Abstract
The aphorism was to develop new chemical entities as potential anticancer, anti-inflammatory, and analgesic agents. The Leuckart synthetic pathway was utilized in development of novel series of 2-(substituted phenoxy)-N-(1-phenylethyl)acetamide derivatives. The compounds containing 1-phenylethylamine as basic moiety attached to substituted phenols were assessed for their anticancer activity against MCF-7 (breast cancer), SK-N-SH (neuroblastoma), anti-inflammatory activity, and analgesic activity. These investigations revealed that synthesized products 3a–j with halogens on the aromatic ring favors as the anticancer and anti-inflammatory activity. Among all, compound 3c N-(1-(4-chlorophenyl)ethyl)-2-(4-nitrophenoxy)acetamide exhibited anticancer, anti-inflammatory, and analgesic activities. In conclusion, 3c may have potential to be developed into a therapeutic agent.
1. Introduction
In the past decade, numerous advances have taken place in the understanding of pathogenesis of cancer and its relationship with inflammation. To date the research work on this ground is substantial, but it still lacks clinical accomplishment, with the existing development facade over the clinical exploitation of drugs with dual acting cyclooxygenase-2 (COX-2) inhibition and antiproliferative potency [1]. Whenever cells in the body are allowed to divide uncontrollably and to metastasize, this results in the formation of cancer. Large scale of studies indicates that overexpression of COX-2 strongly appeared in human breast carcinomas approximately 40% and in colorectal carcinomas approximately 60%. In particular, COX-2 is found during overexpression of human epidermal growth factor receptor 2 (HER2/neu). The amplification of this oncogene plays an important role in the development of some aggressive types of breast cancer. COX-2 is also been reported in early tumors by stromal cells [2] and in larger tumors by dysplastic epithelium [3]. It has been proved that various inflammatory cells, cytokines, chemokines, and enzymes facilitate the development of cancers from inflammation [4]. A few COX-2/LOX-2 (lipooxygenase-2) inhibitors such as sulindac, celecoxib, licofelone, and aspirin analogues have also been reported as suppressors of malignant growth of cells in vitro and in vivo [5] and Alzheimer's disease [6]. This designates that there is an unfamiliar association involving cancer and inflammation [7].
Nowadays no data are available concerning the potential use of anticancer agents as COX/LOX inhibitors. Recent molecular targets for the treatment of cancer are the relation between arachidonic acid (AA) and carcinogenesis because of the regulation of AA by two enzymes, cyclooxygenases (COXs) and lipoxygenases (LOXs). Prostaglandin E2 (PGE2), the main product of COX-2, is found in high concentration in tumor cells [8] and is synthesized by various human breast cancer cell lines. The goal of this research was to achieve a novel series of agents that could have potent anticancer efficacy in association with the suppression of COX/LOX pathways.
In the present paper a novel and efficient strategy has been developed to synthesize N-(1-(4-chlorophenyl) ethyl)-2-(substituted phenoxy)acetamide derivatives and 2-(substituted phenoxy)-N-(1-(p-tolyl)ethyl)acetamide derivatives with excellent yields. This synthetic pathway was established by the Leuckart reaction. The Leuckart reaction is a process for the reductive amination of aldehydes and ketones by formamide, ammonium formate, or formic acid with formamide [9]. This reaction has been used for the aromatic compounds, but very little work has been reported for the synthesis of aliphatic compounds (synthesis of 2-heptylamine, propylamine, and isopropylamine) [10]. Apart from the aliphatic compounds, 1,4-benzodiazepines were synthesized by the Leuckart Wallach reaction containing the benzodiazepine scaffold and were originated with various biological activities (benzodiazepine family) comprised mainly of central nervous system (CNS) suppressant due to its anxiolytic, anticonvulsant, sedative, and muscle relaxant activities. It is used in various marketed drugs such as alprazolam, bromazepam, chlorazepate, and valium [11]. 1,4-Benzodiazepines also demonstrate therapeutic activities and are used as antibiotics [12], antiulcers [13], and anti-HIV agents [14]. They are also used as farnesyltransferase inhibitors [15]. Compounds with 2-phenoxy-N-phenylacetamide core nucleus as in Figure 1 (1a and 1b) have shown remarkable research and demonstrated a variety of biological activities such as antimycobacterial [16], antiparasitic [17], antiviral [18], and anticancer [19]activities [20–23]. These compounds are also reported with inhibition of Pgp efflux transporters which are beneficial in the treatment of multidrug resistant strains of cancer cells. The phenoxy-N-phenylacetamide compounds are reported to be less toxic and more effective as that of potent P-gp inhibitors (coumarin analogues, 2-adamantyl analogues); see Figure 1 (1c) [24]. Now, we are reporting a new series of agents that have potent anticancer activities. These agents possibly may act upon COX/LOX pathways which may be included as a part of our future research program.
Figure 1.
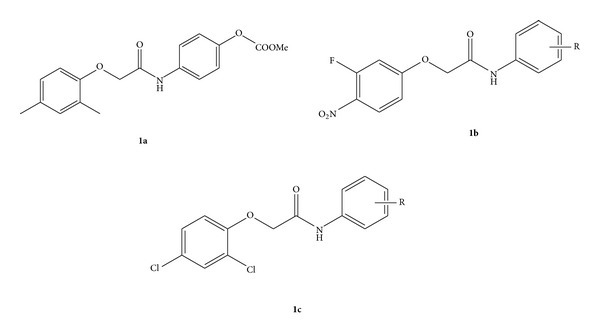
The commonly used structures (1a, 1b 2-Phenoxy-N-phenylacetamide core nucleus with antimycobacterial activity) (1c phenoxy-N-phenylacetamide compounds with potent P-gp inhibitor activities).
These compounds were further biologically evaluated for analgesic activity, and few of them showed activity. For the development of novel anticancer agents with lower toxic effect and higher efficiency, we carried out high throughput screening, which resulted in the discovery of titled compounds. Now, we report structure activity relationships and biological assessment of the titled compounds.
2. Experiment
2.1. General Considerations
All research chemicals were purchased from CDH (Central Drug House P. Ltd., New Delhi, India) and used as such for the reactions. These were used without further purification. Purification of the synthesized compounds was carried out by the recrystallization with appropriate solvent in case of solids but by distillation in case of liquids. Purity of the compounds and completion of reactions were monitored by thin layer chromatography (TLC) using silica gel G as the stationary phase and mobile phases used were n-hexane : ethyl acetate (1 : 1). Spots were visualized by exposure to iodine vapour. Melting points were determined in open capillaries on Thomas Hoover apparatus and are uncorrected. IR spectra were recorded on a Shimadzu IR-435 spectrophotometer using KBr pellets and 1H-NMR spectra were recorded on a Bruker 400 MHz spectrometer (Bruker Corporation, Massachusetts, USA) instrument using tetramethylsilane (TMS) as an internal standard and DMSO-d6 as a solvent. Mass spectra were recorded on Micromass Q-Tof Micro (Waters Corporation Massachusetts, USA). Chemical shifts are given in parts per million (ppm). The anticancer activity was carried out at Tata Memorial Hospital, Mumbai. The anti-inflammatory and analgesic screenings are carried out at pharmacology laboratory of College of Pharmacy IFTM University, Moradabad. The anti-inflammatory activity was carried out using digital plethysmometer (Orchid Scientific, Maharashtra, India). The analgesic activity was carried out by Eddy hot plate method using analgesiometer (Sanghmeshwar International, Ambala, India). All the animal experiments were approved by Institutional Animal Ethical Committee (IAEC).
2.2. Synthesis
2.2.1. General Method for the Synthesis of 1-(4-Chlorophenyl)ethanamine (1a) and 1-(p-Tolyl)ethanamine (1b) from 1-(4-Chlorophenyl)ethanone and 1-(p-Tolyl)ethanone, Respectively
This procedure of the Leuckart reaction was used for the preparation of amines from ketones. Ammonium carbonate (215 gm, 4 mols) was placed in a 1 litre three-necked round bottom flask, which was fitted with a thermometer, a dropping funnel, and a bent tube attached for distillation to a short condenser. Formic acid (98%, 109 mL) was taken in the dropping funnel and added dropwise. When the reaction subsided, the mixture was heated slowly until the temperature of the reaction increased to about 165°C. The ketone (1 mol) was added in one lot and the temperature was slowly raised to 180–185°C. Ammonia, water, carbon dioxide, and some of the unreacted ketones distilled over. The distilled ketone was separated and returned to the reaction mixture. The mixture which gradually became homogenous was maintained at 180–185°C for 4-5 hours. When the reaction was complete, the mixture was cooled and stirred thoroughly with twice its volume of water. The aqueous layer was separated and the formyl derivative of the amine (nonaqueous layer) so obtained was refluxed with 100–150 mL of concentrated hydrochloric acid for 2-3 hours. After the hydrolysis, the reaction mixture was cooled and extracted with ether to remove any unreacted ketone. The aqueous solution was made strongly alkaline with 30% sodium hydroxide solution and the separated amine was extracted with ether. The ethereal extract was dried over anhydrous sodium sulphate, and after removal of the solvent, the product distilled under reduced pressure.
2.2.2. General Method for the Synthesis of 2-Chloro-N-(1-(4-chlorophenyl)ethyl)acetamide (2a) and 2-Chloro-N-(1-(p-tolyl)ethyl)acetamide (2b) from (1a) and (1b), Respectively
An ice-cooled aqueous solution of sodium hydroxide (50 mL, 10%) was taken in two different well-corked conical flasks; then, 0.1 mol of synthesized compounds 1a and 1b was added in both the flasks very slowly followed by addition of chloroacetyl chloride (11.93 mL, 0.15 mol) dropwise with constant stirring and shaking. The reaction was strongly shaken until odor of chloroacetyl chloride moved out. The pH of reaction mixture was kept around 9-10 by the addition of sodium hydroxide solution. Filter off the product and solid amides 2a and 2b that formed were washed thoroughly with water, dried, and recrystallized from ethanol.
2.2.3. General Method for the Synthesis of N-(1-(4-Chlorophenyl)ethyl)-2-(substituted phenoxy)acetamide (3a–e) and 2-(Substituted phenoxy)-N-(1-(p-tolyl)ethyl)acetamide (3f–j) Derivatives from (2a) and (2b), Respectively
Phenoxy acetamide derivatives were prepared by reacting (2a-2b) (0.01 mol) with different substituted phenols (0.01 mol) in presence of anhydrous potassium carbonate (0.01 mol) and catalytic amount of potassium iodide in refluxing dry acetone. In some cases unreacted phenol was removed from the final product by treating the substance with 10% w/v sodium carbonate solution in water. The compound was then filtered and washed thoroughly with water and recrystallised from appropriate solvent. The completion of the reaction was monitored by TLC.
(1) N-(1-(4-Chlorophenyl)ethyl)-2-phenoxyacetamide ( 3a ). IR (KBr, cm−1). 3205, 3112, 3014, 2928, 1711, 1644, 1318, 1235. 1H NMR (400 MHz, DMSO-d6, δ ppm): 8.15 (s, 1H, NH), 7.52–6.90 (m, 4H, H-2′, H-3′, H-5′, H-6′), 6.81 (t, 2H, H-3′′, H-5′′), 6.20–5–93 (m, 3H, H-2′′, H-4′′, H-6′′), 5.80–5.47 (m, 1H, CH-3), 5.21 (s, 2H, CH2-2), 1.74 (d, 3H, CH3-4). 13C NMR (DMSO-d6, δ ppm): 166.4 (C=O, NHCO), 159.8 (C, C-1′′), 141.7 (C, C-1′), 135.4 (C, C-4′), 130.1 (CH, C-3′′, 5′′), 125.1 (CH, C-3′, 5′), 124.4 (CH, C-2′, 6′), 120.8 (CH, C-4′′), 116.1 (CH, C-2′′, 6′′), 68.4 (CH2, C-2), 55.9 (CH, C-3), 25.9 (CH3, C-4). mass: m/z 289 (M+), 290 (M+1, 17.5%), 291 (M+2, 33.4%). Anal. Calc. For C16H16ClNO2: C 66.32, H 5.57, N 4.83. Found: C 66.28, H 5.53, N 4.85.
(2) 2-(4-Bromophenoxy)-N-(1-(4-chlorophenyl)ethyl)acetamide ( 3b ). IR (KBr, cm−1): 3245, 3056, 3017, 2915, 1678, 1622, 1335, 1105. 1H NMR (400 MHz, DMSO-d6, δ ppm): 8.25 (s, 1H, NH), 7.81–7.12 (m, 6H, H-2′, H-3′, H-5′, H-6′, H-3′′, H-5′′), 6.80 (d, 2H, H-2′′, H-6′′), 6.31–6.24 (m, 1H, CH-3), 5.34 (s, 2H, CH2-2), 1.63 (d, 3H, CH3-4). 13C NMR (DMSO-d6, δ ppm): 170.1 (C=O, NHCO), 162.5 (C, C-1′′), 142.4 (C, C-1′), 139.8 (C, C-4′), 138.7 (CH, C-3′′, 5′′), 127.2 (CH, C-3′, 5′), 123.8 (CH, C-2′, 6′), 120.8 (CH, C-2′′, 6′′), 111.9 (C, C-4′′), 66.7 (CH2, C-2), 50.9 (CH, C-3), 23.8 (CH3, C-4). mass: m/z 369 (M+), 367 (M-2, 76.5%), 371 (M+2, 25.3%). Anal. Calc. For C16H15BrClNO2: C 52.13, H 4.10, N 3.80. Found: C 52.09, H 4.13, N 3.77.
(3) N-(1-(4-Chlorophenyl)ethyl)-2-(4-nitrophenoxy)acetamide ( 3c ). IR (KBr, cm−1): 3255, 3218, 3122, 2817, 1686, 1533, 1528, 1213, 1110. 1H NMR (400 MHz, DMSO-d6, δ ppm): 8.25 (d, 2H, H-3′′, H-5′′), 7.92 (s, 1H, NH), 7.81–7.35 (m, 4H, H-2′, H-3′, H-5′, H-6′), 7.17 (d, 2H, H-2′′, H-6′′), 5.78–5.31 (m, 1H, CH-3), 4.92 (s, 2H, CH2-2), 2.11 (d, 3H, CH3-4). 13C NMR (DMSO-d6, δ ppm): 170.5 (C=O, NHCO), 163.7 (C, C-1′′), 148.4 (C, C-4′′), 145.5 (C, C-1′), 139.1 (C, C-4′), 132.4 (CH, C-3′, 5′), 130.4 (CH, C-2′, 6′), 129.7 (CH, C-3′′, 5′′), 120.7 (CH, C-2′′, 6′′), 71.4 (CH2, C-2), 69.1 (CH, C-3), 35.4 (CH3, C-4). mass: m/z 334 (M+), 335 (M+1, 18.7%), 336 (M+2, 34.1%). Anal. Cacl. For C16H15ClN2O4: C 57.41, H 4.52, N 8.37. Found: C 57.45, H 4.48, N 8.32.
(4) 2-(4-(Tert-butyl)phenoxy)-N-(1-(4-chlorophenyl)ethyl)acetamide ( 3d ). IR (KBr, cm−1): 3217, 3181, 2973, 2810, 1688, 1611, 1290, 1176. 1H NMR (400 MHz, DMSO-d6, δ ppm): 8.22 (s, 1H, NH), 8.10–7.37 (m, 6H, H-2′, H-3′, H-5′, H-6′, H-3′′, H-5′′), 7.21 (d, 2H, H-2′′, H-6′′), 5.20–5.13 (m, 1H, CH-3), 4.94 (s, 2H, CH2-2), 3.11 (d, 3H, CH3-4), 2.83 (s, 9H, (CH3)3). 13C NMR (DMSO-d6, δ ppm): 169.8 (C=O, NHCO), 159.4 (C, C-1′′), 151.8 (C, C-4′′), 137.8 (C, C-1′), 131.8 (C, C-4′), 130.4 (CH, C-3′, 5′), 128.1 (CH, C-2′, 6′), 123.4 (CH, C-3′′, 5′′), 110.4 (CH, C-2′′, 6′′), 64.5 (CH2, C-2), 61.8 (CH, C-3), 41.4 (C, C-(CH3)3), 40.9 ((CH3)3), 19.7 (CH3, C-4). mass: m/z 345 (M+), 346 (M+1, 22.3%), 347 (M+2, 32.4%). Anal. Calc. For C20H24ClNO2: C 69.45, H 6.99, N 4.05. Found: C 69.41, H 6.94, N 4.10.
(5) N-(1-(4-Chlorophenyl)ethyl)-2-(4-methoxyphenoxy)acetamide ( 3e ). IR (KBr, cm−1): 3216, 3122, 2988, 2917, 2713, 1677, 1609, 1200, 1174. 1H NMR (400 MHz, DMSO-d6, δ ppm): 8.67 (s, 1H, NH), 7.94–7.36 (m, 4H, H-2′, H-3′, H-5′, H-6′), 7.20 (s, 4H, H-2′′, H-3′′, H-5′′, H-6′′), 5.43–5.21 (m, 1H, CH-3), 5.11 (s, 2H, CH2-2), 4.64 (s, 3H, OCH3), 2.36 (d, 3H, CH3-4). 13C NMR (DMSO-d6, δ ppm): 172.9 (C=O, NHCO), 154.2 (C, C-4′′), 152.8 (C, C-1′′), 139.8 (C, C-1′), 137.2 (C, C-4′), 131.2 (CH, C-3′, 5′), 130.1 (CH, C-2′, 6′), 121.1 (CH, 2′′, 3′′, 5′′, 6′′), 73.3 (CH2, C-2), 61.4 (CH3, OCH3), 60.2 (CH, C-3), 30.1 (CH3, C-4). mass: m/z 319 (M+), 320 (M+1, 19.6%), 321 (M+2, 34.1%). Anal. Calc. For: C17H18ClNO3: C 63.85, H 5.67, N 4.38. Found: C 63.81, H 5.70, N 4.32.
(6) 2-Phenoxy-N-(1-(p-tolyl)ethyl)acetamide ( 3f ). IR (KBr, cm−1): 3266, 3033, 2895, 1654, 1622, 1278, 1112.1H NMR (400 MHz, DMSO-d6, δ ppm): 8.32 (s, 1H, NH), 8.11 (t, 2H, H-3′′, H-5′′), 7.46–7.31 (m, 4H, H-2′, H-3′, H-5′, H-6′), 7.12–6.93 (m, 3H, H-2′′, H-4′′, H-6′′), 6.81-6.78 (m, 1H, CH-3), 6.61 (s, 2H, CH2-2), 3.36 (s, 3H, CH3-4′), 2.45 (d, 3H, CH3-4). 13C NMR (DMSO-d6, δ ppm): 171.8 (C=O, NHCO), 167.4 (C, C-1′′), 151.2 (C, C-1′), 150.1 (C, C-4′), 132.4 (CH, C-3′′, 5′′), 130.6 (CH, C-3′, 5′), 129.8 (CH, C-2′, 6′), 127.1 (CH, C-4′′), 122.7 (CH, C-2′′, 6′′), 66.8 (CH2, C-2), 57.1 (CH, C-3), 20.9 (CH3, C-4), 18.7 (CH3, CH3-C4′). mass: m/z 269 (M+), 270 (M+1, 19.6%), 271 (M+2, 2.7%). Anal. Calc. For C17H19NO2: C 75.81, H 7.11, N 5.20. Found: C 75.84, H 7.05, N 5.24.
(7) 2-(4-Bromophenoxy)-N-(1-(p-tolyl)ethyl)acetamide ( 3g ). IR (KBr, cm−1): 3256, 3078, 2895, 1674, 1556, 1279, 1090. 1H NMR (400 MHz, DMSO-d6, δ ppm): 8.45 (s, 1H, NH), 8.21 (d, 2H, H-3′′, H-5′′), 7.81–7.73 (m, 4H, H-2′, H-3′, H-5′, H-6′), 7.47 (d, 2H, H-2′′, H-6′′), 5.61–5.53 (m, 1H, CH-3), 5.44 (s, 2H, CH2-2), 4.13 (s, 3H, CH3-4′), 2.78 (d, 3H, CH3-4). 13C NMR (DMSO-d6, δ ppm): 169.2 (C=O, NHCO), 161.8 (C, C-1′′), 142.9 (C, C-1′), 141.4 (C, C-4′), 139.9 (CH, C-3′′, 5′′), 126.4 (CH, C-3′, 5′), 123.8 (CH, C-2′, 6′), 116.2 (CH, C-1′′, 6′′), 114.8 (C, C-4′′), 68.6 (CH2, C-2), 51.3 (CH, C-3), 26.7 (CH3, C-4), 25.8 (CH3, CH3-C4′). mass: m/z 347 (M+), 348 (M+1, 18.2%), 349 (M+2, 99.8%), Anal. Calc. For C17H18BrNO2: C 58.63, H 5.21, N 4.02. Found: C 58.69, H 5.17, N 4.08.
(8) 2-(4-Nitrophenoxy)-N-(1-(p-tolyl)ethyl)acetamide ( 3h ). IR (KBr, cm−1): 3219, 3078, 2917, 1678, 1596, 1534, 1267, 1034. 1H NMR (400 MHz, DMSO-d6, δ ppm): 8.37 (d, 2H, H-3′′, H-5′′), 8.13 (s, 1H, NH), 7.92 (d, 2H, H-2′′, H-6′′), 7.79–7.53 (m, 4H, H-2′, H-3′, H-5′, H-6′), 6.78–6.57(m, 1H, CH-3), 5.30 (s, 2H, CH2-2), 3.39 (s, 3H, CH3-4′), 2.84 (d, 3H, CH3-4). 13C NMR (DMSO-d6, δ ppm): 168.4 (C=O, NHCO), 167.1 (C, C-1′′), 161.9 (C, C-4′′), 157.8 (C, C-1′), 139.6 (C, C-4′), 131.6 (CH, C-3′, 5′), 129.1 (CH, C-3′′, 5′′), 127.4 (CH, C-2′, 6′), 120.4 (CH, C-2′′, 6′′), 71.8 (CH2, C-2), 68.4 (CH, C-3), 44.6 (CH3, C-4), 41.9 (CH3, CH3-C4′). mass: m/z 314 (M+), 315 (M+1, 19.1%), 316 (M+2, 2.9%). Anal. Calc. For C17H18N2O4: C 64.96, H 5.77, N 8.91. Found: C 64.91, H 5.78, N 8.97.
(9) 2-(4-(Tert-butyl)phenoxy)-N-(1-(p-tolyl)ethyl)acetamide ( 3i ). IR (KBr, cm−1): 3243, 3056, 2978, 2943, 1635, 1575, 1266, 1044. 1H NMR (400 MHz, DMSO-d6, δ ppm): 8.51 (s, 1H, NH), 8.33 (d, 2H, H-3′′, H-5′′), 8.27–8.11 (m, 4H, H-2′, H-3′, H-5′, H-6′), 7.53 (d, 2H, H-2′′, H-6′′), 5.11–4.28 (m, 1H, CH-3), 4.37 (s, 2H, CH2-2), 3.81 (s, 3H, CH3-4′), 3.16 (d, 3H, CH3-4), 2.76 (s, 9H, (CH3)3).13C NMR (DMSO-d6, δ ppm): 170.8 (C=O, NHCO), 158.4 (C, C-1′′), 151.3 (C, C-4′′), 139.1 (C, C-1′), 138.2 (C, C-4′), 136.4 (CH, C-3′, 5′), 134.1 (CH, C-3′′, 5′′), 130.8 (CH, C-2′, 6′), 122.6 (CH, C-2′′, 6′′), 74.6 (CH2, C-2), 71.4 (CH, C-3), 51.7 (C, C-(CH3)3), 46.2 ((CH3)3), 38.1 (CH3, C-4), 36.7 (CH3, CH3-C4′). mass: m/z 325 (M+), 326 (M+1, 23.1%), 327 (M+2, 3.6%). Anal. Calc. For C21H27NO2: C 77.50, H 8.36, N 4.30. Found: C 77.43, H 8.40, N 4.36.
(10) 2-(4-Methoxyphenoxy)-N-(1-(p-tolyl)ethyl)acetamide ( 3j ). IR (KBr, cm−1): 3233, 2935, 2907, 2811, 1644, 1576, 1308, 1017. 1H NMR (400 MHz, DMSO-d6, δ ppm): 8.44 (s, 1H, NH), 8.21-8.13 (m, 4H, H-2′, H-3′, H-5′, H-6′), 7.69 (s, 4H, H-2′′, H-3′′, H-5′′, H-6′′), 5.75–5.61 (m, 1H, CH-3), 5.32 (s, 2H, CH2-2), 4.23 (s, 3H, OCH3), 2.78 (s, 3H, CH3-4′), 2.34 (d, 3H, CH3-4). 13C NMR (DMSO-d6, δ ppm): 171.2 (C=O, NHCO), 168.8 (C, C-4′′), 161.4 (C, C-1′′), 159.8 (C, C-1′), 158.4 (C, C-4′), 161.4 (C, C-1′′), 157.1 (CH, C-3′, 5′), 152.3 (CH, C-2′, 6′), 148.2 (CH, C-2′′, 3′′, 5′′, 6′′), 76.1 (CH2, C-2), 71.7 (CH3, OCH3), 65.4 (CH, C-3), 32.8 (CH3, C-4), 31.7 (CH3, CH3-C4′). mass: m/z 299 (M+), 300 (M+1, 19.2%), 301 (M+2, 2.8%). Anal. Calc. For C18H21NO3: C 72.22, H 7.07, N 4.68. Found: C 72.29, H 7.01, N 4.62.
2.3. Pharmacological Screening
2.3.1. Animals
Wister albino rats of either gender weighing 140–180 g were obtained. The animals were divided into several groups of five animals each. All the animals were housed under standard ambient environment of temperature (25 ± 2°C) and relative humidity of 50 ± 5%. A 12 : 12 hour light : dark cycle was maintained. All the animals were allowed to have free access to water and standard palletized laboratory animal diet 12 hours prior to pharmacological studies. All the experimental procedures and protocols used in this study were reviewed and approved by the Institutional Animal Ethical Committee (IAEC).
2.3.2. Preparation of Test Compounds
Test samples and the reference drugs were prepared as a suspension in 1% tween 80. Control group received 0.1 mL of tween 80 suspension orally. The second group (reference) received a dose of 50 mg/kg suspension of diclofenac sodium. Test groups were treated with a dose of 100 mg/kg of final synthesized compounds.
2.3.3. Acute Toxicity
The acute toxicity study was carried out according to OECD guidelines [25]to found the successful dose of the test compounds after getting ethical clearance. Wister albino rats of either sex weighing between 140 and 180 g were divided into several groups of 5 animals each. Animals were starved for 12 hours prior to test. On the day of the experiment, animals were treated with different compounds to different groups in an increasing order of 10, 20, 100, 200, and 1000 mg/kg body weight orally. The animals were then observed continuously for 3 hours for common behavioral and autonomic profiles and then every 30 min for next 4 hours and finally for the next 24 hours or till death.
As per above toxicity test, it was observed that at a highest dose of 1000 mg/kg body weight, animals were found to be safe. But few changes were found in the behavioral reaction like touch response, alertness, and restlessness. Therefore, 1/10th of the highest tolerated dose, that is, 100 mg/kg body weight (b.w.), was chosen for the studies.
2.3.4. Anticancer Activity
Compounds 3a–j were biologically evaluated for in vitro cytotoxicity using sulforhodamine B assay (SRB) based cellular protein content determination against two human cancer cell lines that contained MCF-7 (breast) and SK-N-SH (Neuroblastoma), respectively. Adriamycin drug was taken as positive control and the results are reported as percent control growth. In this current protocol, each cell line is preincubated on microtitre plate. Results for each test compound are reported as the percent growth of treated cells is compared with the untreated control cells. The compounds which reduce growth of cell lines to 32% or less are considered active.
2.3.5. Anti-Inflammatory Activity
Carrageenan induced rat paw edema method [26] was employed for screening of the anti-inflammatory activity of the synthesized compounds listed in Table 1. The animals were divided into twelve groups of five each. One hour after oral administration of the drug, acute inflammation was produced by preparing aqueous suspension of carrageenan (1 %w/v, 0.1 mL) which was injected in the right hind paw in the subplanter region of each rat. A mark was applied on the leg at the malleolus to facilitate subsequent readings. The paw volume was measured plethysmometrically at 30 min, 2 hr, and 4 hr after the injection of carrageenan. The % inhibition was calculated by applying the Newbould formula [27]:
| (1) |
where V t and V c are the mean change in paw volume of treated and control rats, respectively.
Table 1.
Characterization data of N-(1-(4-chlorophenyl)ethyl)-2-(Substituted phenoxy)acetamide derivatives (3a–e) and 2-(substituted phenoxy)-N-(1-(p-tolyl)ethyl)acetamide derivatives (3f–j).
| Compound | R | Yield (%) | Melting point∗ (°C) | Rf value# | Molecular formula |
|---|---|---|---|---|---|
| 3a | H | 61.4 | 171–173 | 0.37 | C16H16ClNO2 |
| 3b | 4-Br | 58.3 | 154–156 | 0.45 | C16H15BrClNO2 |
| 3c | 4-NO2 | 59.1 | 165–167 | 0.41 | C16H15ClN2O4 |
| 3d | 4-C-(CH3)3 | 62.7 | 205–207 | 0.50 | C20H24ClNO2 |
| 3e | 4-OCH3 | 71.0 | 126–128 | 0.38 | C17H18ClNO3 |
| 3f | H | 67.8 | 154–156 | 0.32 | C17H19NO2 |
| 3g | 4-Br | 57.2 | 189–191 | 0.46 | C17H18BrNO2 |
| 3h | 4-NO2 | 74.5 | 148–150 | 0.51 | C17H18N2O4 |
| 3i | 4-C-(CH3)3 | 65.9 | 172–174 | 0.39 | C21H27NO2 |
| 3j | 4-OCH3 | 70.4 | 142–144 | 0.47 | C18H21NO3 |
∗Recrystallization with ethanol. #Stationary phase: silica gel; mobile phase: n-hexane : ethyl acetate (1 : 1); iodine vapors as visualizing agent.
2.3.6. Analgesic Activity
The compounds exhibited an important analgesic activity as per Eddy's hot plate method [28]. Animals were individually placed on a hot plate maintained at a constant temperature (55°C) and the reaction of animals such as paw licking or jump response (whichever appears first) was taken as the end point. A cut-off time of 15 seconds was taken as maximum analgesic response to avoid any injury of the paws. The reference group was administered with a dose of 50 mg/kg of the suspension of diclofenac sodium (standard). The reaction time for each animal was noted on the hot plate at 30, 60, and 90 minutes after drug administration.
2.3.7. Statistical Analysis
The results of anticancer, anti-inflammatory, and analgesic activities are shown in the Tables 2, 3, and 4, respectively. The results were expressed as mean ± SEM and were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett's t-test. The probability of 0.05 or less was considered statistically significant. Statistical analysis was computed with the GraphPad Prism software version 5.01, GraphPad Software Inc. USA.
Table 2.
Result of anticancer activity.
| Compound | % Control growth | Activity | |
|---|---|---|---|
| MCF-7 (breast) | SK-N-SH (neuroblastoma) | ||
| 3a | 88.9 | 88.2 | Inactive |
| 3b* | 30.5 | 67.6 | Active |
| 3c*** | 31.6 | 32.3 | Active |
| 3d | 54.0 | 72.1 | Inactive |
| 3e | 88.0 | 95.7 | Inactive |
| 3f*** | 71.9 | 32.1 | Active |
| 3g | 44.4 | 69.1 | Inactive |
| 3h | 70.0 | 71.8 | Inactive |
| 3i | 58.3 | 73.1 | Inactive |
| 3j | 74.0 | 80.1 | Inactive |
∗Active against MCF-7 (breast) cell line.
∗∗Active against SK-N-SH (neuroblastoma) cell line.
∗∗∗Active against both MCF-7 (breast) and SK-N-SH (neuroblastoma) cell lines.
Growth percentages less than 32 are considered as active.
Table 3.
Result of anti-inflammatory activity.
| Compound | Mean changes in paw edema (mL) mean ± SEM | % Inhibition | ||||
|---|---|---|---|---|---|---|
| 30 min | 2 hr | 4 hr | 30 min | 2 hr | 4 hr | |
| Control | 0.612 ± 0.014 | 0.754 ± 0.051 | 0.621 ± 0.013 | — | — | — |
|
| ||||||
| Diclofenac sodium | 0.232 ± 0.021 | 0.277 ± 0.019 | 0.277 ± 0.027 | 62.09 ± 0.095 | 63.26 ± 0.095 | 55.39 ± 0.095 |
|
| ||||||
| 3a** | 0.403 ± 0.024 | 0.462 ± 0.026 | 0.384 ± 0.013 | 34.15 ± 0.011 | 38.72 ± 0.010 | 38.16 ± 0.019 |
|
| ||||||
| 3b** | 0.265 ± 0.033 | 0.292 ± 0.05 | 0.256 ± 0.020 | 56.69 ± 0.033 | 61.27 ± 0.050 | 58.77 ± 0.039 |
|
| ||||||
| 3c* | 0.272 ± 0.009 | 0.341 ± 0.041 | 0.290 ± 0.030 | 55.55 ± 0.041 | 54.77 ± 0.023 | 53.30 ± 0.013 |
|
| ||||||
| 3d** | 0.366 ± 0.043 | 0.433 ± 0.009 | 0.371 ± 0.010 | 40.19 ± 0.014 | 42.57 ± 0.063 | 40.25 ± 0.019 |
|
| ||||||
| 3e* | 0.425 ± 0.013 | 0.526 ± 0.019 | 0.437 ± 0.011 | 30.55 ± 0.033 | 30.23 ± 0.011 | 29.62 ± 0.024 |
|
| ||||||
| 3f* | 0.442 ± 0.039 | 0.556 ± 0.103 | 0.463 ± 0.019 | 27.77 ± 0.134 | 26.25 ± 0.028 | 25.44 ± 0.020 |
|
| ||||||
| 3g** | 0.258 ± 0.024 | 0.322 ± 0.09 | 0.271 ± 0.17 | 57.84 ± 0.006 | 57.29 ± 0.008 | 56.36 ± 0.04 |
|
| ||||||
| 3h* | 0.359 ± 0.009 | 0.442 ± 0.009 | 0.369 ± 0.024 | 41.33 ± 0.039 | 41.37 ± 0.020 | 40.57 ± 0.024 |
|
| ||||||
| 3i** | 0.428 ± 0.020 | 0.524 ± 0.024 | 0.453 ± 0.07 | 30.06 ± 0.041 | 30.50 ± 0.09 | 27.05 ± 0.009 |
|
| ||||||
| 3j* | 0.466 ± 0.041 | 0.564 ± 0.061 | 0.471 ± 0.011 | 23.85 ± 0.043 | 25.19 ± 0.041 | 24.15 ± 0.011 |
*P < 0.05 significant from control.
**P < 0.01 significant from control.
Table 4.
Result of analgesic activity.
| Compound | Reaction time (S) after drug administration (mean ± SEM) | % Inhibition | ||||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 30 min | 60 min | 90 min | |
| Control | 2.67 ± 0.020 | 2.69 ± 0.025 | 2.70 ± 0.03 | — | — | — |
|
| ||||||
| Diclofenac sodium | 5.31 ± 0.03 | 5.67 ± 0.019 | 5.82 ± 0.014 | 98.87 ± 0.214 | 110.78 ± 0.217 | 115.55 ± 1.620 |
|
| ||||||
| 3a* | 3.11 ± .004 | 3.76 ± 0.019 | 3.72 ± 0.07 | 16.47 ± 0.019 | 39.77 ± 0.033 | 37.77 ± 0.041 |
|
| ||||||
| 3b** | 5.43 ± 0.019 | 5.74 ± 0.033 | 6.11 ± 0.010 | 103.37 ± 0.043 | 113.38 ± 0.027 | 126.29 ± 0.90 |
|
| ||||||
| 3c* | 5.34 ± 0.041 | 5.46 ± 0.011 | 5.88 ± 0.028 | 100 ± 0.006 | 102.97 ± 0.011 | 117.77 ± 0.081 |
|
| ||||||
| 3d** | 4.89 ± 0.043 | 4.66 ± 0.010 | 4.49 ± 0.041 | 83.14 ± 0.071 | 73.23 ± 0.063 | 66.29 ± 0.038 |
|
| ||||||
| 3e** | 4.92 ± 0.011 | 5.13 ± 0.020 | 5.10 ± 0.063 | 84.26 ± 0.023 | 90.70 ± 0.006 | 88.88 ± 0.075 |
|
| ||||||
| 3f* | 3.37 ± 0.05 | 3.22 ± 0.008 | 3.20 ± 0.019 | 26.21 ± 0.014 | 19.70 ± 0.023 | 18.51 ± 0.010 |
|
| ||||||
| 3g** | 5.35 ± 0.023 | 5.76 ± 0.09 | 5.91 ± 0.020 | 100.37 ± 0.041 | 114.12 ± 0.020 | 118.88 ± 0.61 |
|
| ||||||
| 3h** | 5.23 ± 0.019 | 5.78 ± 0.063 | 6.13 ± 0.011 | 95.88 ± 0.05 | 114.86 ± 0.039 | 127.03 ± 0.071 |
|
| ||||||
| 3i** | 5.15 ± 0.010 | 4.88 ± 0.014 | 4.85 ± 0.028 | 92.88 ± 0.023 | 81.41 ± 0.043 | 79.62 ± 0.91 |
|
| ||||||
| 3j* | 3.22 ± 0.020 | 3.39 ± 0.041 | 3.57 ± 0.019 | 20.59 ± 0.008 | 26.02 ± 0.041 | 32.22 ± 0.38 |
*P < 0.05 significant from control.
**P < 0.01 significant from control.
3. Results and Discussion
A series of titled derivatives N-(1-(4-chlorophenyl)ethyl)-2-(substituted phenoxy)acetamide 3(a–e) derivatives and 2-(substituted phenoxy)-N-(1-(p-tolyl)ethyl)acetamide 3(f–j) derivatives (Figures 2 and 3) was synthesized as per scheme. 1-(4-chlorophenyl)ethanone and 1-(p-tolyl)ethanone were separately treated with ammonium carbonate and formic acid resulting in the formation of amines 1a and 1b, respectively. These amines on chloroacetylation with chloroacetyl chloride at 0°C in 10% sodium hydroxide medium give chloro compounds 2a and 2b which converted to (3a–j) by the reaction with different substituted phenols in presence of potassium iodide and potassium carbonate in dry acetone as a solvent. The structure of newly synthesized compounds (Figures 2 and 3) was confirmed by spectral data (IR, 1H NMR, 13C-NMR and mass); Table 1 shows the physical data of compounds (3a–j).
Figure 2.
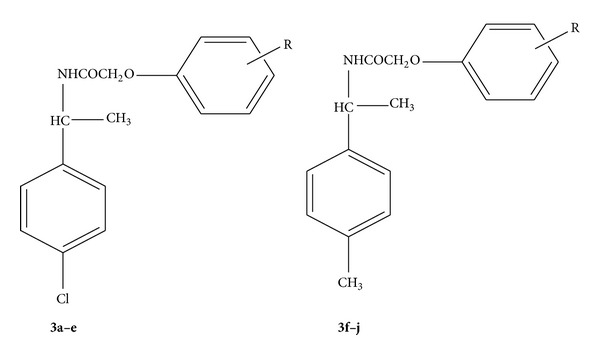
General structure of the synthesized compounds.
Figure 3.
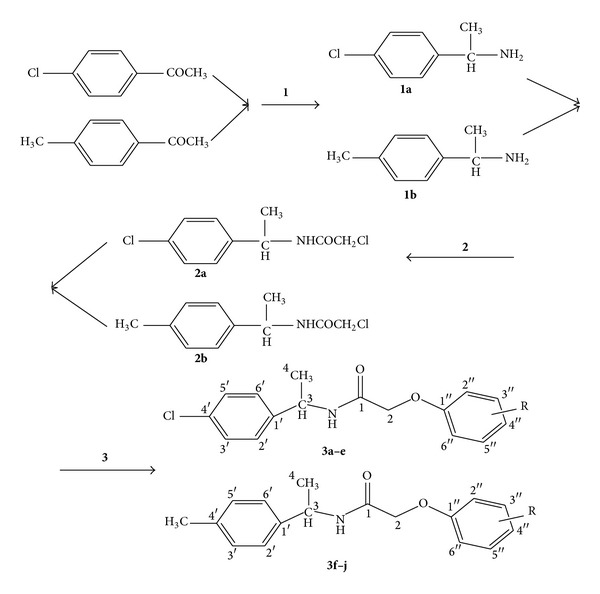
Synthesis of N-(1-(4-chlorophenyl)ethyl)-2-(substituted phenoxy)acetamide (3a–e) derivatives and 2-(substituted phenoxy)-N-(1-(p-tolyl)ethyl)acetamide (3f–j) derivatives.
Further IR spectrum of compounds (3a–j) showed characteristic absorption bands at range of 3205–3266 cm−1 were attributed to NH, 1635–1711 cm−1 accounting for C=O of amide group and 1533–1644 for C=C in the aromatic ring. Two peaks at range of 1200–1335 and range of 1017–1235 indicate the presence of C–O–C linkage. The structure of compounds was further supported by mass spectral data.
All compounds 3a–j were subjected for preliminary toxicity test as per organization for Economic Co-operation and Development (OECD) guidelines in rats and 100 mg/kg was used as therapeutic dose. Acute anti-inflammatory activity was performed by carrageenan induced rat paw edema method by Newbould [26]. Diclofenac sodium was used as a reference standard. Compounds 3b, 3c, and 3g exhibited potent anti-inflammatory activity similar to the standard (Table 3, Figure 4). The analgesic effects of compounds 3b, 3c, 3e, 3g, and 3h were found to be nearly of standard (Table 4, Figure 5).
Figure 4.
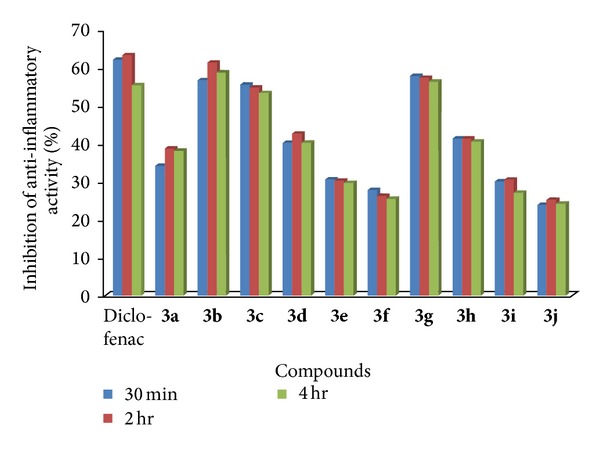
Graphical representation of (% inhibition) of anti-inflammatory activity.
Figure 5.
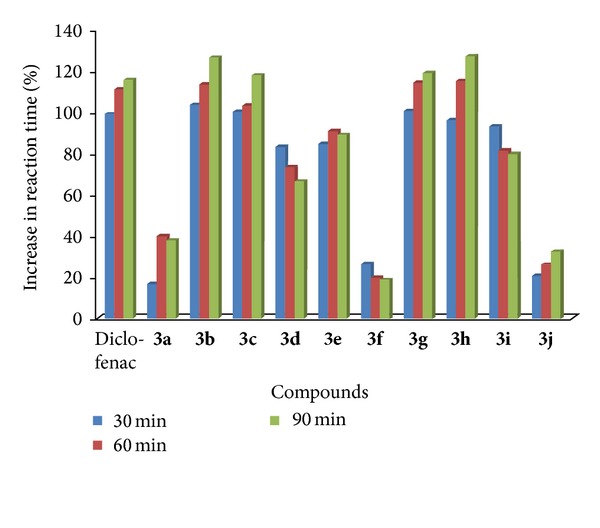
Graphical representation of % increase in reaction time for analgesic activity.
Compounds 3c were found to possess anticancer, anti-inflammatory, and analgesic activities nearly to the standard because of the presence of NO2 group and Br groups at position 4 of the phenoxy ring.
From the comprehensive analysis of the results in current studies, we conclude that synthesized compounds have anticancer, anti-inflammatory, and analgesic activities because of the presence of 1-phenylethylamine as basic ring. In analysis of these observations, we conclude that this series (3a–j) could be developed and explored as a novel class of anticancer and NSAIDs. However, further detailed pharmacological program is required to recognize the potent molecule without various side effects.
3.1. Structure Activity Relationship (SAR)
By comparing the cytotoxic potency of newly synthesized compounds 3a–j, a few inferences could be drawn as follows. (i) The presence of tert-butyl, methoxy, and nitro substituent at 4 positions does not contribute towards cytotoxicity as can be seen in compounds 3d, 3j, and 3h which showed poor growth inhibition against both of the cancer cell lines used. (ii) The presence of Br group at 4 positions contributes significantly towards the enhancement of inhibitory activity. (iii) The presence of NO2 group at 4 positions in the phenoxy nucleus attached to 1-(4-chlorophenyl)ethanamine makes the compound potent antiproliferative agent with dual inhibition of both MCF-7 and SK-N-SH cell lines.
After carrying out the anti-inflammatory and analgesic screenings of synthesized compounds, the following conclusions are drawn. (i) Presence of Br and NO2 groups at 4 positions in the phenoxy nucleus contributes towards anti-inflammatory activity. (ii) Presence of tert-butyl and methoxy substituent at 4 positions does not contribute towards anti-inflammatory activity. It is also observed that compound 3c with nitro substituent in the 4 positions of phenoxy nucleus contributed effectively towards anticancer, anti-inflammatory, and analgesic activities. The analgesic activities may be due to inhibition of COX/LOX pathways which are the possible mechanisms of induction of pain. In our future research programme, these pathways which are highly active in malignant cancerous growth would be targeted. This research would be a new breakthrough in the area of oncology where enzyme mediators and COX-2 and LOX-2 inhibitors would be utilized in suppression of tumor growth.
Scheme I. Synthesis of N-(1-(4-chlorophenyl)ethyl)-2-(substituted phenoxy)acetamide derivatives (3a–e) and 2-(substituted phenoxy)-N-(1-(p-tolyl)ethyl)acetamide derivatives (3f–j). Reagents and conditions: (1) ammonium carbonate and formic acid, heat very slowly till 165°C→Ketone, Heat, 4-5 h, 180–185°C→Reflux, concentrated hydrochloric acid, 2-3 hour, water bath→extraction, diethylether→Strongly alkaline, 30% sodium hydroxide→extraction; Diethylether (2) Chloroacetyl chloride, 10% sodium hydroxide, ice bath, pH 9-10. (3) Substituted phenols, dry acetone, Potassium Carbonate, Potassium Iodide, Reflux.
In the series of ten compounds for activity against MCF-7 (breast) cell line, compounds 3b and 3c showed active inhibition of the cancer cells. These two compounds were found to be highly active against the cancer cell lines. Compounds 3d, 3h, and 3i showed mild to moderate cytotoxic activities, whereas the remaining compounds were found to be completely inactive.
Out of ten compounds, 3f and 3c were found to be modestly active against SK-N-SH (neuroblastoma) cell line, and the remaining compounds were found to be inactive.
Acknowledgment
The authors are grateful to Professor R. M. Dubey, Vice Chancellor of IFTM University, for providing necessary facilities for this research work.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Johnsen JI, Lindskog M, Ponthan F, et al. Cyclooxygenase-2 is expressed in neuroblastoma, and nonsteroidal anti-inflammatory drugs induce apoptosis and inhibit tumor growth in vivo. Cancer Research. 2004;64(20):7210–7215. doi: 10.1158/0008-5472.CAN-04-1795. [DOI] [PubMed] [Google Scholar]
- 2.Sonoshita M, Takaku K, Oshima M, Sugihara K, Taketo MM. Cyclooxygenase-2 expression in fibroblasts and endothelial cells of intestinal polyps. Cancer Research. 2002;62(23):6846–6849. [PubMed] [Google Scholar]
- 3.Sheehan KM. The relationship between cyclooxygenase-2 expression and colorectal cancer. Journal of American Medical Association. 1999;282:1254–1257. doi: 10.1001/jama.282.13.1254. [DOI] [PubMed] [Google Scholar]
- 4.Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Seminars in Cancer Biology. 2004;14(6):433–439. doi: 10.1016/j.semcancer.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Kalgutkar AS, Zhao Z. Discovery and design of selective cyclooxygenase-2 inhibitors as non-ulcerogenic, anti-inflammatory drugs with potential utility as anti-cancer agents. Current Drug Targets. 2001;2(1):79–106. doi: 10.2174/1389450013348830. [DOI] [PubMed] [Google Scholar]
- 6.Pasinetti GM. Cyclooxygenase and Inflammation in Alzheimers disease: experimental approaches and clinical interventions. Journal of Neuroscience Research. 1998;54(1):1–6. doi: 10.1002/(SICI)1097-4547(19981001)54:1<1::AID-JNR1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrey MP, Patel KV. Prostaglandin E2 production and metabolism in human breast cancer cells and breast fibroblasts. Regulation by inflammatory mediators. British Journal of Cancer. 1995;72(6):1412–1419. doi: 10.1038/bjc.1995.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill DT, Loev B. 4-(2-Thienyl)-5-methylpyrimidine. An anomalous Leuckart product. Journal of Organic Chemistry. 1973;38(11):2102–2103. [Google Scholar]
- 10.Bach RD. Preparation of tertiary N,N-dimethylamines by the leuckart reaction. The Journal of Organic Chemistry. 1968;33(4):1647–1649. [Google Scholar]
- 11.Sternbach LH. The benzodiazepine story. Journal of Medicinal Chemistry. 1979;22(1):1–7. doi: 10.1021/jm00187a001. [DOI] [PubMed] [Google Scholar]
- 12.Thurston DE, Bose DS. Synthesis of DNA-interactive pyrrolo[2,1-c][1,4]benzodiazepines. Chemical Reviews. 1994;94(2):433–465. doi: 10.1021/cr100120f. [DOI] [PubMed] [Google Scholar]
- 13.Gupta MB, Nath R, Gupta GP, Bhargava KP. A study of the anti-ulcer activity of diazepam and other tranquillosedatives in albino rats. Clinical and Experimental Pharmacology and Physiology. 1985;12(1):61–66. doi: 10.1111/j.1440-1681.1985.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 14.Lokensgard JR, Chao CC, Gekker G, Hu S, Peterson PK. Benzodiazepines, glia, and HIV-1 neuropathogenesis. Molecular Neurobiology. 1998;18(1):23–33. doi: 10.1007/BF02741458. [DOI] [PubMed] [Google Scholar]
- 15.Ding CZ, Batorsky R, Bhide R, et al. Discovery and structure-activity relationships of imidazole-containing tetrahydrobenzodiazepine inhibitors of farnesyltransferase. Journal of Medicinal Chemistry. 1999;42(25):5241–5253. doi: 10.1021/jm990391w. [DOI] [PubMed] [Google Scholar]
- 16.Ang W, Lin Y, Yang T, et al. Synthesis and biological evaluation of 2-(3-Fluoro-4-nitro phenoxy)-N-phenylacetamide derivatives as novel potential affordable antitubercular agents. Molecules. 2012;17(2):2248–2258. doi: 10.3390/molecules17022248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedstrom LK, Striepen B. IMP dehydrogenase inhibitors for treating mammalian gastrointestinal parasitic infections. WO. 2007;(2007143557)
- 18.Sun A, Prussia A, Zhan WQ, et al. Nonpeptide inhibitors of measles virus entry. Journal of Medicinal Chemistry. 2006;49(17):5080–5092. doi: 10.1021/jm0602559. [DOI] [PubMed] [Google Scholar]
- 19.Tipparaju SK, Muench SP, Mui EJ, et al. Identification and development of novel inhibitors of toxoplasma gondii enoyl reductase. The Journal of Medicinal Chemistry. 2010;53(17):6287–6300. doi: 10.1021/jm9017724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K, Roh SH, Xia Y, Kang KW. Synthesis and biological evaluation of phenoxy-N-phenylacetamide derivatives as novel P-glycoprotein inhibitors. Bulletin of the Korean Chemical Society. 2011;32(10):3666–3674. [Google Scholar]
- 21.Pal D, Banerjee S, Ghosh AK. Dietary-induced cancer prevention: An expanding research arena of emerging diet related to healthcare system. Journal of Advanced Pharmaceutical Technology and Research. 2012;3(1):16–24. doi: 10.4103/2231-4040.93561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal D, Mitra S. A preliminary study on the in vitro antioxidant activity of the stems of Opuntia vulgaris . Journal of Advanced Pharmaceutical Technology and Research. 2010;1(2):268–272. [PMC free article] [PubMed] [Google Scholar]
- 23.Nayak AK, Pal D, Pany DR, Mohanty B. Evaluation of Spinacia oleracea L. leaves mucilage as an innovative suspending agent. Journal of Advanced Pharmaceutical Technology and Research. 2010;1(3):338–341. doi: 10.4103/0110-5558.72430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.OCED/OCDC. Guidelines for testing of chemicals. Revised draft guidelines 423, acute oral toxicity class method, Revised document. 2000.
- 25.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proceedings of the Society for Experimental Biology and Medicine. 1962;3:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 26.Newbould BB. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. British Journal of Pharmacology and Chemotherapy. 1963;21:127–136. doi: 10.1111/j.1476-5381.1963.tb01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eddy NB, Leimbach DJ. Synthetic analgesics II. Dithienylbutenyl and dithienylbutylamines. Journal of Pharmacology and Experimental Therapeutics. 1953;107(3):385–393. [PubMed] [Google Scholar]
- 28.Mathew V, Keshavayya J, Vaidya VP, Giles D. Studies on synthesis and pharmacological activities of 3,6-disubstituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and their dihydro analogues. European Journal of Medicinal Chemistry. 2007;42(6):823–840. doi: 10.1016/j.ejmech.2006.12.010. [DOI] [PubMed] [Google Scholar]


