Summary
Although repair of double-strand breaks (DSBs) by gene conversion is the most accurate way to repair such lesions, in budding yeast there is a thousand-fold increase in accompanying mutations, including interchromosomal template switches (ICTS) involving highly mismatched (homeologous) ectopic sequences. Although such events are rare and appear at a rate of 2×10−7 when template jumps occur between 71% identical sequences, they are surprisingly frequent (0.3% of all repair events) when the second template is identical to the first, revealing the remarkable instability of repair DNA synthesis. With homeologous donors, ICTS uses microhomologies as small as 2 bp. Cells lacking mismatch repair proteins Msh6 and Mlh1 form chimeric recombinants with two distinct patches of microhomology, implying that these proteins are crucial for strand discrimination of heteroduplex DNA formed during ICTS. We identify the chromatin remodeler Rdh54 as the first protein required for template switching that does not affect simple gene conversion.
Introduction
Although eukaryotic cells suffer multiple spontaneous chromosomal double-strand breaks (DSBs) every time they replicate their genome, homologous recombination mechanisms preserve the integrity of the genome. DSBs can be a product of natural replication process when replication fork collapses due to DNA lesions, physical impediments or dNTP pool depletion (Branzei and Foiani, 2010; Poli et al., 2012). Such stalled or broken forks can be restarted or rescued by the number of homologous recombination mechanisms (reviewed by (Yeeles et al., 2013). Recently attention has been focused on recombination coupled to replication that result in changes in gene copy number variation (CNV) and in remarkable complex chromosomal rearrangements known as chromothripsis in mammalian cells (Forment et al., 2012).
A DSB can be repaired by using an identical, unbroken sister chromatid or identical or nearly identical sequences found on a homologous chromosome or at an ectopic location. One well-studied example of DSB repair by an ectopic donor is S. cerevisiae mating-type gene switching. Mating type is dictated by expression of a or α alleles at the MAT locus located on chromosome 3 (Chr3). MAT can switch to the opposite mating-type by gene conversion repair of a site-specific DSB induced by HO endonuclease, using one of two complete, but silent, donor copies – HML and HMR – located near telomeres on the same chromosome (reviewed by (Haber, 2012). Galactose-inducible expression of HO (Herskowitz and Jensen, 1991) creates a DSB in nearly all cells simultaneously in the population, allowing a detailed examination of DSB repair (Connolly et al., 1988; Hicks et al., 2011; White and Haber, 1990).
Recently we reported that while gene conversion is the most conservative pathway to preserve the genome, repair is associated with a thousand-fold increase in mutations in the newly copied DNA (Hicks et al., 2010). These mutations were detected during the gene conversion repair of an HO-induced DSB in MAT by using Kluyveromyces lactis URA3 sequences embedded within the silent HMR locus (hmr::Kl-URA3). While gene conversion should produce a Ura+ outcome, because the Kl-URA3 sequences can be expressed at MAT, but not at HMR, Ura− mutants can readily be selected. Many of these mutations have a distinctive signature, including frameshifts within homonucleotide runs, quasipalindrome alterations and – most surprising – interchromosomal template switches (ICTS) involving highly divergent copies of the template sequence. ICTS occurred when the nascent DNA strand dissociated from hmr::Kl-URA3 and jumped to a different chromosome carrying the S. cerevisisae’s ura3-52 allele, which is intact but not expressed because of a retrotransposon insertion at the 5’ end of the gene. Sc-ura3-52 is only 71% identical to Kl-URA3. Template switching requires a second jump back to the original hmr::Kl-URA3 template to acquire the remaining sequences necessary to complete repair at MAT. ICTS as well as the other types of template switches resulted predominantly from errors of DNA polymerase δ (Hicks et al., 2010).
In the original study, only 2% of the Ura− mutations arising during switching were ICTS. To study these events in much greater detail we modified the system so that only ICTS events would yield a selectable product, creating a functional chimeric Kl-Sc-Kl-URA3 gene. This strategy enabled us to characterize the sizes of microhomology used in template switching and the extent of new DNA synthesis from the second template. We find that template switching is 10,000 times more frequently when the donor on Chr3 is completely homologous to the template on Chr5 than when it is homeologous, amounting to 0.3% of all recombination events. Deletion of the RDH54 chromatin remodeler has no effect on normal DSB repair by gene conversion but reduces ICTS 70-fold; this is the first protein with a specific role in template switching. Our analysis of ICTS between homeologous sequences has also revealed novel roles for the Msh6 and Msh3 mismatch repair proteins in the discrimination of the template and nascent strands within heteroduplex DNA.
Results
Heterochromatin at HMR does not affect ICTS
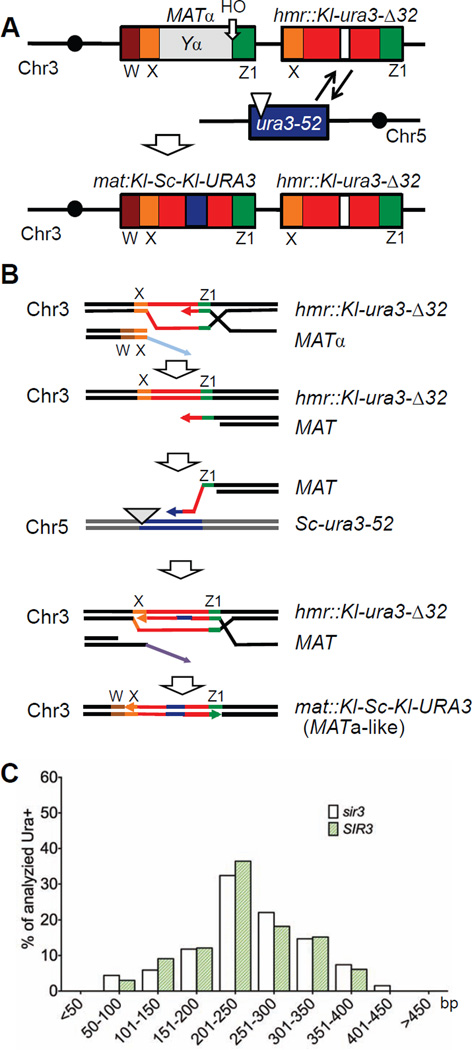
To examine ICTS, we modified the previously described system so that other DSB repair events would be excluded (Fig 1A and 1B). Specifically, we deleted the SIR3 gene, thus unsilencing HMR, and then created a 32-bp deletion in the Kl-URA3 sequences inserted in HMR (hmr::Kl-ura3-Δ32), so that gene conversion repair of the HO-induced DSB at MATα would result in Ura− outcomes, i.e. mat::Kl-ura3-Δ32. In contrast, an ICTS, in which a DSB repair event that began copying at hmr::Kl-ura3-Δ32 but jumped to the homeologous Sc-ura3-52 sequences on Chr5 and then back to HMR, could produce a Ura+ outcome (Fig 1B). As shown in Table 1, we found that in a wild type strain lacking Sir3 the frequency of ICTS was similar to that estimated previously, about 2×10−7. Because all of these events occur in a single cell division, these frequencies are equivalent to rates of recombination.
Figure 1. Selection of ICTS during gene conversion repair.
A. Schematic representation of the ICTS assay used in this study. An HO endonuclease-induced DSB at MATα initiates gene conversion repair with an HMR donor containing K. lactis ura3 sequences with a 32-bp deletion. Rare ICTS events copy sequences from S. cerevisiae ura3-52 and generate a Ura3+ chimeric sequence in MAT. Brown box: W region; orange box: X region; green box: Z1 region; red boxes separated by white box in HMR is Ya::Kl-ura3-Δ32. B. Proposed molecular events during ICTS. After the DSB is induced at MAT, the 5’ to 3’ resected end strand invades the HMR donor in the Z1 region of shared homology and begins to copy the Kl-ura3-Δ32 sequences. The nascent strand can dissociate and then anneal to ectopic homeologous ura3-52 sequences on Chr5. After copying a small patch including the 32 bp missing in the HMR donor, the nascent strand dissociates again, anneals back to HMR and copies the remaining portion Kl-ura3-Δ32 and at least part of the X sequences shared between HMR and MAT. The annealing of this nascent strand at MAT completes repair and results in a chimeric Kl-Sc-Kl-URA3 gene. These Ura+ outcomes can be selected on plates lacking uracil. C. Length of the copied ura3-52 donor in sir3Δ (TOY43) and SIR3 (TOY19) strains.
Table 1.
Rate of interchromosomal template switches, average length of copied ectopic donor and average length of 3’ and 5’ junction sites in different strains*
| Genotype | Rate of ICTS, ×10−7 ** |
Relative rate of ICTS versus wild type |
Average length of copied ura3-52 |
Average length of 5’ junction (bp) |
Average length of 3’ junction (bp) |
Number of Ura+ clones analyzed |
|
|---|---|---|---|---|---|---|---|
| sir3Δ | 2.3 ± 0.1 | na | 247 ± 9 | 7.3 ± 0.4 | 9.2 ± 0.6 | 94 | |
| SIR3 | 1.4 ± 0.4 | 0.6 | 246 ±10 | 8.7 ± 0.5 | 9.4 ± 0.7 | 48 | |
| helicase | sgs1Δ | 2.4 ± 0.3 | 1.0 | 253 ± 9 | 8.0 ± 0.5 | 9.4 ± 0.7 | 48 |
| mph1Δ | 2.5 ± 0.2 | 1.1 | 258 ± 11 | 9.5 ± 0.5 | 11.3 ± 1.0 | 48 | |
| sgs1Δ mph1Δ | 3.3 ± 0.3 | 1.4 | 269 ± 8 | 9.0 ± 0.4 | 9.9 ± 0.6 | 59 | |
| MMR | mlh1Δ | 5.2 ± 0.9 | 2.3 | 245 ± 6 | 7.4 ± 0.4 | 10.4 ± 0.6 | 96 |
| msh3Δ | 3.7 ± 0.7 | 1.6 | 250 ± 7 | 7.8 ± 0.4 | 10.0 ± 0.6 | 96 | |
| msh6Δ | 4.8 ± 0.8 | 2.1 | 231 ± 10 | 6.9 ± 0.4 | 8.6 ± 0.6 | 70 | |
| msh6Δ msh3Δ | 3.1 ± 0.2 | 1.3 | 233 ± 6 | 7.6 ± 0.4 | 11.3 ± 0.5 | 92 | |
| msh6Δ mlh1Δ | 2.7 ± 0.6 | 1.2 | 247 ± 12 | 7.4 ± 0.3 | 10.1 ± 0.6 | 94 | |
| rad1Δ | 1.1 ± 0.2 | 0.5 | 239 ± 8 | 7.6 ± 0.3 | 10.9 ± 0.6 | 92 | |
| double mutant | msh6Δ mph1Δ | 10.0 ± 1.2 | 4.3 | 242 ± 15 | 7.8 ± 0.5 | 9.8 ± 0.7 | 48 |
| msh6Δ rad1Δ | 2.7 ± 0.5 | 1.2 | 264 ± 9 | 7.9 ± 0.4 | 9.8 ± 0.7 | 68 | |
all strains are sir3Δ unless otherwise noted;
p-values >0.05
It was possible that dissociation of the newly copied strand during gene conversion from the heterochromatic HMR locus might result from difficulties in repair DNA synthesis caused by the ordered nucleosome structure of the donor (Weiss and Simpson, 1998). However, the heterochromatic state of HMR did not affect the rate of Ura+ events and did not alter the sites of the homeologous junctions formed between Kl-ura3 and Sc-ura3 sequences nor the length of the Sc-ura3 ectopic sequences copied (Table 1, Fig 1C and Table S1). Therefore we conducted the rest of our experiments in a sir3Δ background, which we will refer to as the wild type strain.
Perfect homology increases template switching
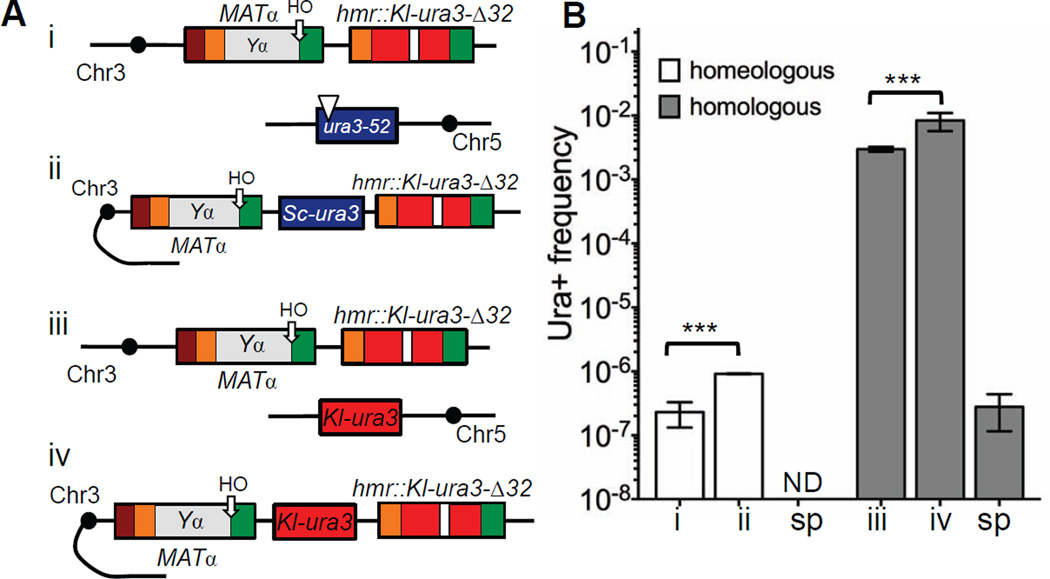
Interchromosomal switches between sequences sharing 71% homology occur rarely, about twice per 107 cells. We asked if the extensive mismatches between these donor templates pose a significant barrier to template jumps. We substituted ura3-52 and its promoter with a promoterless Kl-ura3 sequence also lacking the initial ATG codon, so that template switching would involve hmr::Kl-ura3-Δ32 and a 100% identical Kl-ura3 template on Chr5 (Fig 2A, compare i and iii). ICTS increased 10,000 times, to a rate of 3×10−3, while spontaneous recombination measured between mat::Kl-ura-Δ32 or hmr::Kl-ura3-Δ32 and Chr5 Kl-ura3 was 2.8×10−7 (Fig 2B, Table S2 and Table S3). Spontaneous recombination between two homeologous templates was lower than 1×10−10.
Figure 2. Increasing homology significantly promotes template switching during gene conversion.
A. Analysis of Ura+ rates in TOY43, TOY79, TOY47 and TOY74 strains. System was converted from inter- (i) to intrachromosomal (ii) template switches between two non-identical sequences. Interchromosomal template switches between two identical sequences can be studied by replacing ura3-52 on Chr5 with Kl-ura3 sequences lacking both a promoter and the ATG start codon (iii). System to study intra chromosomal template switches between two identical sequences (iv). B. Analysis of template switches in strains described in A. sp = spontaneous. ND = not detected; less than 1×10−10. Data are represented as mean +/− SEM.
We also inserted a donor with perfect Kl-ura3 homology onto Chr3 between MAT and HMR in the same orientation as HMR, thus allowing us to examine intrachromosomal template switches (Fig 2A, compare iii and iv). The frequency of intrachromosomal template switches increased 3 times compared to ICTS (Fig 2B and Table S2). When a promoterless and ATG-less S. cerevisiae ura3 gene was placed at the same location on Chr3 (Fig 2A, compare i and ii), the frequency of homeologous template switches increased 4 times compared to ICTS (Fig 2B and Table S2). With perfect homology, intrachromosomal template switching occurred in almost 1 in 100 gene conversion events (8×10−3). This result reveals the remarkable instability of the repair DNA replication machinery during gene conversion.
ICTS can occur at many different homeologous junctions
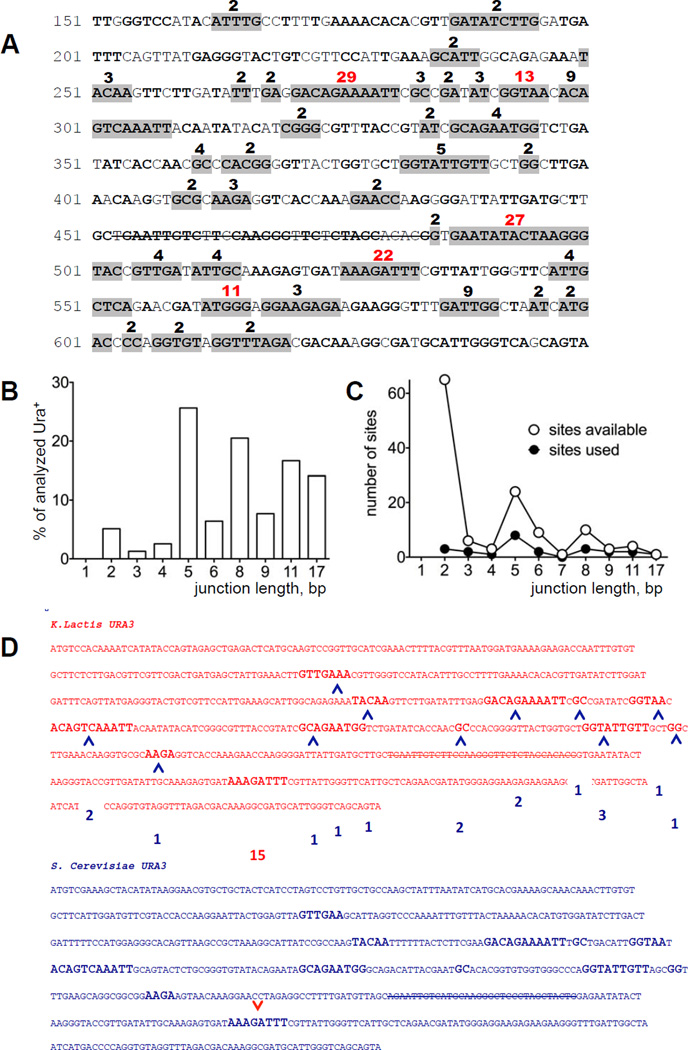
To analyze the precise events that occurred in ICTS, we sequenced chimeric URA3 genes at MAT after HO induction. In each case, the ICTS had copied the 32 bp missing from the HMR donor. The entry and exit points of each event (that is, the junctions on the 3’ and 5’ sides of the deletion, respectively) were determined by locating the regions of shared homology between the Kl-ura3-Δ32 and Sc-ura3-52 sequences. Both the entrance and exit points were spread over most of the URA3 sequence (Fig 3A). Some junctions are much more preferred then others and are among the longest regions of microhomology, but other microhomologies of similar length are used much less often (Fig 3A–C and Table S1). The sizes of microhomology were not significantly different for entry and exit sites (Table 1); moreover there was no apparent relation between the exit sites used for any particular entry site. An example of the different exit sites used for one particular entry site is shown in Fig 3D. Among 142 examples analyzed from both sir3Δ and SIR3 strains, the sequences copied from the ura3-52 ectopic donor ranged between 76 and 412 bp, with a mean size of 246 and 247 bp, respectively (Table 1 and Fig 1C). Thus the heterochromatic state of the HMR donor did not alter the spectrum of repair events.
Figure 3. A number of microhomologies are used for ICTS.
A. Sequence of K. lactis URA3 from 150 to 650 bp. In-frame, shared microhomologies between Kl-URA3 and Sc-URA3 are in bold. Gray-highlighted microhomologies were found among 94 Ura+ colonies in sir3Δ strain (TOY43). The strike-through sequence represents the 32-bp deletion (Δ32). Numbers over the sequence are the percentage of microhomology usage among the Ura+ colonies analyzed. The five most frequently used sites are shown in red. B. Length distribution of microhomologies shown in A. C. Distribution of available shared microhomologies between Kl-URA3 and Sc-URA3. Open circles illustrate the number of possible junctions of a particular length; filled circles represent the number of different junctions of each particular length used for template switches. D. Among 15 template switches involving one specific 3’ junction (red arrow), a second template switch occurred at each of the indicated 5’ junctions (blue arrows), with their frequency of usage shown in blue boxes.
Genes involved in heteroduplex rejection and mismatch repair do not affect the rate of ICTS
Several types of homeologous recombination, including gene conversion, are inhibited by mismatch repair proteins and the Sgs1 helicase via heteroduplex DNA rejection (reviewed by (Boiteux and Jinks-Robertson, 2013). We tested whether deletions of different genes involved in heteroduplex rejection and mismatch repair, as well as the 3’ to 5’ DNA helicases, Sgs1 and Mph1, affected the efficiency of ICTS (Table 1). None of the tested mutants exhibited more than a 2–3 fold increase or decrease in ICTS rates, confirming that mismatch repair proteins are not predominantly responsible for the barrier to recombinat ion between highly diverged sequences (Dutta et al. 1996). Our observations do not allow us to determine if the rules for template switching between non-identical sequences are distinctly different from those governing initial strand invasion in gene conversion. We note that the lengths and distribution of microhomology junctions for template switching do not differ significantly from the wild-type strain in any of these mutants (Table 1, Fig S1A and S1B and Table S1). The strains lacking helicase activities had fewer short tracts (Fig S1B); however, none of the distributions was significantly different from the tracts in the sir3Δ strain (p > 0.05).
Deletion of MSH6 leads to multiple patches after gene conversion repair
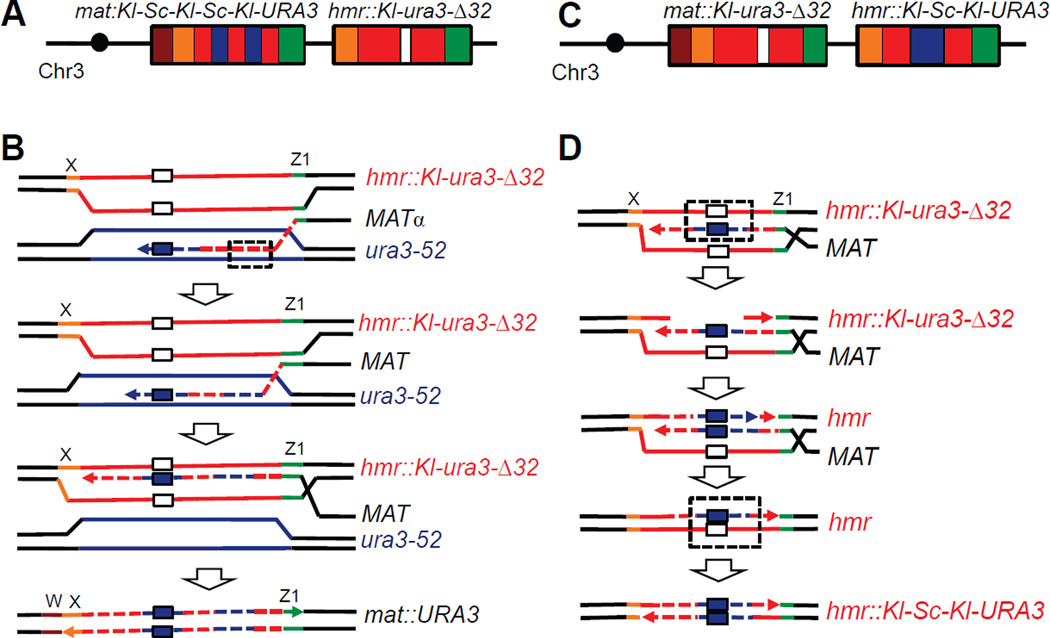
Despite the fact that deleting MSH6 increased the rate only 2 fold and did not affect the general characteristics of ICTS events (Table 1 and Table S1), we were surprised to discover that 11% of msh6Δ Ura+ clones possessed a chimeric URA3 gene having two distinct patches of ura3-52 sequence embedded in the Kl-URA3 sequence at MAT (Fig 4A and Fig S2). One segment corrects the 32-bp deletion. The second Sc-ura3 patch was found 3’ of the deletion correction in 7 out of 8 of the cases, i.e. in sequences that were copied after the MAT end invaded HMR but prior to reaching the 32-bp deletion. These second patches were substantially shorter (19 to 64 bp) than those correcting the 32-bp deletion (mean length 231 bp; see Table 1). Such outcomes could be result of mismatch repair of a long heteroduplex formed after dissociation of the nascent repair strand from hmr::Kl-ura3-Δ32 and invasion of this strand into Sc-ura3-52 (Fig 4B). The remaining case had a second patch located 5’ of the 32-bp correction (Fig S3) that could also reflect heteroduplex correction or a second ICTS. Similar Kl-Sc-Kl-Sc-Kl-URA3 chimeric genes in MAT were found among 7% of Ura+ outcomes from the msh6Δ mph1Δ strain.
Figure 4. Msh6 is crucial for strand discrimination during interchromosomal template switch.
A. Multiple Sc-ura3 patches identified in a Ura+ at MAT in a msh6Δ strain (TOY44). B. Scheme illustrating appearance of a Kl-Sc-Kl-Sc-Kl pattern in a URA3 chimeric gene in MAT. When the nascent repair strand dissociates from HMR and anneals to ura3-52 on Chr5, a heteroduplex DNA (hDNA) is formed (dashed box). hDNA is recognized and corrected by Msh6-independent mismatch repair using the ura3-52 strand as a template. C. A footprint of ura3-52 was also found replacing the 32-bp deletion at hmr::Kl-ura3-Δ32 in some sir3Δ cells lacking Msh6 and/or Mlh1. D. A possible explanation of ura3-52 footprint in HMR. The nascent repair strand dissociates from HMR and copies a patch from the ectopic donor, including the 32-bp segment on Chr5. After re-invasion into HMR, hDNA is formed (dashed box) which is recognized and used by mismatch repair to replace the deleted 32-bp segment and create an expressed chimeric URA3 gene. Such events were only found in msh6 and/or mlh1 cells that apparently are not able to discriminate the intact from the invading strand. White box: Δ32; dark blue box: sequence corresponding Δ32 copied from Sc-ura3-52.
Previously it was shown that heteroduplex loops (as opposed to single-bp mismatches) arising in meiotic recombination were repaired by a Msh2 and Msh3-dependent, but Mlh1 and Pms1-independent, process (Kearney et al., 2001). The patches of microhomology found in our study would produce a “bubble” of mismatched sequences rather than a single loop. In keeping with the idea that Msh3 would be required to introduce a second patch of S. cerevisiae sequences into the repaired product, we did not find any multiple patches in a msh6Δ msh3Δ double mutant. We also did not find any multiple patches among 94 isolates of the msh6Δ mlh1Δ double mutant. However, additional results presented below suggest that the MSH3-dependent multiple patches can be formed in the absence of Mlh1.
Deletion of MSH6 or MLH1 leaves a footprint in the ectopic HMR donor
Surprisingly, some of msh6Δ and mlh1Δ Ura+ clones analyzed in the sir3Δ background, where HMR locus is unsilenced, should not have been Ura+ because the sequenced MAT locus contained the Kl-ura3 sequence harboring the 32-bp deletion. These clones could however be Ura+ if the expressed hmr::Kl-ura3-Δ32 locus had been converted to a functional sequence or if the Sc-ura3-52 locus had somehow become reverted to URA3. Indeed, sequencing of HMR revealed that 4% of all Ura+ analyzed in msh6Δ (4 out of 96) and 2% of the Ura+ recovered in mlh1Δ (2 out of 96) had been converted to Ura+ by the copying a segment of Sc-ura3-52 to replace the 32-bp deletion at HMR (Fig 4C). The length of inserted sequence ranged from 127 to 322 bp. We believe these events are the result of mismatch repair after re-invasion of nascent strand dissociating from Sc-ura3-52 and invading back into hmr::Kl-ura3-Δ32, followed by mismatch correction of the parental strand.
In the msh6Δ mlh1Δ double mutant, 3% of Ura+ clones (3 out of 92 sequenced) had a footprint of ura3-52 in HMR. No such footprints were found in 94 analyzed msh6Δ msh3Δ double mutants, compared to 6 of 118 examples where absence of Msh6 activity leads to ura3-52 footprint in HMR. Altogether, 20 of 212 events in msh6Δ strains had either multiple Sc-ura3-52 patches at MAT or a footprint of Sc-ura3-52 in HMR. We conclude that Msh6 plays crucial role preventing such multiple events while Msh3 is required to establish them in the msh6Δ background. We suggest that these events can occur when cells fail to distinguish which strand is the intact donor and which is the incomplete nascent strand (Fig 4D).
To support our hypothesis that alterations of HMR or the multiple conversion tracts at MAT resulted from the repair of “bubbles” of mismatched sequences, we deleted RAD1 in the msh6Δ strain, as the repair of heteroduplex loops has previously been shown to require Msh2-Msh3 and Rad1-Rad10 (Kearney et al., 2001). The absence of Rad1 did not affect the characteristics of ICTS but decreased survival, consistent with the role of Rad1 in removing nonhomologous 3’-ended tails during MAT switching (Lyndaker et al., 2008) (Table 1, Table S1 and Table S4). Without Rad1, multiple patches of Sc-ura3 in MAT and footprints of ura3-52 in HMR were absent in msh6Δ strain a result consistent with a loop-repair mechanism.
All the above described events were likely due to the repair of the DSB in the MAT as the rate of spontaneous recombination between Sc-ura3-52 and either mat::Kl-ura3-Δ32 or hmr::Kl-ura3-Δ32 where HO endonuclease cannot act was 0.7×10−10 (Table S3), compared to the HO-induced rate of about 2×10−8 (0.04×4.8×10−7). Although a previous study found increased spontaneous recombination between diverged sequences in msh3Δ strains (Datta et al., 1996), we did not find any Sc-ura3-52 patches in MAT or HMR in msh3Δ strain. We suggest that the multiple patches of ura3-52 sequence at MAT or the Ura+ conversions at HMR result from MSH3-dependent repair of extensive heteroduplex DNA formed in the absence of Msh6, as described above.
Overexpression of Rad51 does not affect ICTS in wild-type strain
MAT switching is eliminated in the absence of the recombination protein Rad51 or its interacting chromatin remodeler, Rad54 (Malkova et al., 1996; Signon et al., 2001). We confirmed here that RAD54 is required for all HO-induced repair events (Table S4). Rad51 is a recombinase that catalyzes homology searching and strand exchange between homologous sequences (Renkawitz et al., 2013; Sung, 1994). Overexpression of Rad51 has been shown to increase the efficiency of break-induced replication (Lydeard et al., 2010). To investigate if increased levels of Rad51 might affect the frequency of ICTS, we transformed the wild type strain with a plasmid carrying RAD51 expressed under a constitutive PGK promoter. Overexpression of RAD51 had no effect on simple gene conversion, which was already >92% efficient with normal levels of Rad51. The rate of ICTS was also unaffected (Table S2).
Rdh54 is required for template switches
It is not evident if ICTS uses only the recombination machinery required for simple gene conversions; consequently we investigated the role of Rdh54 and Rad59 in this process as both proteins had been implicated earlier in a Rad51-independent repair mechanism (Ira and Haber, 2002). Rdh54 (Tid1) is a homologue of Rad54, but has distinct functions in DSB repair. Although it plays a major role in meiotic recombination in combination with the Rad51 homolog, Dmc1, it has very little effect on mitotic recombination (Holzen et al., 2006; Klein, 1997; Shinohara et al., 2000). Moreover, Rdh54, along with both Rad54 and a third homolog, Uls1, has been implicated in dissociating Rad51 from double-stranded DNA (Shah et al., 2010). We confirmed that deleting RDH54 had no discernable effect on the very efficient repair of HO-cut MATα with the hmr::Kl-ura3-Δ32 donor, as simple gene conversion was not decreased (Table S4). It was therefore astonishing that ICTS in an rdh54Δ strain was decreased 70 fold compared to the wild type strain (Fig 5A and Table S2). Although the rdh54Δ mutant is adaptation-defective, when there is no repair, it is proficient in resuming cell cycle progression after repair (Lee et al., 2001). Nevertheless, to show that rdh54Δ was not defective in resuming cell cycle progression, we measured ICTS in an rdh54Δ chk1Δ strain where its checkpoint defect is suppressed. The frequency of ICTS in such a strain remained as low as for rdh54Δ strain (data not shown) indicating that failure to produce Ura+ colonies is not due to inability to resume growth after healing a DSB.
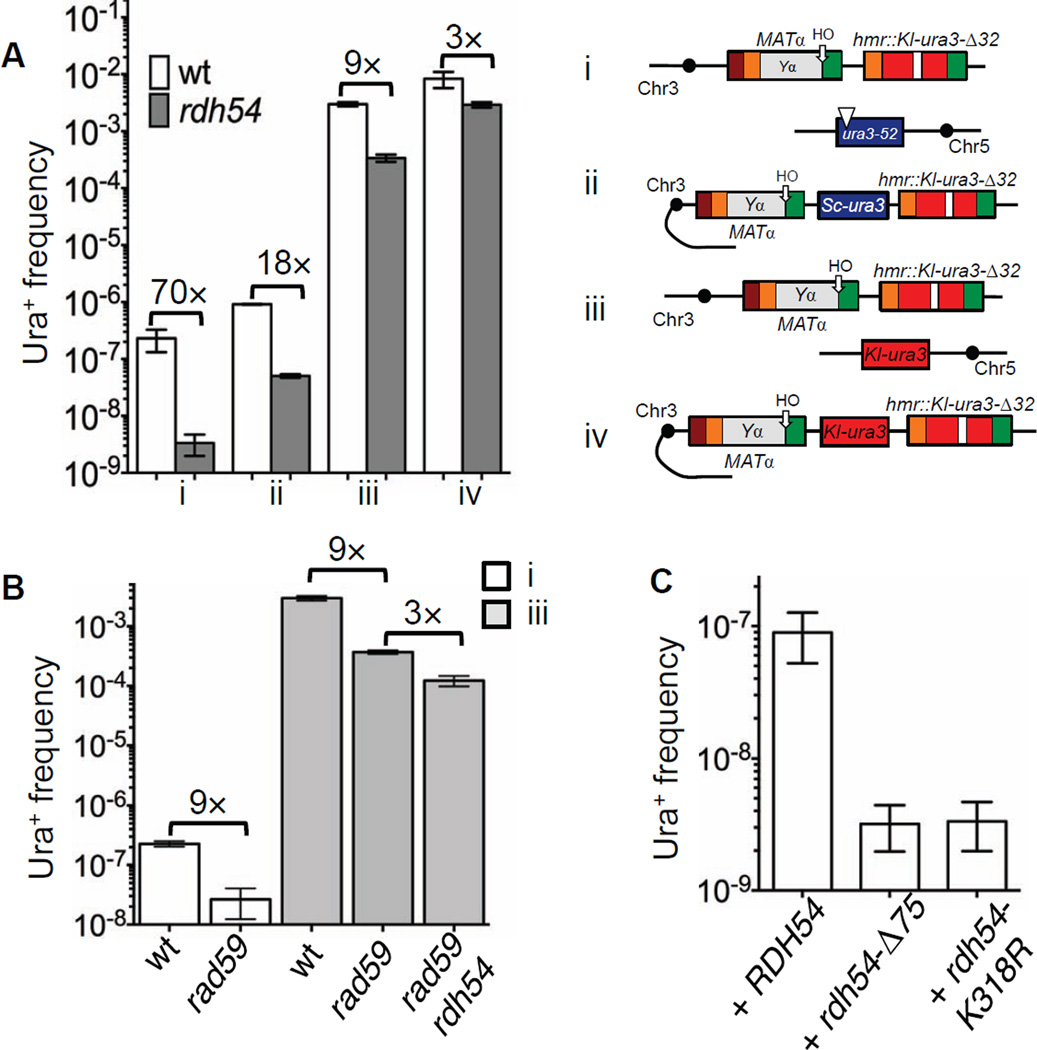
Figure 5. Rdh54 is important for template switching.
A. In an rdh54Δ strain, template switching between inter- (TOY62) and intachromosomal (TOY83) homeologous sequences is reduced 70-fold and 18-fold correspondingly compared to the wild type (i and ii). Deletion of RDH54 also reduces homologous template switching 10-fold to 3×10−4 (TOY64) (iii) and has a similar effect on intrachromosomal homologous events reducing rates to the 3×10−3 (TOY77) (iv). B. Analysis of Ura+ rates in TOY43, TOY47, TOY85, TOY90 and TOY91 strains. In rad59Δ strain, frequency of ICTS both between homeologous and homologous templates is reduced compared to wild type (compare i and iii). Absence of Rad59 in rdh54Δ further decreases ICTS even for homologous system. C. Both ATPase activity of Rdh54 and its interaction with Rad51 are required for ICTS. Data are represented as mean +/− SEM.
When the homeologous donor in rdh54Δ strain was placed on Chr3, rdh54Δ still inhibited template switching by a factor of 20 (Fig 5A, compare i and ii, and Table S2). The strong role of Rdh54 in ICTS was seen even when the Chr5 donor carried a fully homologous Kl-ura3 sequence, where ICTS in rdh54Δ was reduced to 10% of the wild type level (Fig 5A and Table S2). When we moved a homologous donor to Chr3 in rdh54Δ strain the frequency of template switching was reduced 3 fold (Fig 5A, compare iii and iv, and Table S2). We conclude that Rdh54 is crucial for the DNA transactions during both inter- and intrachromosomal template switches between divergent sequences even though it has no significant role in simple gene conversion.
We asked if Rdh54’s role was connected to two of its previously identified functions. Rdh54 plays a central role in meiosis with the Rad51 homolog, Dmc1, which should not be expressed in mitotic cells (Dresser et al., 1997; Petukhova et al., 2000). To be certain that Dmc1 was not involved, we measured ICTS in dmc1Δ and found no effect (Table S2 and Table S4). In addition, Rdh54 acts along with its homologs Rad54 and Uls1 to displace Rad51 from dsDNA (see above). Rad54 is essential for all DSB-provoked gene conversion (Table S4), but uls1Δ has no effect on gene conversion and ICTS (Table S2 and Table S4).
Spontaneous recombination measured between Kl-ura3 on Chr5 and mat::Kl-ura3-Δ32 or hmr::Kl-ura3-Δ32 in rdh54Δ strain was similar to the wild type strain arguing that rdh54Δ cells are proficient for recombination but deficient for ICTS (Table S3). These data identify rdh54 as the first mutation to specifically impair ICTS without affecting gene conversion per se.
Finally, we show that Rad59, another protein implicated in a Rad51-independent recombinational repair process (Ira and Haber, 2002), is also important for ICTS (Fig 5B). However, unlike rdh54Δ, deletion of RAD59 also affects the efficiency of gene conversion, reducing it to about 30% (Table S4). Spontaneous recombination between two identical sequences in both rad59Δ and rad59Δ rdh54Δ strains were also lower than in wild type (Table S3), whereas deletion of RDH54 does not affect either gene conversion or spontaneous recombination.
The frequency of ICTS between homeologous sequences was reduced 9 fold in rad59Δ compared to the wild type (Fig 5B, system i). ICTS between homologous sequences was also reduced 9 fold compared to wild type, from 3.0×10−3 to 3.7×10−4 (Fig 5B and Table S2). This reduction is comparable to that seen with rdh54Δ (3.4×10−4). When we deleted RDH54 in the rad59Δ background, the rate of ICTS between homologous sequences decreased a further 3 fold, arguing that deleting these two genes may affect more than one pathway (Fig 5B and Table S2).
ATPase activity and interaction with Rad51 are important for the role of Rdh54 in template switching
Rdh54 is ATP-dependent DNA translocase and interacts with Rad51 protein through its N-terminus (Chi et al., 2006; Santa Maria et al., 2013). To elucidate if translocase activity or interaction with Rad51 is required for template switch, we transformed rdh54Δ strain with plasmids carrying full-length RDH54 (pHK489), rdh54 lacking first 75 amino acids required for interaction with Rad51 (pHK549) or rdh54-K318R (pRB101), all expressed from their native promoter (Santa Maria et al., 2013). Full length RDH54 restored ICTS to the wild-type level, while neither rdh54-Δ75 nor rdh54-K318R constructs were able to rescue the rdh54 phenotype (Fig 5C and Table S2). We conclude that both ATPase activity and interaction with Rad51 are required for ICTS during gene conversion repair.
As the Rad51-interacting domain of Rdh54 is required for ICTS, we asked if overexpression of Rad51 would affect ICTS in the rdh54Δ strain. As constitutive overexpression of RAD51 is deleterious in a rdh54Δ strain ((Santa Maria et al., 2013) and data not shown), we transformed rdh54Δ with galactose-inducible RAD51 on a multicopy plasmid pHK317(3) (Santa Maria et al., 2013) and simultaneously induced a DSB and RAD51 overexpression for five hours. Transient Rad51 overproduction did not affect the rate of ICTS in the rdh54Δ strain (Table S2), but significantly reduced its survival to 47% compared to the rdh54Δ strain transformed with an empty vector (Table S4). Finally, we found that overexpression of RDH54 under a native promoter from a multicopy plasmid (pOT23) did not affect ICTS (Table S2).
The kinetics of the first and second template switches are similar
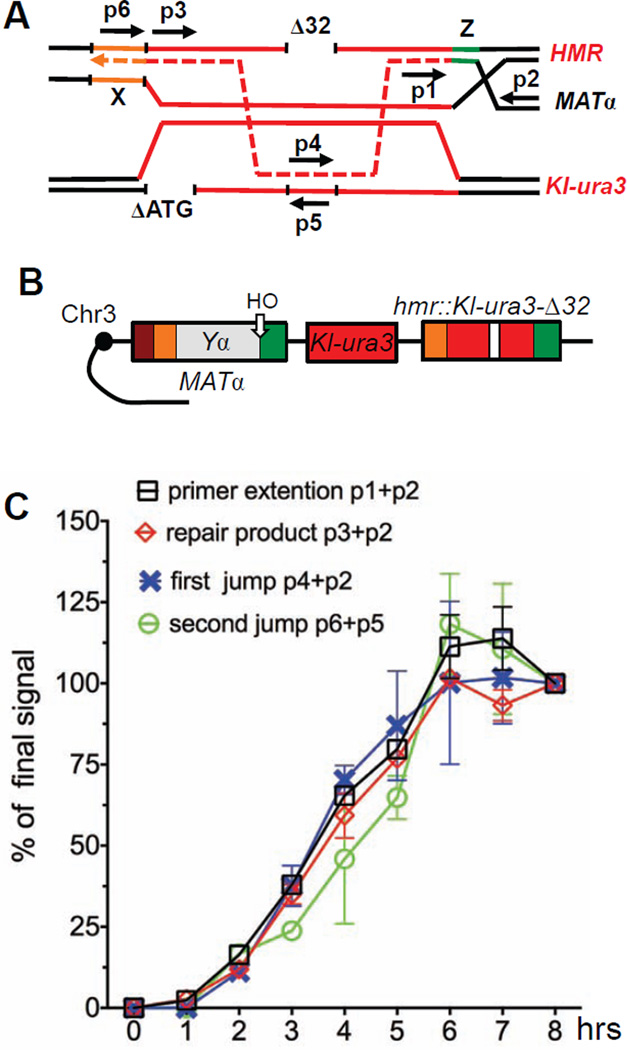
We analyzed the timing of intrachromosomal jumps by semi-quantitative PCR analysis. Appropriate dilutions of template DNA were assessed by the intensity of PCR bands that were quantified after agarose gel resolution (see Experimental Procedures). We designed a set of primers to detect kinetics of the simple MATα → mat::Kl-ura3-Δ32 switch (Fig 6A). Two other primer pairs were created to detect (a) the copying of the 32-bp segment after MAT end invaded into Kl-ura3 donor and (b) the completion of template switch, when the 32-bp region from Kl-ura3 was joined to MAT-proximal sequences (Fig 6A).
Figure 6. Kinetics of template switching between homologous sequences.
A. Primers used to analyze the kinetics of simple gene conversion repair and template switch. B. Template switching strains to analyze homologous intrachromosomal events (TOY74). C. Kinetics of gene conversion repair and template switch in TOY74. Data are represented as mean +/− SEM.
In the homologous intrachromosomal system (Fig 6B) the kinetics of the simple gene conversion was similar to those described previously (Hicks et al., 2011): the PCR product for primer extension and the repaired product at MAT were detected as early as 1 h after HO cut and DSB formation (Fig 6C). Repair was complete by 6 h and nearly 100% of cells completed repair (Fig 6C).
The timing of both the first and second steps of template switch were similar to that much more abundant gene conversion repair product, suggesting that there are no long delays in the search for homology in template switching, and the second template switch immediately follows the first template switch (Fig 6C).
Discussion
Accurate repair of DSBs is essential to ensure genome stability. Although gene conversion – inserting a small patch of newly synthesized DNA to seal up a DSB – is a conservative way to accomplish repair, it is not without risk. There is a 1000-fold increase in mutations within the newly copied sequences (Hicks et al., 2010). Many of these mutations apparently result from dissociations of the partially copied strand from its donor template and then its inaccurate re-association, yielding frameshift mutations in homonucleotide runs, quasipalindrome mutations and – most remarkably – interchromosomal template switches between highly divergent templates. In this paper we have focused on ICTS and documented a number of remarkable aspects about its molecular mechanism.
One concern in our original work was that the initial donor sequences (hmr::Kl-URA3) were heterochromatic, so that it was possible that the high rate of ICTS was prompted by the difficulty in the repair DNA polymerase to copy these sequences, although normal MAT switching using these heterochromatic sequences is nearly 100% efficient (Haber, 2012). Here we show that ICTS is not significantly affected when the donor is unsilenced compared to the silent, heterochromatic state. These results suggest that neither the initial dissociation nor the re-invasion of the nascent strand after copying sequences from the interchromosomal donor is altered by the heterochromatic state of HMR.
ICTS between 71% identical templates was readily detectable by selection, and represented 2–4% of all the mutations we recovered (Hicks et al., 2010), but the rate of these interchromosomal jumps was only about 2 in 107 cells. We now find that this low rate reflects the barriers imposed by the extensive sequence divergence of the K. lactis and S. cerevisiae URA3 sequences, because when the Chr5 donor was changed to be identical to the sequences in hmr::Kl-ura3-Δ32, the rate of ICTS increased 10,000 times, to about 3×10−3. In fact when the donor was moved from Chr5 to Chr3, so that all the transactions were intrachromosomal, the rate of template switching was 8×10−3 (Fig 2B and Table S2). These kinds of template jumps vastly exceed the rates of simple frameshift or quasipalindrome mutations, which simply involve realignments of the nascent strand on a single template, which were found at a rate of about 3×10−5 (Hicks et al. 2010). Our results emphasize the remarkable instability of the repair replication process, such that nearly 1 in 100 events was accompanied by the pair of template jumps. We note that we only detect events that copy the region correcting the 32-bp deletion; there could easily be an equal or larger number of events that copied other segments of the template but would not be scored in our assay. In any case it is evident that ICTS is very common, indicating that DNA synthesis machinery is extremely unstable and prone to dissociation from a template strand. If we assume that the rate of dissociation from the template and invasion in a second donor is the same for both of the required two jumps to complete repair, then the rate of dissociation and ectopic invasion occurs once in every ten events.
Template switching was also described earlier in another type of recombination repair – break-induced replication (BIR), when invading strand turns to replication fork and continues DNA synthesis to the very end of a chromosome (Deem et al., 2008; Smith et al., 2007). Despite parallels between gene conversion repair and BIR, such as highly inaccurate DNA synthesis (Deem et al., 2011; Hicks et al., 2010) and a conservative mechanism of DNA replication involving a migrating D-loop (Donnianni and Symington, 2013; Ira et al., 2006; Saini et al., 2013), the template switch machinery might be distinct from either of these two processes. First, we showed that GC-associated template switching is not affected by the overproduction of Rad51 recombinase, whereas ectopic BIR is increased (Lydeard et al., 2010). Second, single or double deletion of MUS81 or YEN1 endonucleases or MPH1 helicase implicated earlier in BIR template switching (Pardo and Aguilera, 2012; Stafa et al., 2014), did not change rates of ICTS in our system (Table 1 and data not shown). Finally, we have previously shown that deleting POL32, which is required for BIR, has no effect on ICTS (Hicks et al., 2010).
The barriers to homeologous ICTS are formidable. We have knocked out many of the mismatch repair (MMR) genes and 3’ to 5’ helicases that have been shown to play some role in rejecting recombination between mismatched sequences, albeit usually less mismatched than the 71% divergence between the sequences used here (Alani, 1996; Nickoloff et al., 1999; Schmidt et al., 2006); none of them exhibited more than a two-fold increase in ICTS (Table 1). These results might mean that ICTS acts in a different fashion, not relying on the MMR genes and the helicases to prevent recombination. Indeed, it is not certain whether the jumps into and out of ura3-52 require the Rad51-mediated strand exchange machinery. Heteroduplex rejection by MMR and helicases might only occur in the context of Rad51-mediated exchange and not if ICTS involved template switches mediated directly by the DNA polymerase still associated with the nascent strand. Alternatively, the extent of divergence might simply be too great for MMR proteins to act efficiently. In a previous study a msh6Δ mutant alone increased homeologous recombination between 91% identical sequences 4-fold and msh6Δ msh3Δ increased the rate almost 70-fold (Spell and Jinks-Robertson, 2003). A similar effect was shown for msh3 and msh2 mutants in recombination between 77% homologous sequences (Datta et al., 1996). However, in ICTS between 71% identical templates, neither msh6Δ nor the double mutant msh6Δ msh3Δ increased the rate by more than about 2-fold (Table 1).
The junctions formed during ICTS are characterized by small patches of microhomology. Many different junctions are used, with an average length of about 8–9 bp. Sites with the longest microhomology (11 bp on one side of the Δ32 deletion and 17 bp on the other) were used more frequently than any others, but sites with only 5–8 bp microhomology were also frequently used and some sites of only 2 bp were among the highest usage. Hence, we do not deduce a very clear rule about where strand invasions into and out of the homeologous template should take place. There also was no evident correlation between the site of invasion into ura3-52 and the site of departure.
It should be noted that the site where we detect microhomology does not mean that the invasion of the displaced strand into the homeologous template was restricted to these few base pairs. A much longer region of heteroduplex DNA might be established and then acted upon by mismatch repair genes to yield a final outcome (reviewed by (Boiteux and Jinks-Robertson, 2013; Chen and Jinks-Robertson, 1998)). This idea is strikingly emphasized by a remarkable phenotype of deleting MSH6.
Although msh6Δ did not significantly change the rate of ICTS, the absence of this gene resulted in nearly 10% of all mat::URA3 outcomes having 2 patches of ura3-52 sequences in the final outcome, each bounded by microhomology junctions (Fig 4A and 4B, Fig S2 and Fig S3). Although these events could reflect four template switches instead of the normal two needed to replace the 32-bp deletion, we believe that these outcomes reflect the formation of a long region of heteroduplex DNA between the divergent sequences, followed by “patchy” mismatch repair. In the absence of MSH6, the length of heteroduplex might be much longer, affording loops of consecutively mismatched bases to be repaired by a Msh6-independent repair process. Indeed heteroduplex loops in meiotic cells have been shown to be repaired by an Msh3-dependent process (Kearney et al., 2001). Here, we find that the frequent instances of two-patch gene conversion in msh6Δ are eliminated in msh6Δ msh3Δ cells. We note that in the meiotic case, the heteroduplex was a single indel loop, whereas in the case we are examining, two mismatched unpaired strands of equal length will form symmetrical loops. The repair of such discontinuities appears to require not only Msh3 but also the Mlh1 (and presumably Pms1) proteins, which were not required for repair of the simpler meiotic loops.
The msh6Δ and mlh1Δ strains also displayed another previously unseen phenotype in which the cells were Ura+ in spite of having copied the Kl-ura3-Δ32 sequence into MAT. These unexpected cases proved to have one patch of ura3-52 sequence copied into the transcribed HMR donor, creating a chimeric Ura+ gene (Fig 4C). Among hundreds of wild type and other mutant strains that we have analyzed we found no cases where the URA3 sequences were not at MAT. We believe these cases represent instances in which the displaced strand, leaving the ura3-52 donor, forms an extensive heteroduplex at hmr::Klura3-Δ32 which leads to mismatch repair of the hmr::Kl-ura3-Δ32 sequence (Fig 4D). These events can only be obtained if cells fail to discriminate which is the nascent strand in two sequential steps during dissociation/strand re-invasion at hmr::Kl-ura3-Δ32, while for the formation of two Sc-ura3 patches at MAT the lack of strand discrimination activity is not required. We conclude that Msh6 and Mlh1 normally play an important role not only in confining the length of heteroduplex but in directing mismatch repair to the nascent strand rather than the uninterrupted template. Further evidence of this “reverse” mismatch repair came from discovering cases in msh6Δ that had a chimeric and functional URA3 gene at MAT but a patch of ura3-52 sequences at the HMR donor as well. As at MAT, these unusual events were eliminated when Msh3 was deleted in the msh6Δ strain.
In addition to identifying novel roles for Msh6 in homeologous ICTS, we have discovered the first protein that is required for ICTS but has no evident role in simple gene conversions. Deletion of RDH54 has no impact on simple gene conversion between MATα and hmr::Kl-ura3-Δ32, but profoundly reduced ICTS. In the homeologous ICTS system rdh54Δ reduced events by 70 fold. In homologous inter- and intrachromosomal assays, rdh54Δ still reduced the rate of Ura+ recovery by 10- and 3-fold, correspondingly.
In meiosis, Rdh54 is required for the efficient strand invasion activity of the Rad51 homolog, Dmc1 (Dresser et al., 1997; Petukhova et al., 2000). Dmc1 is not expressed in mitotic cells, but Rdh54 may adopt a similar role with Rad51 in these unusual circumstances. Indeed our analysis showed that the rdh54-Δ75 mutation which eliminates its interaction with Rad51 (Santa Maria et al., 2013) behaves as a null mutation (Fig 5C).
We do not yet know how Rdh54 might specifically facilitate ICTS, but it seems possible that Rdh54 is required to facilitate the strand invasions at the distant donor (Renkawitz et al., 2013; Sung, 1994). Alternatively, Rdh54 is required to dissociate nascent strand from either hmr::Kl-ura3-Δ32 or Kl-ura3 donor to execute either first or second template jump.
Experimental Procedures
Yeast strains and plasmids
All strains used in this study are isogenic to JKM153 (Hicks et al., 2010). Table S5 notes only the allele(s) that differ from the JKM153 genotype.
The 32-bp deletion (Δ32) in Kl-URA3 was created by PCR mutagenesis using primers OT09, OT10, OT12 and OT13 (see Table S6). An hmr::Kl-URA3 construct lacking the HO cut site was used a template (Hicks et al., 2010) to create Ya::Kl-ura3-Δ32 cassette.
To introduce Kl-ura3-KANMX, Kl-ura3-TRP1 or Sc-ura3-KANMX into Chr 3 or 5, a pRS315-based plasmids were created by in vivo recombination (Oldenburg et al., 1997) where K. lactis or S. cerevisiae URA3 ORFs were placed adjacent to KANMX or TRP1 markers resulting in plasmids pRS314-pLEU2-Kl-URA3-KANMX6 (pOT04), pRS314-pLEU2-Kl-URA3-TRP1 (pOT05) or pRS314-pLEU2-Sc-URA3-KANMX6 (pOT14). Appropriate PCR fragments were transformed into yeast strains (Table S6).
To express RDH54, rdh54-Δ75 or rdh54-K318R from their native promoters, previously described plasmids pHK489, pHK549 and pRB101 were used (Santa Maria et al., 2013). To overexpress RDH54, a full length RDH54 under its native promoter was PCR-amplified using pHK489 (Santa Maria et al., 2013) as a template and then inserted into the 2µ YEp13-LEU2 vector (Broach et al., 1979) by in vivo recombination creating pOT23. To overproduce Rad51, the previously described pSJ5 was used (Lydeard et al., 2010).
Analysis of template switching frequencies and survival
Yeast cells were grown in YP media supplemented with raffinose to mid-log phase, induced 4 h with galactose, collected and appropriate dilutions were plated on YPD plates and plates lacking uracil. For survival analysis, an equal number of cells were plated on YP+Dextrose and YP+Galactose. Plates were incubated 3 days at 30°C and the number of colonies were counted. Three repetitions per strain were performed; 3 clones were analyzed per repetition.
To measure spontaneous recombination frequencies in different strains, a Ura− MATa-like strain (mat::Kl-ura3-Δ32 hmr::Kl-ura3-Δ32) was used. The HO-cut site is absent at both mat and hmr, therefore HO-induced events are excluded. Spontaneous recombination can occur between the ectopic donor on Chr5 and either mat::Kl-ura3-Δ32 or hmr::Kl-ura3-Δ32; we scored total Ura+ colonies and did not distinguish between the two loci. Frequencies were scored as described above.
Error bars indicate the standard error of the mean. To assess if difference between strains is significant, t-test analysis was performed.
Sequencing analysis of Ura+ colonies
Genomic DNA was extracted from Ura+ colonies with a MasterPure Yeast DNA purification kit (Epicentre) and MAT or HMR loci were PCR amplified using MATX_F + MATDp8, or OT09 + OT10 primers, correspondingly (Table S6). PCR products were sequenced with MATXp1 and KlUra3p1 primers, correspondingly. Chimeric URA3 sequences were aligned against S. cerevisiae URA3 and junction sites were identified at the border of homology.
Analysis of kinetics of template switches by semi-quantitative PCR
Yeast were grown in YP supplemented with raffinose to OD ≈ 0.4, nocodazole-arrested for 3 hours at 20µg/ml, and HO-induced by adding 2% of galactose. Approximately 2×108 cells were collected by centrifugation and DNA was purified with MasterPure Yeast DNA purification kit (Epicentre). DNA concentration was normalized according to PCR with primers to unrelated sequence SLX1 (Table S6) and appropriate dilutions of a DNA were subjected to PCR with primers p1 (OT88), p2 (MATdist_2R), p3 (Kl URA3p1), p4 (OT70), p5 (OT71) and p6 (MATXp1). Three standards with known DNA concentrations were run in parallel. To avoid saturation of PCR products in test samples and standards, different number of amplification cycles were applied and DNA standards of different concentrations were used for every set of primers. To analyze simple gene conversion, samples were amplified for 20–22 cycles and standard DNA range 0.1×10−4–1.0×10−4 pg/µl was used for primers p1 and p2 and 0.1×10−3–1.0×10−3 pg/µl for primers p3 and p2. To analyze template switch, samples were amplified for 42–44 cycles and standard DNA concentration of 0.1×10−6–1.0×10−6 pg/µl or 0.1×10−7–1.0×10−7 pg/µl was used. DNA fragments were resolved by 2% agarose gel stained with ethidium bromide, checked for saturation with Quantity One 1-D software (Bio-Rad) and then quantified with same software.
Supplementary Material
Highlights.
Interchromosomal template switches (ICTS) occur in nearly 1% of DSB repair events
ICTS is not impaired by heterochromatin
Mismatch repair proteins Msh6 and Mlh1 direct strand-repair of heteroduplex DNA
The chromatin remodeler Rdh54 is required for ICTS but not simple gene conversion
Acknowledgements
We thank H. Klein for plasmids, N. Sugawara and other members of the Haber group for advice and discussion. OT is supported by an International postdoc fellowship from The Swedish Research Council, reference number 2011–6805. Research in the Haber lab is funded by NIH grants GM20056 and GM76020.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alani E. The Saccharomyces cerevisiae Msh2 and Msh6 proteins form a complex that specifically binds to duplex oligonucleotides containing mismatched DNA base pairs. Mol Cell Biol. 1996;16:5604–5615. doi: 10.1128/mcb.16.10.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S, Jinks-Robertson S. DNA repair mechanisms and the bypass of DNA damage in Saccharomyces cerevisiae. Genetics. 2013;193:1025–1064. doi: 10.1534/genetics.112.145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol. 2010;11:208–219. doi: 10.1038/nrm2852. [DOI] [PubMed] [Google Scholar]
- Broach JR, Strathern JN, Hicks JB. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979;8:121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Chen W, Jinks-Robertson S. Mismatch repair proteins regulate heteroduplex formation during mitotic recombination in yeast. Mol Cell Biol. 1998;18:6525–6537. doi: 10.1128/mcb.18.11.6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Kwon Y, Seong C, Epshtein A, Lam I, Sung P, Klein HL. Yeast recombination factor Rdh54 functionally interacts with the Rad51 recombinase and catalyzes Rad51 removal from DNA. J Biol Chem. 2006;281:26268–26279. doi: 10.1074/jbc.M602983200. [DOI] [PubMed] [Google Scholar]
- Connolly B, White CI, Haber JE. Physical monitoring of mating type switching in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:2342–2349. doi: 10.1128/mcb.8.6.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Adjiri A, New L, Crouse GF, Jinks Robertson S. Mitotic crossovers between diverged sequences are regulated by mismatch repair proteins in Saccaromyces cerevisiae. Mol Cell Biol. 1996;16:1085–1093. doi: 10.1128/mcb.16.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem A, Barker K, Vanhulle K, Downing B, Vayl A, Malkova A. Defective break-induced replication leads to half-crossovers in Saccharomyces cerevisiae. Genetics. 2008;179:1845–1860. doi: 10.1534/genetics.108.087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem A, Keszthelyi A, Blackgrove T, Vayl A, Coffey B, Mathur R, Chabes A, Malkova A. Break-induced replication is highly inaccurate. PLoS Biol. 2011;9:e1000594. doi: 10.1371/journal.pbio.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnianni RA, Symington LS. Break-induced replication occurs by conservative DNA synthesis. Proc Natl Acad Sci U S A. 2013;110:13475–13480. doi: 10.1073/pnas.1309800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresser ME, Ewing DJ, Conrad MN, Dominguez AM, Barstead R, Jiang H, Kodadek T. DMC1 functions in a Saccharomyces cerevisiae meiotic pathway that is largely independent of the RAD51 pathway. Genetics. 1997;147:533–544. doi: 10.1093/genetics/147.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forment JV, Kaidi A, Jackson SP. Chromothripsis and cancer: causes and consequences of chromosome shattering. Nat Rev Cancer. 2012;12:663–670. doi: 10.1038/nrc3352. [DOI] [PubMed] [Google Scholar]
- Haber JE. Mating-type genes and MAT switching in Saccharomyces cerevisiae. Genetics. 2012;191:33–64. doi: 10.1534/genetics.111.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I, Jensen RE. Putting the HO gene to work: practical uses for mating-type switching. Methods Enzymol. 1991;194:132–146. doi: 10.1016/0076-6879(91)94011-z. [DOI] [PubMed] [Google Scholar]
- Hicks WM, Kim M, Haber JE. Increased mutagenesis and unique mutation signature associated with mitotic gene conversion. Science. 2010;329:82–85. doi: 10.1126/science.1191125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks WM, Yamaguchi M, Haber JE. Real-time analysis of double-strand DNA break repair by homologous recombination. Proc Natl Acad Sci U S A. 2011;108:3108–3115. doi: 10.1073/pnas.1019660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzen TM, Shah PP, Olivares HA, Bishop DK. Tid1/Rdh54 promotes dissociation of Dmc1 from nonrecombinogenic sites on meiotic chromatin. Genes Dev. 2006;20:2593–2604. doi: 10.1101/gad.1447106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira G, Haber JE. Characterization of RAD51-independent break-induced replication that acts preferentially with short homologous sequences. Mol Cell Biol. 2002;22:6384–6392. doi: 10.1128/MCB.22.18.6384-6392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira G, Satory D, Haber JE. Conservative inheritance of newly synthesized DNA in double-strand break-induced gene conversion. Mol Cell Biol. 2006;26:9424–9429. doi: 10.1128/MCB.01654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney HM, Kirkpatrick DT, Gerton JL, Petes TD. Meiotic recombination involving heterozygous large insertions in Saccharomyces cerevisiae: formation and repair of large, unpaired DNA loops. Genetics. 2001;158:1457–1476. doi: 10.1093/genetics/158.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein HL. RDH54, a RAD54 homologue in Saccharomyces cerevisiae, is required for mitotic diploid-specific recombination and repair and for meiosis. Genetics. 1997;147:1533–1543. doi: 10.1093/genetics/147.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Pellicioli A, Malkova A, Foiani M, Haber JE. The Saccharomyces recombination protein Tid1p is required for adaptation from G2/M arrest induced by a double-strand break. Curr Biol. 2001;11:1053–1057. doi: 10.1016/s0960-9822(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Lydeard JR, Lipkin-Moore Z, Jain S, Eapen VV, Haber JE. Sgs1 and exo1 redundantly inhibit break-induced replication and de novo telomere addition at broken chromosome ends. PLoS Genet. 2010;6:e1000973. doi: 10.1371/journal.pgen.1000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyndaker AM, Goldfarb T, Alani E. Mutants defective in Rad1-Rad10-Slx4 exhibit a unique pattern of viability during mating-type switching in Saccharomyces cerevisiae. Genetics. 2008;179:1807–1821. doi: 10.1534/genetics.108.090654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova A, Ivanov EL, Haber JE. Double-strand break repair in the absence of RAD51 in yeast: a possible role for break-induced DNA replication. Proc Natl Acad Sci U S A. 1996;93:7131–7136. doi: 10.1073/pnas.93.14.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff JA, Sweetser DB, Clikeman JA, Khalsa GJ, Wheeler SL. Multiple heterologies increase mitotic double-strand break-induced allelic gene conversion tract lengths in yeast. Genetics. 1999;153:665–679. doi: 10.1093/genetics/153.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg KR, Vo KT, Michaelis S, Paddon C. Recombination-mediated PCR-directed plasmid construction in vivo in yeast. Nucleic Acids Res. 1997;25:451–452. doi: 10.1093/nar/25.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo B, Aguilera A. Complex chromosomal rearrangements mediated by break-induced replication involve structure-selective endonucleases. PLoS Genet. 2012;8:e1002979. doi: 10.1371/journal.pgen.1002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petukhova G, Sung P, Klein H. Promotion of Rad51-dependent D-loop formation by yeast recombination factor Rdh54/Tid1. Genes Dev. 2000;14:2206–2215. doi: 10.1101/gad.826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli J, Tsaponina O, Crabbe L, Keszthelyi A, Pantesco V, Chabes A, Lengronne A, Pasero P. dNTP pools determine fork progression and origin usage under replication stress. Embo J. 2012;31:883–894. doi: 10.1038/emboj.2011.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawitz J, Lademann CA, Kalocsay M, Jentsch S. Monitoring homology search during DNA double-strand break repair in vivo. Mol Cell. 2013;50:261–272. doi: 10.1016/j.molcel.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Saini N, Ramakrishnan S, Elango R, Ayyar S, Zhang Y, Deem A, Ira G, Haber JE, Lobachev KS, Malkova A. Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature. 2013;502:389–392. doi: 10.1038/nature12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa Maria SR, Kwon Y, Sung P, Klein HL. Characterization of the interaction between the Saccharomyces cerevisiae Rad51 recombinase and the DNA translocase Rdh54. J Biol Chem. 2013;288:21999–22005. doi: 10.1074/jbc.M113.480475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KH, Wu J, Kolodner RD. Control of translocations between highly diverged genes by Sgs1, the Saccharomyces cerevisiae homolog of the Bloom's syndrome protein. Mol Cell Biol. 2006;26:5406–5420. doi: 10.1128/MCB.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PP, Zheng X, Epshtein A, Carey JN, Bishop DK, Klein HL. Swi2/Snf2-related translocases prevent accumulation of toxic Rad51 complexes during mitotic growth. Mol Cell. 2010;39:862–872. doi: 10.1016/j.molcel.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Gasior SL, Bishop DK, Shinohara A. Tid1/Rdh54 promotes colocalization of rad51 and dmc1 during meiotic recombination. Proc Natl Acad Sci U S A. 2000;97:10814–10819. doi: 10.1073/pnas.97.20.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signon L, Malkova A, Naylor ML, Klein H, Haber JE. Genetic requirements for RAD51- and RAD54-independent break-induced replication repair of a chromosomal double-strand break. Mol Cell Biol. 2001;21:2048–2056. doi: 10.1128/MCB.21.6.2048-2056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, Llorente B, Symington LS. Template switching during breakinduced replication. Nature. 2007;447:102–105. doi: 10.1038/nature05723. [DOI] [PubMed] [Google Scholar]
- Spell RM, Jinks-Robertson S. Role of mismatch repair in the fidelity of RAD51- and RAD59-dependent recombination in Saccharomyces cerevisiae. Genetics. 2003;165:1733–1744. doi: 10.1093/genetics/165.4.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafa A, Donnianni RA, Timashev LA, Lam AF, Symington LS. Template switching during break-induced replication is promoted by the Mph1 helicase in Saccharomyces cerevisiae. Genetics. 2014;196:1017–1028. doi: 10.1534/genetics.114.162297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science. 1994;265:1241–1243. doi: 10.1126/science.8066464. [DOI] [PubMed] [Google Scholar]
- Weiss K, Simpson RT. High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating type locus HML alpha. Mol Cell Biol. 1998;18:5392–5403. doi: 10.1128/mcb.18.9.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CI, Haber JE. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. Embo J. 1990;9:663–673. doi: 10.1002/j.1460-2075.1990.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeeles JT, Poli J, Marians KJ, Pasero P. Rescuing stalled or damaged replication forks. Cold Spring Harb Perspect Biol. 2013;5:a012815. doi: 10.1101/cshperspect.a012815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.