a vaccine candidate for immunotherapy. PLoS One 2011;6:e20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plotkin SA. Vaccines: the fourth century. Clin Vaccine Immunol 2009;16:1709–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy PS, Burroughs KD, Hales LM, et al. Seneca Valley virus, a systemically deliverable oncolytic picornavirus, and the treatment of neuroendocrine cancers. J Natl Cancer Inst 2007;99:1623–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato S, Zhang L, Kim J, et al. A neutralization site of DA strain of Theiler's murine encephalomyelitis virus important for disease phenotype. Virology 1996;226:327–337 [DOI] [PubMed] [Google Scholar]
- 26.Schirmbeck R, Reimann J, Kochanek S, and Kreppel F. The immunogenicity of adenovirus vectors limits the multispecificity of CD8 T-cell responses to vector-encoded transgenic antigens. Mol Ther 1996;16:1609–1616 [DOI] [PubMed] [Google Scholar]
- 27.Sette A, and Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 1999;50:201–212 [DOI] [PubMed] [Google Scholar]
- 28.Smith CL, Dunbar PR, Mirza F, et al. Recombinant modified vaccinia Ankara primes functionally activated CTL specific for a melanoma tumor antigen epitope in melanoma patients with a high risk of disease recurrence. Int J Cancer 2005;113:259–266 [DOI] [PubMed] [Google Scholar]
- 29.Smith CL, Mirza F, Pasquetto V, et al. Immunodominance of poxviral-specific CTL in a human trial of recombinant-modified vaccinia Ankara. J Immunol 2005;175:8431–8437 [DOI] [PubMed] [Google Scholar]
- 30.Tatsis N, and Ertl HC. Adenoviruses as vaccine vectors. Mol Ther 2004;10:616–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webby RJ, Andreansky S, Stambas J, et al. Protection and compensation in the influenza virus-specific CD8+ T cell response. Proc Natl Acad Sci U S A 2003;100:7235–7240 [DOI] [PMC free article] [PubMed] [Google Scholar]