Abstract
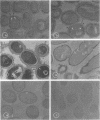
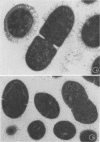
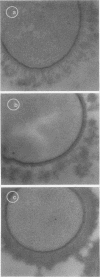
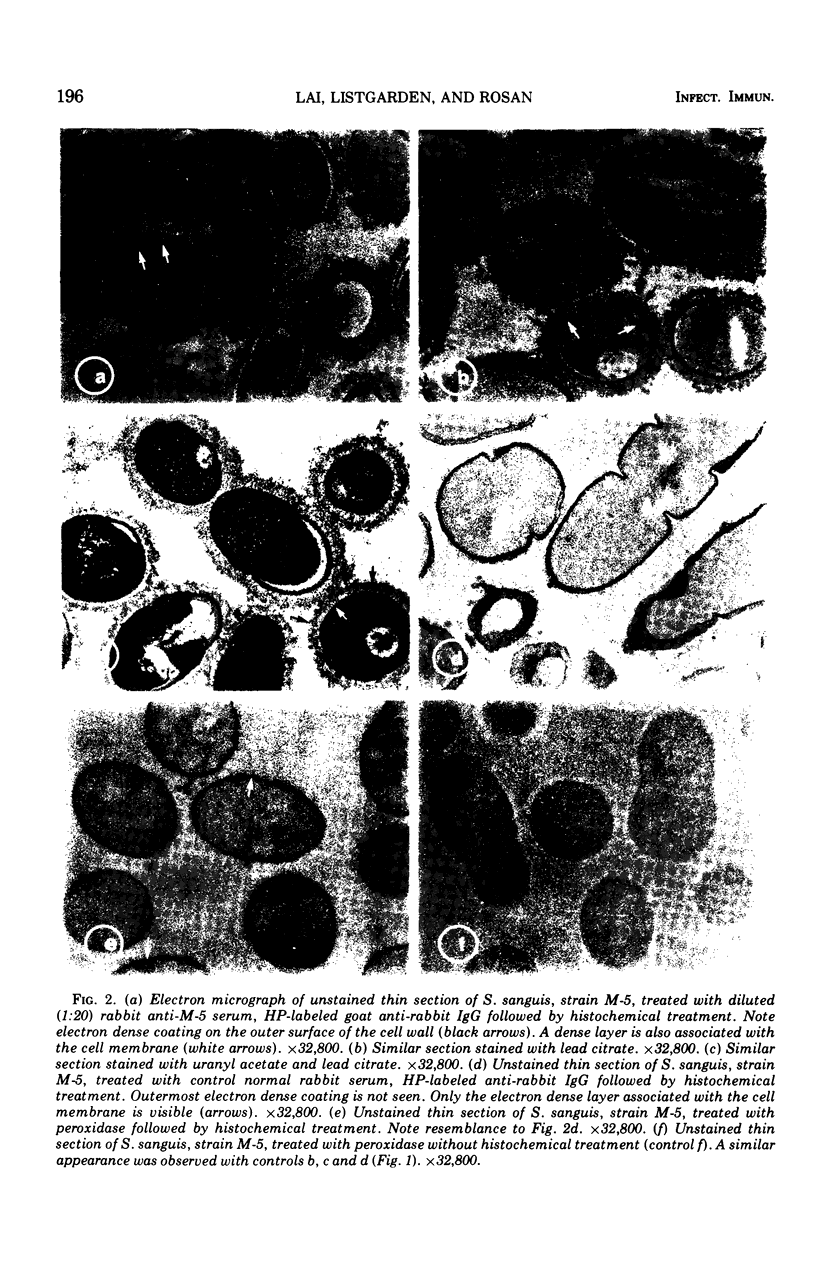
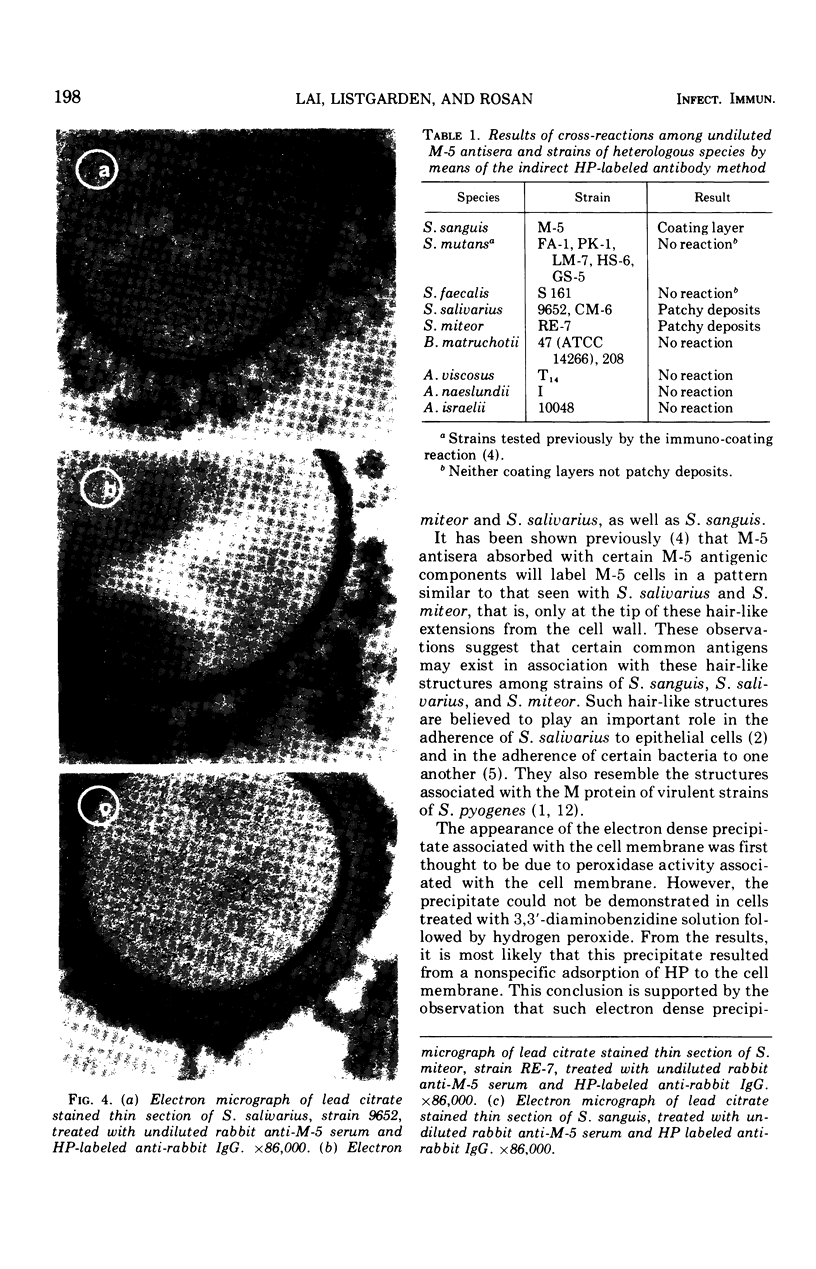
An indirect method of localizing antigens with horseradish peroxidase-labeled antibody was used to identify and localize surface antigens of Streptococcus sanguis at the ultrastructural level. An electron dense layer surrounding the cell wall could be distinguished without any additional electron microscope staining. This labeled layer represents an immune complex consisting of bacterial surface antigens, specific rabbit antisera, and peroxidase-labeled goat anti-rabbit globulins. Although with undiluted antisera slight cross-reactions occurred with S. salivarius and S. miteor (mitis), these could be readily distinguished from the more intense homologous reaction by their patchiness and the difference in distribution of the label. These cross-reactions were eliminated by appropriate dilutions of the antiserum. No cross-reactions occurred with S. mutans, S. faecalis, Actinomyces species, or Bacterionema, microorganisms wents indicated that horseradish peroxidase can become non-specifically adsorbed to the membrane of certain bacterial cells. Appropriate controls must, therefore, be included for localization of membrane associated antigens with horseradish peroxidase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Van Houte J., Liljemark W. F. Parameters that effect the adherence of Streptococcus salivarius to oral epithelial surfaces. J Dent Res. 1972 Mar-Apr;51(2):424–435. doi: 10.1177/00220345720510023101. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C., Listgarten M., Rosan B. Serology of Streptococcus sanguis: localization of antigens with unlabeled antisera. Infect Immun. 1973 Sep;8(3):475–481. doi: 10.1128/iai.8.3.475-481.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A., Mayo H., Amsterdam M. Ultrastructure of the attachment device between coccal and filamentous microorganisms in "corn cob" formations of dental plaque. Arch Oral Biol. 1973 May;18(5):651–656. doi: 10.1016/0003-9969(73)90105-2. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Pierce G. B., Jr Enzyme-labeled antibodies for the light and electron microscopic localization of tissue antigens. J Cell Biol. 1967 May;33(2):307–318. doi: 10.1083/jcb.33.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALADE G. E. A study of fixation for electron microscopy. J Exp Med. 1952 Mar;95(3):285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B. Antigens of Streptococcus sanguis. Infect Immun. 1973 Feb;7(2):205–211. doi: 10.1128/iai.7.2.205-211.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanini M., De Martino C., Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967 Oct 14;216(5111):173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]