Abstract
Magnetic resonance imaging (MRI) and functional MRI (fMRI) continue to advance because creative physicists, engineers, neuroscientists, clinicians, and physiologists find new ways for extracting more information from the signal. Innovations in pulse sequence design, paradigm design, and processing methods have advanced the field and firmly established fMRI as a cornerstone for understanding the human brain. In this article, the field of fMRI is described through consideration of the central problem of separating hemodynamic from neuronal information. Discussed here are examples of how pulse sequences, activation paradigms, and processing methods are integrated such that novel, high-quality information can be obtained. Examples include the extraction of information such as activation onset latency, metabolic rate, neuronal adaptation, vascular patency, vessel diameter, vigilance, and subvoxel activation. Experimental measures include time series latency, hemodynamic shape, MR phase, multivoxel patterns, ratios of activation-related R2* to R2, metabolic rate changes, fluctuation correlations and frequencies, changes in fluctuation correlations and frequencies over time, resting correlation states, echo time dependence, and more.
Key words: : blood, brain, fMRI, hemodynamic, high-resolution, multimodal, neurovascular coupling
Introduction
I never forgot one of the first sentences uttered to me by Jim Hyde as I sat in his office, interviewing for graduate school admission. To paraphrase his words: “The MR signal has a wealth of information and we're in the business of extracting it.” This was both an inspiration and a challenge, and completely hooked me into wanting to work in his group. This is the essence of magnetic resonance imaging (MRI) development. Since those first words that Jim Hyde spoke to me, it's been my business as well—and pretty much all that I think about in the context of functional MRI (fMRI).
MRI has indeed been unique in that the potential contrasts and dimensions of advancement are generally greater in number than those of other imaging techniques. Starting with the precession of nuclei—the most common being protons—that change their relaxation rates depending on their immediate environment, pulse sequences extract and reveal differences and similarities in these proton pools. MRI is sensitive to specific metabolites and molecules and is able to differentiate spins based on their molecular environment, magnetic field environment, motion, and diffusion characteristics. These fundamental measures allow a wealth of neuronal and physiologic information to be extracted (Moonen et al., 1990). The ability to scan at higher resolution and higher field and with multiple coils thus allows ever more fine delineation of differences in these characteristics.
MRI is sensitive to macroscopic and microscopic susceptibility variations created by iron, ferritin, oxygen, air–tissue interfaces, and other materials. With advances in susceptibility imaging pulse sequences, enhanced by high field and high gradient magnitudes and slew rates, anatomical and diffusion images provide whole brain information rivaling that of histology slides (Yang et al., 2013). The information obtainable by MRI has outstripped the ability of the vendors and end-users to make use of it—we are all trying to catch up with what is possible.
It is the extreme sensitivity to subtle changes in blood susceptibility arising from changes in blood oxygenation following localized activation-induced flow changes that allows blood oxygen level-dependent (BOLD) fMRI to be a viable functional contrast (Ogawa et al., 1990). With this sensitivity, one can extract substantial neuronal and physiologic information. A primary challenge is not only to become aware of what aspects of the signal contain information but also how to actually separate out the information.
The practice in fMRI has been to perform blocked activation designs with simple and stylized stimuli, then to smooth the 3D statistical significance maps of activation blobs, and then spatially average activation significance maps with multiple other subjects in a common coordinate system to arrive at maps describing common activation across what is hoped to be a homogenous population. Most studies stop there, reporting the coordinates of the centers of mass.
While this practice is reasonable, it falls far short of what is possible today. Each task, each time series, each voxel, each subtle fluctuation, and each individual contains extractable neuronal and physiologic information. This article is about how this information has been and may be extracted.
The following topics are covered: the hemodynamic response, calibration and quantitation, resting-state fMRI, novel contrasts, multimodal synergy, multivoxel patterns, and high spatial resolution.
The Hemodynamic Response
The fMRI signal changes—both the magnitude and dynamics—are influenced by the interplay of changes in cerebral metabolic rate, blood flow, and blood volume (Blockley et al., 2013). The hemodynamic response is a nonlinear function of neuronal activity at very brief neuronal activity durations or at very low neuronal activity magnitudes (Birn and Bandettini, 2005; Birn et al., 2001; Logothetis et al., 2001). A postundershoot in the signal is commonly observed—lasting up to 40 sec (Poser et al., 2011; Yacoub et al., 2006). A preundershoot is much more rare—lasting about 500 msec (Hu et al., 1997).
The postundershoot has received an upsurge of interest, not necessarily because it holds promise of being extremely useful, but because it is a robust fluctuation in the signal that is not fully understood and that perhaps sheds light on the entire neuronal–hemodynamic coupling process. Some groups suggest that the poststimulus undershoot is mostly caused by a continuation of elevated blood volume (Buxton et al., 2004; Mandeville et al., 1998), while others suggest a continuation of elevated cerebral metabolic rate (Frahm et al., 2008). Others suggest that the postundershoot is related to ongoing neuronal activity (Mullinger et al., 2013, 2014). Still others suggest that it is due, at least in part, to an undershoot in flow. So far, there is no clear consensus; however, this phenomenon will likely continue to be something that physics/contrast mechanism researchers gnaw on as they think deeply about the details of neurovascular coupling and if neuronal information can ever truly be separated from hemodynamic using fMRI alone.
The magnitude of the fMRI signal is most highly influenced also by the baseline venous blood volume in each voxel (Bandettini and Wong, 1997; Davis et al., 1998; Hoge et al., 1999) that can be inferred by using a stressor that causes a uniform global flow change. Such maps of venous volume may be used for segmentation or may be compared in a voxelwise manner with gray matter maps to determine relative oxygen extraction rates.
The magnitude of the fMRI signal—as it varies with a parametric variation of a task within a time series—provides information about the rate of relative rate neuronal firing or relative population of activated neurons. Parametric variations in visual stimulation, motor tasks, and cognitive load lead to corresponding changes in the magnitude; however, as inferred above, the magnitude variation over space of the signal change with a single task is primarily sensitive to venous blood volume per voxel. Neuronal information can really only be gleaned from changes associated with a parametric modulation of the task.
The latency of the fMRI signal, similar to magnitude, is mostly influenced by the relative lag associated with hyperoxygenated blood as it travels through the venous vasculature. The spread of latencies over space are on the order of±2.5 sec. Modulation of the timing of stimuli or task and comparison of this lag have successfully extracted neuronal latencies (Bellgowan et al., 2003; Formisano and Goebel, 2003; Henson et al., 2002; Menon et al., 1998), revealing regions that show a corresponding systematic shift in relative onset latency or even width.
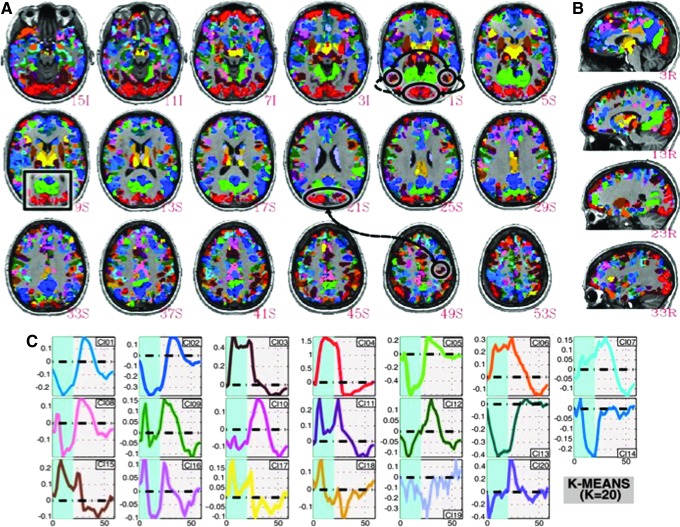
Other transients and nonlinearities are commonly observed. These have been attributed to neuronal habituation (Birn and Bandettini, 2005; Logothetis et al., 2001) or transient on or off responses. Two studies highlight the importance of paying attention to the transients. The first is by Uludag (2008), which demonstrates that with a visual stimulus, the transients at the beginning and at the end of the stimulus block—otherwise missed by standard canonical reference functions—were able to be mapped onto distinct cortical regions. A second study by Gonzalez-Castillo demonstrated that, with enough averaging and with complete relaxation of the expected model function, the entire brain demonstrated activation associated with the pressing of a button in response to the perception of a letter or number within a visual stimulus (Gonzalez-Castillo et al., 2012). Figure 1 shows the representative results from this study. These seemingly noisy signals with odd temporal shapes clustered cleanly in specific cortical and subcortical regions. There is definitely more information there. The null hypothesis appears to not exist.
FIG. 1.
Whole-brain activation revealed by 9 h of averaging and model relaxation. Note that the responses, while diverse, cluster according to specific brain regions. (A) Axial view of a map of the significant signal changes throughout the entire cortex to a simple motor response to a visual decision task. (B) Corresponding Saggital view of the same map. (C) Time courses corresponding to colored regions in the brain. Note that all the different neuronal response timings that pass threshold. Reproduced with permission from Figure 4 of Gonzalez-Castillo and coworkers (2012).
Grill-Spector et al. originated an entire class of fMRI paradigms, known as fMRI adaptation paradigms, where the amount of signal adaption is modulated by the amount of similarity between successive stimuli (Grill-Spector, 2006; Grill-Spector and Malach, 2001). In this study, a stimulus or task is briefly given and then at short intervals, either a similar or different stimulus or task is given in a repetitive manner. If the same stimulus is repetitively presented, then the signal changes will adapt or generally show lower amplitudes. If different stimuli are presented, then the signal will not adapt thus being manifest as a signal that shows no such attenuation. The difference in signal attenuation between the repeating versus nonrepeating stimuli reveals the population that adapted to the repeating stimuli. This task—adaptation modulation—allows titration of stimulus- or task-selective neurons within a voxel, thus providing a boost to the specificity and spatial resolution of fMRI.
Taking a step back, there still appears to be much to learn by studying closely the hemodynamic response as it varies on a voxelwise basis. Hemodynamic latency, width, pre- and postundershoot, and response behavior with varying task on/off rates have all been studied. In a very early study, this group performed simple Fourier analysis of a blocked design time series data set and found peaks not only at the expected on/off frequencies but also multiple harmonics in different voxels, suggesting subtle differences in transient activity across voxels. Figure 2 shows the hemodynamic response and the harmonics from a simple motor task.
FIG. 2.
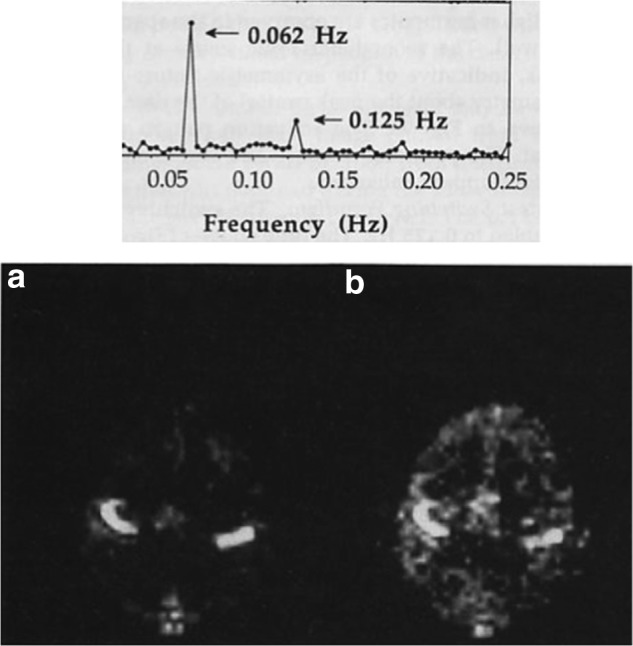
Bilateral finger tapping at an on/off rate of 0.062 Hz reveals a peak at the fundamental frequency and peaks out to later harmonics, suggesting transient activity. Maps of the spectral density, “a” is the fundamental and “b” is the first harmonic—show different regions associated with each. Reproduced with permission from Figure 9 of Bandettini and associates (1993).
Calibration and Quantitation
Since just after the start of fMRI, it was understood that the BOLD signal magnitude was a function of—among other things—baseline venous blood volume, change in cerebral oxidative metabolic rate (CMRO2), change in blood flow, volume, and oxygen extraction rate (Buxton and Frank, 1997; van Zijl et al., 1998).
Since first tentative efforts to normalize the fMRI response magnitude and location to the variation in venous blood volume by subtraction of fMRI maps from a simple hypercapnia response (Bandettini and Wong, 1997), progress has been made to estimate CMRO2 changes (Davis et al., 1998; Hoge et al., 1999) and blood oxygenation changes (Haacke et al., 1997). Lu and Ge (2008) have developed a novel method involving arterial spin labeling and multiecho imaging to select and quantitate blood oxygenation. More recently, Bulte and associates (2012) have refined their calibration approaches to allow assessment of baseline CMRO2. Several groups have also derived maps of vessel size (Bulte et al., 2012; Jochimsen et al., 2010).
These quantitative maps, combined perhaps with vascular territory maps (Wong, 2013), may be an effective complement to current methods that assess vascular patency or susceptibility for stroke or even predict stroke recovery. A shortcoming to most calibration approaches is that they involve hypercapnia or hyperoxia induction—which can be problematic for uncooperative or physically limited patients. These issues may become less problematic with the emergence of alternative more efficient global manipulations such as assessment of spontaneous depth changes (Birn et al., 2008b).
Resting-State fMRI
The discovery by Bharat Biswal that the voxelwise correlations of time series from resting brains contained information related to functional organization (Biswal et al., 1995) was paradigm shifting, opening up—after almost a decade of quiescence—an entirely new field of fMRI (Biswal, 2012). The field of resting-state fMRI has spawned new journals, primarily the present one, and has become the dominant topic of study at the annual Organization for Human Brain Mapping Meeting. Figure 3 shows the overall increase in fMRI publications and the relative increase in publications associated with resting-state fMRI.
FIG. 3.
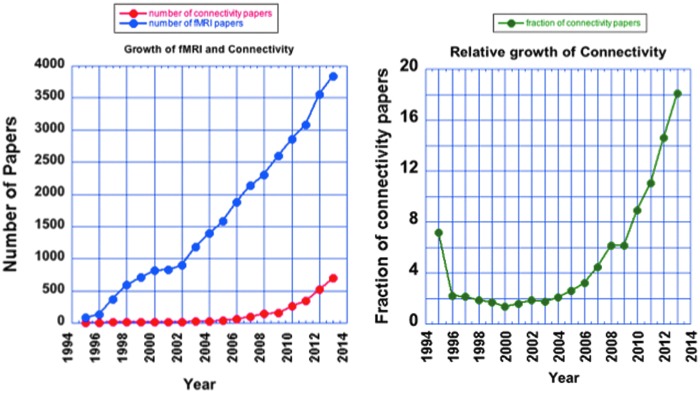
The growth of functional magnetic resonance imaging (fMRI) and connectivity fMRI. Data obtained using Scopus and search terms “fMRI” or “functional MRI” for fMRI and “resting state fMRI” or “connectivity AND fMRI” or “spontaneous fluctuations AND fMRI.”
A first thought was that this signal was either an artifact of imaging or purely vasomotion. The clear success of the method in identifying known functional networks and predicting activation patterns helped to foster acceptance among the brain imaging community that, while the signal of course was vascular and very slow, the correlations indeed represent underlying neuronal fluctuations. The irony to resting-state fMRI is that it relies on the spontaneous and transient activity of the brain for functional contrast. When functionally connected regions show a brief spontaneous activity, the fMRI signal in each of these regions increases in synchrony, demonstrating, after some amount of averaging, a higher correlation than signals that are not functionally related. Using this basic idea, connectivity maps of the brain have been created. From this basic observation, the field has expanded rapidly as researchers have been developing better ways to obtain this connectivity information, more meaningful interpretations, and new ways in which to apply it.
A first observation about resting-state connectivity is that the frequencies of these correlations are extremely low (<0.1 Hz). While there are hints that each network or even complete cortical regions have predominant signature frequencies, all resting-state signals manifest in a very broad band region below 0.1 Hz (Cordes et al., 2001; Niazy et al., 2011), suggesting perhaps that the temporal characteristics of resting state are not well characterized by Fourier analysis.
A second observation is that, either using a seed voxel approach or using mathematical separation methods such as independent component analysis (ICA), a multitude of well-characterized networks consistently reveal themselves. The number of networks or correlated nodes is not well known, having increased in number—due to better processing methods and more sensitivity—from about 5 upward to 350 (Craddock et al., 2012). It is likely that they will continue to grow—perhaps until columnar organization is reached—as the ability to delineate fine detail increases.
A third observation is that the functional correlations change to some degree over time (Chang and Glover, 2010; Hutchison et al., 2013) suggesting network interaction changes or brain state changes. The brain appears to move from state to state. A powerful method to capture all the resting-state correlations is with the creation of pairwise correlation matrices (Allen et al., 2014). A matrix is created by first dividing the brain into regions from which time series are averaged, and then calculating the correlation of the time series from every segment with that of every other segment. Currently, it is not clear precisely how to segment the brain to best define this pairwise correlation matrix. It does appear that the more the segments correspond to functionally homogenous regions of the brain, the more information-rich the pairwise correlation matrices tend to be.
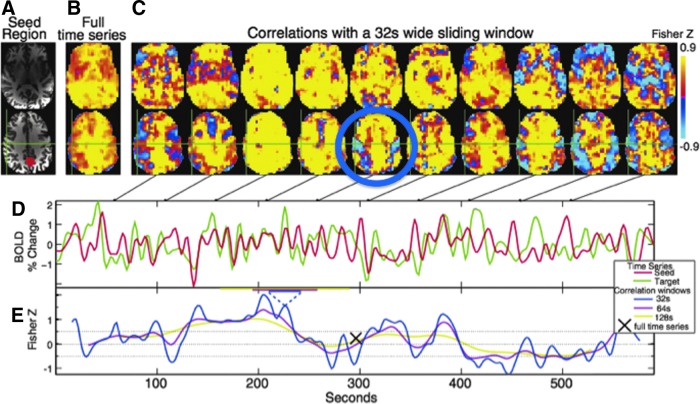
The authors have really only scratched the surface of what information is contained in resting-state signal and how it relates to conscious and unconscious processing. Resting-state fMRI is continuously growing and shifting as the methods and questions become more sophisticated. Many open questions exist regarding resting-state fMRI. Among the most pressing are the following: (1) What is the purpose of resting-state fluctuations? What evolutionary need do they satisfy? (2) What temporal characteristics define each resting-state network? Can they be defined are characterized in this way at all? (3) What about latencies? Accounting for hemodynamic latency variations across the brain may increase the detectability and interpretability of resting-state fMRI data. (4) How many brain states exist? What are the time constants or sequence of these states? Do these measured states correspond to actual states of mind? Are they sensitive to pathology? (5) How finely delineated are these networks? (6) How sensitive is resting-state signal to pathology and prediction of treatment? (7) How much of the resting-state signal is hemodynamic (i.e., vasomotion) versus neuronal in origin? (8) What predominant neuronal oscillation frequency does resting-state fMRI signal correspond to? (9) How is connectivity truly represented through correlation? This conceptual jump between temporal correlation and functional connectivity is wrought with assumptions, including the significant assumption that if a region is oscillating with or has a beat frequency with another region it is connected. This group has observed networks that demonstrate a clear periodic beating, as shown in Figure 4 (Handwerker et al., 2012). However, the authors are unable to say that the two networks are communicating in that any two low-frequency and broadband oscillators can have multiple beat frequencies without actually interacting. In addition, changes in connectivity are typically inferred by changes in correlation. A change in correlation—in the presence of noise—could also be brought on by a simple change in the amplitude of one signal or the other or a simple change in the signal to noise ratio (SNR) of one signal or the other. (10) Last, a controversy that has been debated over the last few years has been regarding whether to regress out the global signal or not to increase sensitivity (Birn et al., 2008a, Murphy et al., 2009). The growing consensus is that one should not remove the global signal as it artificially induces negative correlations between previously uncorrelated regions—thus complicating interpretation. However, a recent study, as shown in Figure 5, has shown that this global signal is highly sensitive to the state of vigilance and removing it may normalize for vigilance variations across subjects and over time (Wong et al., 2013). So the question remains, what should one do about the global signal? It appears to depend on what one wants to do with the time series. Much work remains to be done!
FIG. 4.
Correlations of anterior cingulate with the brain. (A) Red highlight shows the seed region. (B) The correlation maps with the entire time series. (C) Windowed correlation maps demonstrating clear changes in correlations over time. Specifically, the blue circled image shows clearly the motor cortex region demonstrating a periodic anticorrelation with anterior cingulate. (D) Time series signal of the anterior cingulate and the motor cortex. (E) Time series of correlation values between the anterior cingulate and motor cortex. While the average correlation is zero, the signals increase and decrease; however, it is difficult to infer that the networks are communicating. They may just be beating against each other. Reproduced with permission from Figure 1 of Handwerker and coworkers (2012).
FIG. 5.
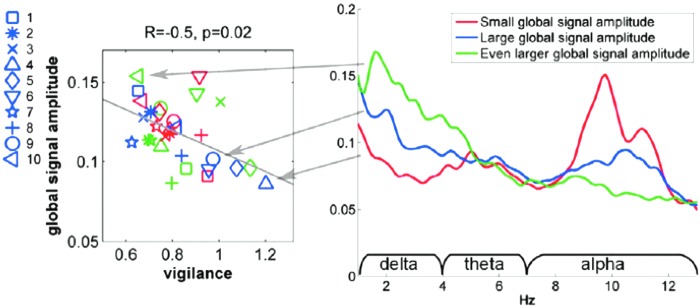
Global signal versus electroencephalography-related vigilance measures showing a clear relationship between global signal amplitude and vigilance. Reproduced with permission from Figure 5 of Wong and associates (2013).
Related to SNR, a tremendous amount of effort has been applied to clean up the time series noise. A goal—that the only variations in the fMRI time series signal are those of neuronally related fluctuations—remains highly elusive yet fundamentally important. This group has achieved a high level of success using a multiecho ICA-based approach (Kundu et al., 2012, 2013). The authors determine if each ICA component is BOLD like by how well it fits a predicted model of what the BOLD signal should be based on the time series signal echo time (TE) dependence. BOLD signal fluctuations show a linear increase with echo time, while non-BOLD fluctuations do not typically show any systematic TE dependence. Using this approach, the authors have been able to create clear maps of resting-state-based functional organization as well as time series movies clearly showing that spontaneous networks arise and wane away in time. They have also been able to create clear resting-state-based functional segregation maps from averaging only a handful of subjects. The authors are hopeful that this approach coupled with methods that eliminate BOLD-like time series artifactual signal changes, such as breathing-related changes, may pave the way for a truly noiseless resting-state time series.
Novel Contrast
Throughout the years, pulse sequences have been introduced to increase specificity to neuronal activity or at least to specific hemodynamics (Boyacioglu et al., 2014; Koopmans et al., 2012; Norris, 2012). Spin-echo sequences may be more sensitive to small compartments but are still sensitive to intravascular signals, such that the large cost in sensitivity does not outweigh any increase in specificity—except perhaps at fields of 7 Tesla or higher, where the intravascular signal is minimal. Another potential advantage of spin-echo imaging is reduced signal dropout as well as slightly reduced pulsatile noise due to the fact that the inversion pulse does not excite rapidly flowing or pulsatile spins after the 90 degree excitation.
Diffusion-weighted fMRI is a means by which intravascular signal is removed with a small amount of diffusion weighting (otherwise known as “velocity nulling”) (Boxerman et al., 1995; Song, 2012; Song et al., 1996). In theory, performing velocity nulling in conjunction with spin-echo sequences should be ideal for selecting only capillaries at lower fields than 7T. However, no successful applications have been reported as the sensitivity is likely below threshold due to both the spin-echo acquisition and the application of diffusion weighting. Perhaps, it is worth trying again at 3T with the latest in radio-frequency coil technology.
With regard to tissue or vessel selectivity, sequences such as VASO (Lu et al., 2003) as well as simultaneous flow-, BOLD-, and volume (Yang et al., 2004)-sensitive sequences have been able to selectively null or tag specific tissue such as venous blood, arterial blood, and inflowing blood. This simultaneous information, along with an activation paradigm involving interleaved global flow challenges and brain stimulation, offers a high degree of flexibility and power to detect, quantify, and separate specific hemodynamic and neuronal changes during activation. If not quantification, these approaches may at least allow improved vascular selectivity, thus increasing the functional, spatial, and temporal resolution, as well as interpretability since the preferentially selected capillary distribution is more homogeneously distributed on the scale of voxels than larger vessels.
Other potential sources of novel nonhemodynamic information reside in (1) neuronal activation-related cell swelling—perhaps detected by diffusion weighting (Kohno et al., 2009; Le Bihan et al., 2006)—although highly controversial (Miller et al., 2007), (2) microscopic magnetic field changes (Petridou et al., 2001, 2006), and (3) temperature changes (Yablonskiy et al., 2000). fMRI sequences tuned to each of these effects have shown suggestive preliminary findings. It will likely be a long time before sensitivity and specificity are improved such that these novel contrasts compete with BOLD contrast—if they do at all.
Multimodal Synergy
The collection of complementary information for fMRI is almost always useful as the other measures have either a higher temporal resolution or more specificity to neuronal activity or information about subject performance or behavior to help guide the analysis (Mantini et al., 2007; Shibasaki, 2008; Sui et al., 2012). Multimodal measures include electroencephalography (EEG) (Reed et al., 2004), magnetoencephalography (MEG) (Brookes et al., 2011), implanted electrodes, electrocorticography (Ojemann et al., 2013; Siero et al., 2013), optical imaging (Huppert et al., 2006; Strangman et al., 2002), behavioral measures, and other MRI measures that may perhaps be more sensitive to a specific aspect of hemodynamics (e.g., perfusion) but too low in sensitivity to be used in itself.
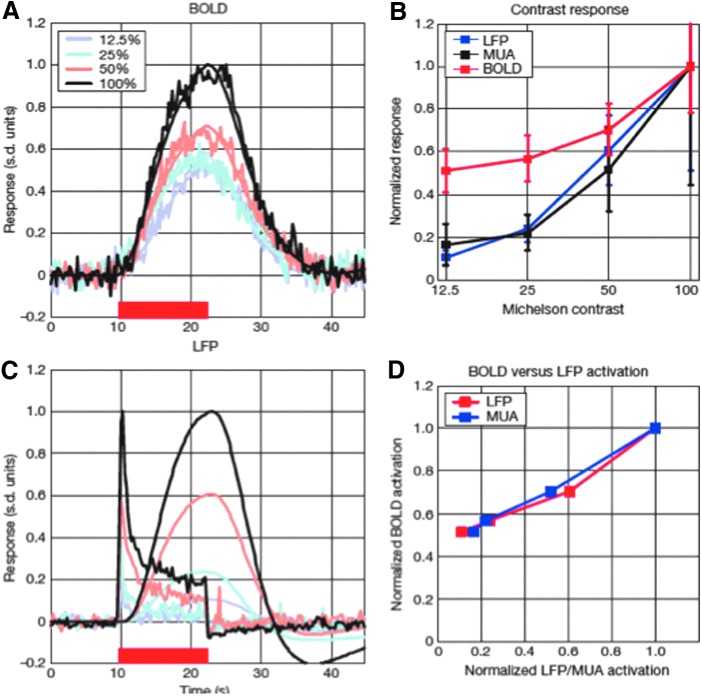
Neuronal correlates of the resting-state and BOLD signal have been uncovered using EEG and MEG, suggesting that a wide range of frequencies drive much of resting-state signal (Shmuel and Leopold, 2008). Multiunit arrays have shed light on the specific neuronal underpinnings of fMRI, showing that the signal, within a specific range, is linearly proportional to neuronal activity, but with very brief or low activity, the hemodynamic response apparently over-represents the neuronal activity (Logothetis et al., 2001). This is shown in Figure 6.
FIG. 6.
BOLD responses to stimuli with four different contrasts. On left (A and C) is the raw signal and on the right (B and D) are the comparisons. Note that in (D), the relationship between the electrophysiologic measures and BOLD is linear, yet, clearly, to reach the zero point, the relationship needs to change, suggesting a high level of nonlinearity at low levels of neuronal activity. Reproduced with permission from Figure 5 of Logothetis and colleagues (2001).
Optical imaging results have shown at extremely high resolution detailed spatial representation specific hemodynamics, motivating and supporting many of the currently ongoing high-resolution fMRI studies (Boas et al., 2001, 2004, 2014; Huppert et al., 2006; Strangman et al., 2002).
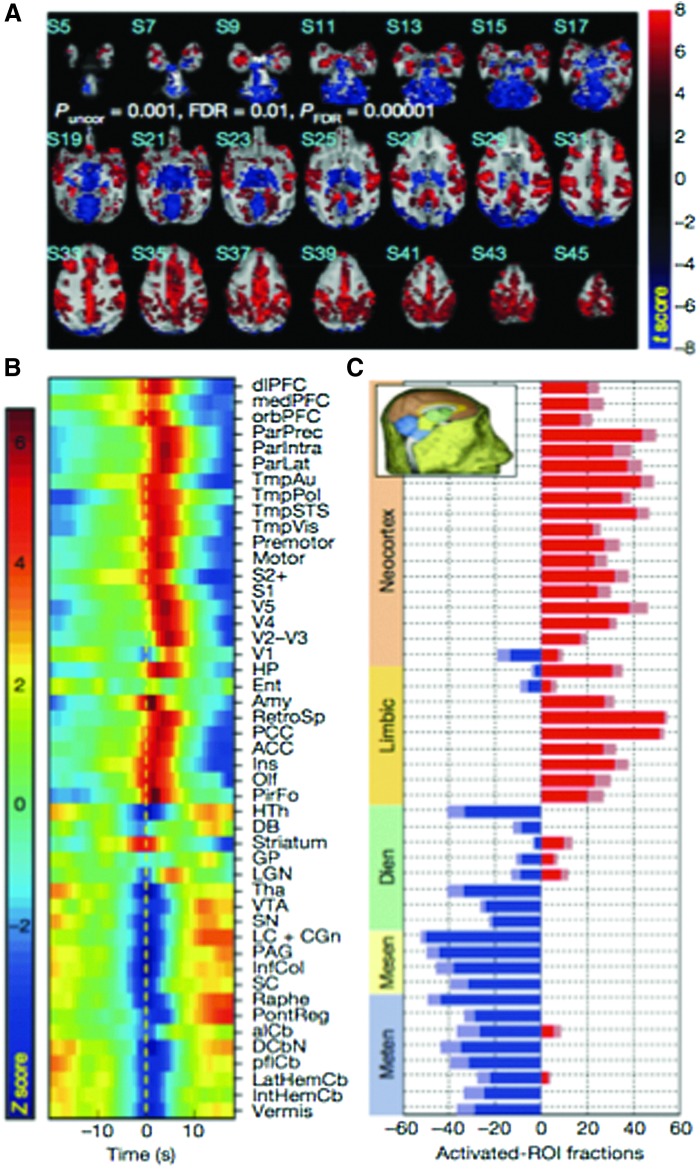
A recent study that beautifully demonstrates the synergy of multimodal imaging and the ability for it to reveal something that the two individual modalities could not, involved the use of electrodes embedded in nonhuman primate hippocampus (Logothetis et al., 2012). The results of this study are shown in Figure 7. During sleep, a specific pattern of transient activity—known as hippocampal ripples—would spontaneously appear. Simultaneous measures of fMRI, binned by the instances of these ripples, revealed quite clearly that these ripples were positively correlated with most of the cortex, and yet they were negatively correlated with most of the subcortical structure activity.
FIG. 7.
Activation maps and relative activations from cortical and subcortical regions that correspond to transient hippocampal ripples during sleep. (A) Shows fMRI activation maps of regions that were both positively and negatively correlated with the hippocampal ripples. (B) Shows the statistical significance and relative phase of the maximum fMRI correlation across multiple regions. (C) Shows the magnitude and sign of the correlation with the hippocampal ripples broken down by hierarchy of the structure. Reproduced with permission from Figure 3 of Logothetis and coworkers (2012).
Multivoxel Patterns
In the very early 2000s, Haxby and coworkers (2001) did something unique. They kept looking at what appeared to be a null result and, in the process, started an entirely new subfield of fMRI. They had been studying object processing, looking at activation of the face area versus house area. It appears that processing faces is so important of an area to humans that it takes up a large swath of cortex, resulting in a clear blob of activation. Most of fMRI up to this point involved happily, describing the center of mass coordinates of blobs of activation. The field of fMRI has been quite fortunate that most activated regions were somewhat blobby and large, lending themselves to easy averaging across subjects and visualization with glass brains. The macroscopic system-level organization of the brain was being fully studied and mapped, until Haxby et al. tried looking at objects with less evolutionary importance such as scissors versus shoes. On presenting these stimuli and analyzing the results, no clear blob—even a small one—distinguished itself. Instead, what they saw was a salt and pepper pattern of activation that was, to the eye, not really different between the two less important or less clearly categorized stimuli. So, instead of moving on to something else, they calculated the activation maps for half of each data set and calculated the spatial correlation between the two scissors maps and the two shoe maps and then between the scissors map and the shoe map and so on. Their result was that even though the patterns looked like noise to the eye accustomed to seeing only clear blobs, the patterns of activation clearly revealed themselves to be unique to the stimuli—showing a much higher spatial correlation with the other half of the same data set than with another data set having different stimuli. This study was likely the first to apply a rudimentary machine learning approach on a voxelwise basis to pull out the unique voxelwise pattern of activation associated with these more subtle stimuli.
Many others have since picked up on this. Kriegeskorte and coworkers (2006) developed a searchlight-based pattern effect mapping. Kamitani and Tong (2005) applied this to discerning which angle of grating a subject was viewing—even without their conscious knowledge. Advancements have included multivariate analysis to push the temporal and spatial resolution of fMRI to sub-TR and subvoxel resolutions.
The discovery that fMRI can detect and map brain activity that is both subvoxel and uniquely distributed across many voxels is a leap of insight not only regarding what fMRI can detect but also regarding how the brain is organized. New paradigms comparing the similarity of stimuli or tasks with the similarity of patterns of activity revealed a close connection between the two. Such analysis did not limit itself to voxels. Pattern similarity mapping has been carried out in the visual cortex of nonhuman primates and compared directly with fMRI patterns in humans (Kriegeskorte et al., 2006). Such an avenue of investigation is literally wide open for new paradigms and new insights into similarities and differences across individuals rather than group averages. In fact, this approach defies spatial averaging of group maps as this fine-grained pattern effect would be washed away.
Further advances in brain reading, decoding, and brain encoding have been carried out, strongly suggesting that information—including object, word, sound, semantic content, and more—is distributed much more widely and finely than most realized. At this point in time, the field of fMRI decoding and encoding and multivariate assessments are full of opportunity as this approach allows us to look beyond blobs to more subtle patterns that may reflect activation of subvoxel-scale neuronal populations and even sub-TR activation timing (Misaki et al., 2013a, 2013b).
High Spatial Resolution
At high resolution, the hemodynamic response variability becomes perhaps less of a confound because of the increased ability for fMRI to look through the draining vein effects—as the draining veins are more clearly delineated. In addition, the SNR necessary for high resolution is most readily available at high field, so the reduced intravascular signal that also results at high field comes as an added benefit. This allows clear delineation of columns and layers of activity (Logothetis et al., 2002; Polimeni et al., 2010; Yacoub et al., 2007, 2008). With layer-specific delineation, comes the promise of disentangling input versus output neuronal activity as specific layers correspond to each. A goal of fMRI, which I think is approaching the realm of possibility, is the delineation of neuronal causality by layer specificity. This ability would open up a new fMRI domain that goes well beyond mapping macroscopic network connections. It would allow exploration of fine-scale interactions on the columnar and layer level—thus having the potential to map out human brain organization in a new and more information-rich manner.
Not only is neuronal information more richly extracted at high resolution, but layer-specific differences in hemodynamic effects have also been revealed. As an example, Goense and colleagues (2012) showed that layer dependence of blood volume and flow changes is differentially sensitive to neuronal activation and neuronal inhibition.
High resolution also represents a paradigm shift in that the research focus is on individual results rather than spatially averaged group results. Spatial variability increases with increased resolution. An analogy is that it is easy to average hands, but much more difficult to average fingerprints without losing all the information. It is easier to average large swaths of brain than average cortical columns. Either one can try to learn the pattern of organization at a finer scale using anatomical landmarks such as gyrification and myeloarchitecture—which is ultimately limited, or work toward understanding how to group and compare results in other ways that do not involve spatial averaging of the results.
Conclusions
In this review, my focus was on the current understanding of neuronal versus hemodynamic influences on the fMRI signal. The specific topics were the hemodynamic response, calibration and quantitation, resting-state fMRI, novel contrasts, multimodal synergy, multivoxel patterns, and high spatial resolution imaging. From these discussions—which by no means comprehensively summarize the currently massive amount of worldwide effort placed in this direction—it is hoped that the essence of most fMRI researchers grapple with every day is fully conveyed.
Working with fMRI inevitably involves gaining a deep appreciation for the unique advantages and limitations of the hemodynamic response. It is an extremely sensitive, powerful, and uniquely whole-brain measure of neuronal activity, but has a variability that is associated with a multiscale and complex vasculature that is subsequently chopped into 1- to 3-mm cubes. Over the years, the ability to extract neuronal information and more precisely interpret the fMRI signal has increased tremendously through the research described above, and by all measures, this progress continues.
Acknowledgment
This work was supported by the Division of Intramural Programs of National Institute of Mental Health (NIMH).
Author Disclosure Statement
No competing financial interests exist.
References
- Allen EA, Plis SM, Erhardt EB, Eichele T, Calhoun VD. 2014. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. 1993. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med 30:161–173 [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC. 1997. A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. NMR Biomed 10:197–203 [DOI] [PubMed] [Google Scholar]
- Bellgowan PSF, Saad ZS, Bandettini PA. 2003. Understanding neural system dynamics through task modulation and measurement of functional MRI amplitude, latency, and width. Proc Natl Acad Sci U S A 100:1415–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Bandettini PA. 2005. The effect of stimulus duty cycle and off duration on BOLD response linearity. Neuroimage 27:70–82 [DOI] [PubMed] [Google Scholar]
- Birn RM, Murphy K, Bandettini PA. 2008a. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum Brain Mapp 29:740–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Saad ZS, Bandettini PA. 2001. Spatial heterogeneity of the nonlinear dynamics in the FMRI BOLD response. Neuroimage 14:817–826 [DOI] [PubMed] [Google Scholar]
- Birn RM, Smith MA, Jones TB, Bandettini PA. 2008b. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage 40:644–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB. 2012. Resting state fMRI: a personal history. Neuroimage 62:938–944 [DOI] [PubMed] [Google Scholar]
- Biswal BF, Yetkin , Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Blockley NP, Griffeth VE, Simon AB, Buxton RB. 2013. A review of calibrated blood oxygenation level-dependent (BOLD) methods for the measurement of task-induced changes in brain oxygen metabolism. NMR Biomed 26:987–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas DA, Dale AM, Franceschini MA. 2004. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. Neuroimage 23(Suppl. 1):S275–S288 [DOI] [PubMed] [Google Scholar]
- Boas DA, Elwell CE, Ferrari M, Taga G. 2014. Twenty years of functional near-infrared spectroscopy: introduction for the special issue. Neuroimage 85:1–5 [DOI] [PubMed] [Google Scholar]
- Boas DA, Gaudette T, Strangman G, Cheng X, Marota JJA, Mandeville JB. 2001. The accuracy of near infrared spectroscopy and imaging during focal changes in cerebral hemodynamics. Neuroimage 13:76–90 [DOI] [PubMed] [Google Scholar]
- Boxerman JL, Bandettini PA, Kwong KK, Baker JR, Davis TL, Rosen BR, Weisskoff RM. 1995. The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo. Magn Reson Med 34:4–10 [DOI] [PubMed] [Google Scholar]
- Boyacioglu R, Schulz J, Muller NC, Koopmans PJ, Barth M, Norris DG. 2014. Whole brain, high resolution multiband spin-echo EPI fMRI at 7T: a comparison with gradient-echo EPI using a color-word Stroop task. Neuroimage 97:142–150 [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Hale JR, Zumer JM, Stevenson CM, Francis ST, Barnes GR, Owen JP, Morris PG, Nagarajan SS. 2011. Measuring functional connectivity using MEG: methodology and comparison with fcMRI. Neuroimage 56:1082–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulte DP, Kelly M, Germuska M, Xie J, Chappell MA, Okell TW, Bright MG, Jezzard P. 2012. Quantitative measurement of cerebral physiology using respiratory-calibrated MRI. Neuroimage 60:582–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB, Frank LR. 1997. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab 17:64–72 [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K. D.ubowitz DJ, Liu TT. 2004. Modeling the hemodynamic response to brain activation. Neuroimage 23Suppl 1:S220–S233 [DOI] [PubMed] [Google Scholar]
- Chang CG, Glover H. 2010. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50:81–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. 2001. Frequencies contributing to functional connectivity in the cerebral cortex in resting-state data. AJNR Am J Neuroradiol 22:1326–1333 [PMC free article] [PubMed] [Google Scholar]
- Craddock RC, James GA, Holtzheimer PE, 3rd, Hu XP, Mayberg HS. 2012. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp 33:1914–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. 1998. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A 95:1834–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano E, Goebel R. 2003. Tracking cognitive processes with functional MRI mental chronometry. Curr Opin Neurobiol 13:174–181 [DOI] [PubMed] [Google Scholar]
- Frahm J, Baudewig J, Kallenberg K, Kastrup A, Merboldt KD, Dechent P. 2008. The post-stimulation undershoot in BOLD fMRI of human brain is not caused by elevated cerebral blood volume. Neuroimage 40:473–481 [DOI] [PubMed] [Google Scholar]
- Goense J, Merkle H, Logothetis N. 2012. High-resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron 76:629–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Castillo J, Saad ZS, Handwerker DA, Inati SJ, Brenowitz N, Bandettini PA. 2012. Whole-brain, time-locked activation with simple tasks revealed using massive averaging and model-free analysis. Proc Natl Acad Sci U S A 109:5487–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K. 2006. Selectivity of adaptation in single units: implications for FMRI experiments. Neuron 49:170–171 [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. 2001. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol 107:293–321 [DOI] [PubMed] [Google Scholar]
- Haacke EM, Lai S, Reichenbach JR, Kuppusamy K, Hoogenraad FG, Takeichi H, Lin W. 1997. In vivo measurement of blood oxygen saturation using magnetic resonance imaging: a direct validation of the blood oxygen level-dependent concept in functional brain imaging. Hum Brain Mapp 5:341–346 [DOI] [PubMed] [Google Scholar]
- Handwerker DA, Roopchansingh V, Gonzalez-Castillo J, Bandettini PA. 2012. Periodic changes in fMRI connectivity. Neuroimage 63:1712–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. 2001. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science 293:2425–2430 [DOI] [PubMed] [Google Scholar]
- Henson RNA, Price CJ, Rugg MD, Turner R, Friston KJ. 2002. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage 15:83–97 [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. 1999. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci U S A 96:9403–9408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XP, Le TH, Ugurbil K. 1997. Evaluation of the early response in fMRI in individual subjects using short stimulus duration. Magn Reson Med 37:877–884 [DOI] [PubMed] [Google Scholar]
- Huppert TJ, Hoge RD, Diamond SG, Franceschini MA, Boas DA. 2006. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage 29:368–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M. Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C. 2013. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80:360–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochimsen TH, Ivanov D, Ott DV, Heinke W, Turner R, Moller HE, Reichenbach JR. 2010. Whole-brain mapping of venous vessel size in humans using the hypercapnia-induced BOLD effect. Neuroimage 51:765–774 [DOI] [PubMed] [Google Scholar]
- Kamitani Y, Tong F. 2005. Decoding the visual and subjective contents of the human brain. Nat Neurosci 8:679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno S, Sawamoto N, Urayama SI, Aso T, Aso K, Seiyama A, Fukuyama H, Le Bihan D. 2009. Water-diffusion slowdown in the human visual cortex on visual stimulation precedes vascular responses. J Cereb Blood Flow Metab 29:1197–1207 [DOI] [PubMed] [Google Scholar]
- Koopmans PJ, Boyacioglu R, Barth M, Norris DG. 2012. Whole brain, high resolution spin-echo resting state fMRI using PINS multiplexing at 7 T. Neuroimage 62:1939–1946 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Goebel R, Bandettini P. 2006. Information-based functional brain mapping. Proc Natl Acad Sci U S A 103:3863–3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Brenowitz ND, Voon V, Worbe Y, Vertes PE, Inati SJ, Saad ZS, Bandettini PA, Bullmore ET. 2013. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc Natl Acad Sci U S A 110:16187–16192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Inati SJ, Evans JW, Luh WM, Bandettini PA. 2012. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage 60:1759–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Urayama SI, Aso T, Hanakawa T, Fukuyama H. 2006. Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proc Natl Acad Sci U S A 103:8263–8268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Eschenko O, Murayama Y, Augath M, Steudel T, Evrard HC, Besserve M, Oeltermann A. 2012. Hippocampal-cortical interaction during periods of subcortical silence. Nature 491:547–553 [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Merkle H, Augath M, Trinath T, Ugurbil K. 2002. Ultra high-resolution fMRI in monkeys with implanted RF coils. Neuron 35:227–242 [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. 2001. Neurophysiological investigation of the basis of the fMRI signal. Nature 412:150–157 [DOI] [PubMed] [Google Scholar]
- Lu H, Ge Y. 2008. Quantitative evaluation of oxygenation in venous vessels using T2-relaxation-under-spin-tagging MRI. Magn Reson Med 60:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Golay X, Pekar JJ, Van Zijl PCM. 2003. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med 50:263–274 [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJA, Kosofsky BE, Keltner JR, Weissleder R, Rosen BR, Weisskoff RM. 1998. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med 39:615–624 [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. 2007. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A 104:13170–13175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon RS, Luknowsky DC, Gati JS. 1998. Mental chronometry using latency-resolved functional MRI. Proc Natl Acad Sci U S A 95:10902–10907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KL, Bulte DP, Devlin H, Robson MD, Wise RG, Woolrich MW, Jezzard P, Behrens TE. 2007. Evidence for a vascular contribution to diffusion FMRI at high b value. Proc Natl Acad Sci U S A 104:20967–20972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M, Luh WM, Bandettini PA. 2013a. Accurate decoding of sub-TR timing differences in stimulations of sub-voxel regions from multi-voxel response patterns. Neuroimage 66:623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M, Luh WM, Bandettini PA. 2013b. The effect of spatial smoothing on fMRI decoding of columnar-level organization with linear support vector machine. J Neurosci Methods 212:355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonen CT, van Zijl PC, Frank JA, Le Bihan D, Becker ED. 1990. Functional magnetic resonance imaging in medicine and physiology. Science 250:53–61 [DOI] [PubMed] [Google Scholar]
- Mullinger KJ, Mayhew SD, Bagshaw AP, Bowtell R, Francis ST. 2013. Poststimulus undershoots in cerebral blood flow and BOLD fMRI responses are modulated by poststimulus neuronal activity. Proc Natl Acad Sci U S A 110:13636–13641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullinger KJ, Mayhew SD, Bagshaw AP, Bowtell R, Francis ST. 2014. Evidence that the negative BOLD response is neuronal in origin: a simultaneous EEG-BOLD-CBF study in humans. Neuroimage 94:263–274 [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. 2009. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44:893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazy RK, Xie J, Miller K, Beckmann CF, Smith SM. 2011. Spectral characteristics of resting state networks. Prog Brain Res 193:259–276 [DOI] [PubMed] [Google Scholar]
- Norris DG. 2012. Spin-echo fMRI: The poor relation? Neuroimage 62:1109–1115 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. 1990. Brain magnetic-resonance-imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 87:9868–9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA, Ramsey NF, Ojemann J. 2013. Relation between functional magnetic resonance imaging (fMRI) and single neuron, local field potential (LFP) and electrocorticography (ECoG) activity in human cortex. Front Hum Neurosci 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridou N, Bodurka J, Loew M, Bandettini PA. 2001. Neuronal current imaging using MRI: a feasibility study. Proceedings of SPIE—The International Society for Optical Engineering San Diego [Google Scholar]
- Petridou N, Plenz D, Silva AC, Loew M, Bodurka J, Bandettini PA. 2006. Direct magnetic resonance detection of neuronal electrical activity. Proc Natl Acad Sci U S A 103:16015–16020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni JR, Fischl B, Greve DN, Wald LL. 2010. Laminar analysis of 7T BOLD using an imposed spatial activation pattern in human V1. Neuroimage 52:1334–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser BA, van Mierlo E, Norris DG. 2011. Exploring the post-stimulus undershoot with spin-echo fMRI: implications for models of neurovascular response. Hum Brain Mapp 32:141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CL, Shoham S, Halgren E. 2004. Neural substrates of tactile object recognition: an fMRI study. Hum Brain Mapp 21:236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H. 2008. Human brain mapping: hemodynamic response and electrophysiology. Clin Neurophysiol 119:731–743 [DOI] [PubMed] [Google Scholar]
- Shmuel AD, Leopold A. 2008. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum Brain Mapp 29:751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siero JCW, Hermes D, Hoogduin H, Luijten PR, Petridou N, Ramsey NF. 2013. BOLD consistently matches electrophysiology in human sensorimotor cortex at increasing movement rates: a combined 7T fMRI and ECoG study on neurovascular coupling. J Cereb Blood Flow Metab 33:1448–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AW. 2012. Diffusion modulation of the fMRI signal: early investigations on the origin of the BOLD signal. Neuroimage 62:949–952 [DOI] [PubMed] [Google Scholar]
- Song AW, Wong EC, Tan SG, Hyde JS. 1996. Diffusion weighted fMRI at 1.5 T. Magn Reson Med 35:155–158 [DOI] [PubMed] [Google Scholar]
- Strangman G, Culver JP, Thompson JH, Boas DA. 2002. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 17:719–731 [PubMed] [Google Scholar]
- Sui J, Adali T, Yu Q, Chen J, Calhoun VD. 2012. A review of multivariate methods for multimodal fusion of brain imaging data. J Neurosci Methods 204:68–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uludag K. 2008. Transient and sustained BOLD responses to sustained visual stimulation. Magn Reson Imaging 26:863–869 [DOI] [PubMed] [Google Scholar]
- van Zijl PCM, Eleff SM, Ulatowski JA, Oja JME, Ulug AM, Traystman RJ, Kauppinen RA. 1998. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med 4:159–167 [DOI] [PubMed] [Google Scholar]
- Wong CW, Olafsson V, Tal O, Liu TT. 2013. The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage 83:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EC. 2013. New developments in arterial spin labeling pulse sequences. NMR Biomed 26:887–891 [DOI] [PubMed] [Google Scholar]
- Yablonskiy DA, Ackerman JH, Raichle ME. 2000. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation (vol 98, pg 7603, 2000). Proc Natl Acad Sci U S A 97:9819–9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Harel N, Ugurbil K. 2008. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci U S A 105:10607–10612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Logothetis N, Ugurbil K. 2007. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. Neuroimage 37:1161–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Ugurbil K, Harel N. 2006. The spatial dependence of the poststimulus undershoot as revealed by high-resolution BOLD- and CBV-weighted fMRI. J Cereb Blood Flow Metab 26:634–644 [DOI] [PubMed] [Google Scholar]
- Yang S, Yang Z, Fischer K, Zhong K, Stadler J, Godenschweger F, Steiner J, Heinze HJ, Bernstein HG, Bogerts B, Mawrin C, Reutens DC, Speck O, Walter M. 2013. Integration of ultra-high field MRI and histology for connectome based research of brain disorders. Front Neuroanat 7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gu H, Stein EA. 2004. Simultaneous MRI acquisition of blood volume, blood flow, and blood oxygenation information during brain activation. Magn Reson Med 52:1407–1417 [DOI] [PubMed] [Google Scholar]