Abstract
Myelin is a vertebrate adaptation that allows for the rapid propagation of action potentials along axons. Specialized glial cells – oligodendrocytes in the central nervous system (CNS) and Schwann cells in the peripheral nervous system (PNS) – form myelin by repeatedly wrapping axon segments. Debilitating diseases result from the disruption of myelin, including Multiple Sclerosis and Charcot-Marie-Tooth peripheral neuropathies. The process of myelination involves extensive communication between glial cells and the associated neurons, and the last few years have seen important progress in understanding the molecular basis of the signals that coordinate the development of these fascinating cells. This review highlights recent advances in myelination deriving from studies in the zebrafish model system, with a primary focus on the PNS. While Neuregulin1-ErbB signaling has long been known to play important roles in peripheral myelin development, work in zebrafish has elucidated its roles in Schwann cell migration and radial sorting of axons sorting in vivo. Forward genetic screens in zebrafish have also uncovered new genes required for development of myelinated axons, including gpr126, which encodes a G-protein coupled receptor required for Schwann cells to progress beyond the promyelinating stage. In addition, work in zebrafish uncovered new roles for Schwann cells themselves, including in regulating the boundary between the PNS and CNS and positioning a nerve after its initial outgrowth.
Introduction
The myelin sheath increases axonal conduction velocity by reducing capacitance of the axonal membrane and allowing saltatory conduction (Hodgkin, 1964; Stampfli, 1954). Thus, myelinated axons of small diameter can transmit information as rapidly as much larger unmyelinated axons. Myelin therefore is an evolutionary innovation that allows the nervous system to increase in speed and complexity without a corresponding increase in size and energy requirements. Although some invertebrate species have myelinated axons, myelin is ubiquitous among the gnathostomes (jawed vertebrates), and this adaptation has surely been essential for the formation of the large, complex nervous systems that distinguish the vertebrates from other groups (Bunge, 1968; Hartline and Colman, 2007).
Disruption of the myelin sheath underlies many debilitating diseases including Multiple Sclerosis, Charcot Marie Tooth disease, and others (Berger et al., 2006; McQualter and Bernard, 2007). Specialized glial cells, oligodendrocytes in the central nervous system (CNS) and Schwann cells in the peripheral nervous system (PNS), wrap their membranes many times around a segment of an axon to form the myelin sheath (Bunge, 1968; Geren and Raskind, 1953; Peters, 1964). Along its length, each axon is ensheathed by multiple myelin segments, which are separated by unmyelinated gaps called nodes of Ranvier (Bunge, 1968; Stampfli, 1954; Tasaki, 1959). Oligodendrocytes interact with and elaborate myelin sheaths around many different axons; in contrast, Schwann cells myelinate only one segment of one axon (Bunge, 1968).
Schwann Cell Development
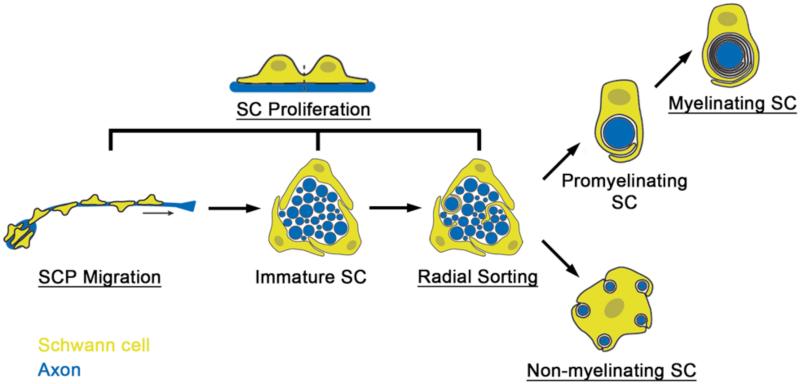
As extensively documented in mammals, Schwann cells originate from the neural crest and undergo a series of developmental transitions that culminates with myelination (Jessen and Mirsky, 2005). Schwann cell precursors form from neural crest progenitors that have delaminated from the dorsal neural tube and associated with axons in peripheral nerves (Jessen et al., 1994; Sauka-Spengler and Bronner, 2010). Schwann cell precursors migrate with axons as they grow towards their targets (Dong et al., 1999). Once migration is complete, Schwann cell precursors differentiate into immature Schwann cells, which associate with many axons organized as a bundle (Jessen and Mirsky, 2005). Through a process termed radial sorting, Schwann cells progressively sort axons from the main bundle, such that a Schwann cell associates with a single axonal segment that is to be myelinated (Webster et al., 1973). At this point the immature Schwann cells associated with a single large caliber axon will become promyelinating Schwann cell, initiate the myelination transcription pathway, and begin to wrap their cytoplasm around the axon to create a myelin sheath (Jessen and Mirsky, 2005; Webster et al., 1973). In contrast, immature Schwann cells associated with several small caliber axons will become non-myelinating Schwann cells, ensheathing each axon in a pocket of its cytoplasm, forming a Remak bundle (Aguayo and Bray, 1975; Aguayo et al., 1976; Hahn et al., 1987; Jessen and Mirsky, 2005; Peters and Muir, 1959).
Much of our current understanding of myelination comes from mammalian studies, and a number of genes essential for Schwann cell development are known. sox10, erbb2, erbb3, and nrg1 are required at multiple steps of Schwann cell development, regulating neural crest migration and specification, as well as Schwann cell proliferation, survival, specification, and myelination (Britsch et al., 2001; Chen et al., 2006; Dong et al., 1995; Finzsch et al., 2010; Garratt et al., 2000b; Jessen and Mirsky, 2005; Kuhlbrodt et al., 1998; Michailov et al., 2004; Morrissey et al., 1995; Newbern and Birchmeier, 2010; Riethmacher et al., 1997; Schreiner et al., 2007; Woldeyesus et al., 1999). Other genes are necessary at discrete stages - oct6, brn2, and krox20 are required for Schwann cells to progress beyond the promyelinating stage and make myelin (Svaren and Meijer, 2008). Components of the extracellular matrix and cytoskeletal regulators are required for radial sorting, including rac1, cdc42, FAK, ILK, β1-integrin, laminin-λ1, laminin-2, and laminin-8 (Benninger et al., 2007; Chen and Strickland, 2003; Feltri et al., 2002; Grove et al., 2007; Nodari et al., 2007; Pereira et al., 2009; Yu et al., 2009; Yu et al., 2005).
Despite important progress in defining key genes that regulate Schwann cell and oligodendrocyte development and myelination, our understanding of the pathways that regulate glial development and myelination is still incomplete (Emery, 2010; Jessen and Mirsky, 2005). In this review, we seek to highlight advances in understanding myelination in the zebrafish model system, which combines powerful genetics with exquisite in vivo imaging. We will focus mainly on the peripheral nervous system, where a more complete pathway of Schwann cell development has emerged, and in particular on the roles of Neuregulin1/ErbB signaling and Gpr126. In addition, we will highlight new roles for Schwann cells that have been discovered in zebrafish.
Neuregulin1/ErbB Signaling
Neuregulin1 (Nrg1) is an EGF-related signal that activates ErbB receptor tyrosine kinases on Schwann cells to regulate several aspects of Schwann cell development, including proliferation, survival, myelination and the formation of Remak bundles (Birchmeier and Nave, 2008; Dong et al., 1995; Garratt et al., 2000b; Lyons et al., 2005; Michailov et al., 2004; Morrissey et al., 1995; Riethmacher et al., 1997; Taveggia et al., 2005; Woldeyesus et al., 1999). ErbB2 and ErbB3 are the main receptors for Nrg1 ligands in Schwann cells (Citri et al., 2003; Newbern and Birchmeier, 2010). There are over 15 different isoforms of Nrg1, but a single isoform, Nrg1 type III, has emerged as a primary regulator of Schwann cell development (Falls, 2003; Michailov et al., 2004; Taveggia et al., 2005). For example, the amount of Nrg1 type III expressed on an axon determines whether the associated Schwann cell will become a myelinating or non-myelinating Schwann cell, as well as the thickness of the myelin sheath (Michailov et al., 2004; Taveggia et al., 2005). Nrg1 can also induce demyelination (Syed et al., 2010; Zanazzi et al., 2001), however, suggesting that the level or location of the signal may help determine how a Schwann cell will respond to it. Additionally, the signaling pathways downstream of Nrg1/ErbB signaling may modulate the response to the signal at different stages; these pathways include PI3K/Akt, MEK/ERK, Calcineurin/NFAT, Cdc42, Shp2, and others (Benninger et al., 2007; Cotter et al., 2010; Goebbels et al., 2010; Grossman et al., 2009; Kao et al., 2009; Ogata et al., 2010; for a more extensive review of Nrg1/ErbB signaling in Schwann cells see Newbern and Birchmeier, 2010).
Analyses in zebrafish have investigated the role of Nrg1/ErbB signaling in Schwann cell migration and subsequent steps in Schwann cell development using mutants, small molecule inhibitors, and timelapse imaging in vivo. A genetic screen for mutants with defects in myelin basic protein (mbp) expression isolated mutations in erbb2 and erbb3, among other genes (Lyons et al., 2005; Pogoda et al., 2006). Similar to previous studies of mouse mutants in ErbB2 and ErbB3, the zebrafish erbb2 and erbb3 mutants lack Schwann cells along peripheral axons, although some Schwann cells do associate with neuronal cell bodies at cranial ganglia (Lyons et al., 2005; Pogoda et al., 2006; Reithmacher et al., 1997; Woldeyesus et al., 1999). BrdU incorporation studies revealed that Schwann cell proliferation is reduced (but not eliminated) in erbb mutants, consistent with many mammalian studies showing that Nrg1 is a Schwann cell mitogen (Garratt et al., 2000b; Lyons et al., 2005; Morrissey et al., 1995; Newbern and Birchmeier, 2010). The use of timelapse imaging combined with chemical inhibitors of ErbB activity also showed that ErbB signaling is required continuously during Schwann cell migration, in addition to its role in Schwann cell proliferation (Lyons et al., 2005). When the ErbB inhibitor AG1478 was applied after the start of migration, some Schwann cells continued to move, but in a misdirected fashion – in some instances even switching nerves. These studies support the possibility that ErbB signaling is important for directed migration of Schwann cells, rather than simply promoting the motility of these cells. Future studies are required to determine the identity of the axonal signal that directs Schwann cell migration, but, given the involvement of ErbB receptors, it is likely that Nrg1 is involved.
A recent study has added to the understanding of ErbB signaling in radial sorting, by analyzing the effects of ErbB inhibitors added after Schwann cell migration is complete (Raphael et al., 2011). Migrating Schwann cells associate with bundles of many axons, but shortly after the end of migration, radial sorting begins (Figure 1). Radial sorting occurs similarly in zebrafish as in mammals, with immature Schwann cells surrounding a bundle of axons, extending processes into the axon bundle and “radially” sorting axons to the periphery of the bundle, at which point one Schwann cell is interacting with one axon and the myelination program can begin (Raphael et al., 2011; Webster et al., 1973). During radial sorting, Schwann cells proliferate extensively, so that they are present in numbers corresponding to the many axonal segments that will be myelinated (Jessen and Mirsky, 2005; Webster et al., 1973). Inhibitors of cell division interfere with radial sorting and the onset of myelination (Lyons et al., 2005; Raphael et al., 2011). Inhibition of ErbB signaling also disrupted radial sorting, as expected in light of the role of Nrg1-ErbB signaling in Schwann cell proliferation (Raphael et al., 2011). Supporting a previous study of mammalian Schwann cells in culture (Taveggia et al., 2005), ErbB signaling also has a role that is independent of Schwann cell number: Schwann cells processes do not extend into axon bundles in fish treated with ErbB inhibitors, in contrast to fish treated with inhibitors of cell division or untreated controls (Raphael et al., 2011). This indicates that, in addition to regulating Schwann cell proliferation during radial sorting, ErbB signaling is also required for Schwann cell process extension. It will be interesting to learn how Nrg1 signals from axons are coordinated with signaling from the basal lamina, which is generated by the Schwann cells themselves, to bring about radial sorting and myelination. In addition, timelapse imaging of the interaction of Schwann cells and their axons in living zebrafish may generate new insights into radial sorting, a dynamic process that is currently understood from electron micrograph time courses.
Figure 1. Multiple roles for Nrg1/ErbB signaling during Schwann cell development.
Schwann cells (yellow) migrate along axons (blue) as Schwann cell precursors (SCP; Jessen and Mirsky, 2005). Once migration is complete, SCPs differentiate into immature Schwann cells (SC) and begin the process of radial sorting by inserting their processes into the axon bundle (Jessen and Mirsky, 2005; Webster et al., 1973). Immature Schwann cells can become either promyelinating and then myelinating Schwann cells, if they are associated with one axon, or non-myelinating Schwann cells, which associate with multiple small axons (Nave and Salzer, 2006). Schwann cell proliferation occurs in SCPs and in immature Schwann cells and during radial sorting; however, once immature Schwann cells differentiate, they exit the cell cycle (Jessen and Mirsky, 2005; Martin and Webster, 1973; Webster et al., 1973). Schwann cells divide parallel to the axons (Martin and Webster, 1973). Steps that require Nrg1/ErbB signaling are underlined and include Schwann cell migration, Schwann cell proliferation, radial sorting, myelination, and the formation of Remak bundles – non-myelinating Schwann cells ensheathing small caliber axons (Dong et al., 1995; Garratt et al., 2000a; Garratt et al., 2000b; Lyons et al., 2005; Michailov et al., 2004; Morrissey et al., 1995; Raphael et al., 2011; Riethmacher et al., 1997; Taveggia et al., 2005; Woldeyesus et al., 1999).
Combined, the zebrafish and mammalian studies have revealed roles for Nrg1-ErbB signaling in migration, survival, proliferation, Remak bundle formation, radial sorting, and myelination (Birchmeier and Nave, 2008; Dong et al., 1995; Garratt et al., 2000b; Lyons et al., 2005; Michailov et al., 2004; Morrissey et al., 1995; Raphael et al., 2011; Riethmacher et al., 1997; Taveggia et al., 2005; Woldeyesus et al., 1999). Future investigation is required to understand how one signal controls so many different aspects of Schwann cell development, but important factors likely include the concentration and source of the ligand, the developmental stage of the Schwann cell receiving the signal, and process specific downstream factors, such as Calcineurin/NFAT, PI3K/Akt, MEK/ERK, Shp2, and Cdc42 (Benninger et al., 2007; Cotter et al., 2010; Goebbels et al., 2010; Grossmann et al., 2009; Kao et al., 2009; Newbern and Birchmeier, 2010; Ogata et al., 2004; Syed et al., 2010).
Gpr126
In addition to finding new roles for genes initially characterized in mammals, zebrafish screens have also uncovered novel genes that regulate myelin formation. A screen for mutants with abnormal expression of mbp identified two mutations in gpr126 (Monk et al., 2009; Pogoda et al., 2006). Gpr126 is an orphan member of the adhesion subfamily of GPCRs, which are characterized by long, extracellular segments N-terminal to the 7-pass transmembrane domain (Bjarnadottir et al., 2004; Fredriksson et al., 2003). In gpr126 mutants, expression of the early Schwann cell marker sox10 is normal but markers of later stages are significantly reduced, including the promyelinating genes oct6 and krox20 (Monk et al., 2009). These marker studies suggested that gpr126 is dispensable for early stages of Schwann cell development, but essential for the onset of myelination. Transmission electron microscopy analysis revealed that Schwann cells arrest at the promyelinating stage in gpr126 mutants, with no more than one and a half wraps of Schwann cell cytoplasm around axons in peripheral nerves. This phenotype is reminiscent of krox20 mutants in both mammals and zebrafish, where mutant Schwann cells also arrest with only one and a half wraps of cytoplasm around an axon (Monk et al., 2009; Topilko et al., 1994). It had been known for many years that cAMP is an important second messenger during myelination and that the addition of forskolin, which elevates cAMP, could initiate myelination in cultured Schwann cells and mimic the presence of an axon (Jessen et al., 1991; Monuki et al., 1989). The endogenous regulation of cAMP, however, was not understood. Many G-protein coupled receptors signal through cAMP (Jalink and Moolenaar, 2010), raising the possibility that Gpr126 activates myelination by elevating levels of cAMP. Application of forskolin rescues myelination in gpr126 mutants, but not in krox20 mutants (Monk et al., 2009). These results support the possibility that Gpr126 acts upstream of cAMP, so that adding forskolin to artificially increase cAMP bypasses the requirement for Gpr126 (Monk et al., 2009). In contrast, krox20 is downstream of cAMP signaling, and these mutants cannot be rescued by elevating the levels of cAMP (Monk et al., 2009). Future studies are required to investigate the pathway downstream of gpr126, the interaction of gpr126 and other key regulators such as Nrg1, and the identity of ligands that may activate gpr126.
Characterization of a gpr126 mutant mouse revealed conservation of its function in myelination, and also new roles for gpr126 in the regulation of other aspects of peripheral nerve development (Monk et al., in press). gpr126 mutant mice have severe congenital hypomyelinating peripheral neuropathy. Similar to the situation in zebrafish, mouse gpr126 mutants have decreased expression of oct6, krox20, and mbp, and the mutant Schwann cells arrest at the promyelinating stage (Monk et al., 2009; Monk et al., in press). The analysis also revealed that there is a delay in radial sorting and a loss of Remak bundles, and thus non-myelinating Schwann cells (Monk et al., in press). Additionally, ectopic perineurial fibroblasts were found inappropriately within the nerve and these fibroblasts segregated the axon fibers in small bundles, forming “minifascicles.” Several aspects of the gpr126 mutant phenotype are similar to mutants for Adam22 and lgi4/claw paw, which also have Schwann cells arrested at the promyelinating stage and axons organized into minifascicles (Darbas et al., 2004; Henry et al., 1991; Nishino et al., 2010; Ozkaynak et al., 2010). Adam22 binds Lgi4, and it is possible that the functions of these proteins are in some way related to Gpr126.
New Roles for Schwann Cells
Mutations in genes that have critical functions in Schwann cells, including erbb2, erbb3, and sox10, have led to important progress in the understanding of the pathways that regulate Schwann cell development. In addition, these mutants have provided useful tools to analyze roles of Schwann cells in peripheral nerve development. The study of zebrafish mutants lacking Schwann cells has defined several new roles for these cells, including preventing the ectopic accumulation of axonal sodium channels in internodal axonal segments, preventing the premature differentiation of sensory organs, and in the proper fasciculation of the nerve (Gilmour et al., 2002; Grant et al., 2005; Voas et al., 2009). Additionally, more recent studies have uncovered roles for Schwann cells in preventing oligodendrocytes from improperly exiting the spinal cord, and repositioning a peripheral nerve across a basement membrane (Kucenas et al., 2009; Raphael et al., 2010).
Timelapse imaging of oligodendrocytes in the absence of Schwann cells revealed that oligodendrocytes can inappropriately exit the spinal cord in the absence of Schwann cells, suggesting that Schwann cells are required to keep oligodendrocytes from crossing the nerve root transition zones between the PNS and CNS (Kucenas et al., 2009). Normally, only axons cross these zones, either exiting or entering the spinal cord, with oligodendrocytes forming a heminode of myelin on the CNS side and Schwann cells forming one on the PNS side (Fraher, 1999). Extensive time-lapse imaging revealed that in the absence of Schwann cells, oligodendrocyte processes project out of the spinal cord along the motor axons, and the cell bodies ultimately follow (Kucenas et al., 2009). These data support the idea that the glial cells themselves regulate the transition zones and that in the absence of Schwann cells, oligodendrocytes are free to exit the spinal cord and myelinate peripheral axons.
Two recent studies in mammals also investigated the role of Schwann cells in restricting the migration of oligodendrocytes in peripheral nerves, suggesting that Schwann cells must progress beyond the promyelinating stage to limit ectopic migration of oligodendrocytes. In krox20 mutant mice, where Schwann cells are arrested at the promyelinating stage, oligodendrocytes exited the CNS and enter peripheral nerves (Coulpier et al., 2010). Similarly, in the study of the gpr126 mutant mouse described above, oligodendrocytes were expanded into peripheral territory at the transition zone of the auditory nerve, despite the presence of Schwann cells arrested at the promyelinating stage (Monk et al., in press). Interestingly, oligodendrocytes did not extend into the periphery in TremblerJ/PMP20 mutant mice, which express Krox20 but lack PNS myelination (Coulpier et al., 2010). This expansion of CNS glia into the PNS has also been observed in a human patient with congenital amyelinating neuropathy, which is characterized by deficits in Krox20 protein in Schwann cells (Coulpier et al., 2011). These results suggest that Krox20, downstream of Gpr126, may play a role in mediating the transition zone and that oligodendrocytes may be sensitive to the myelination state of adjoining Schwann cells.
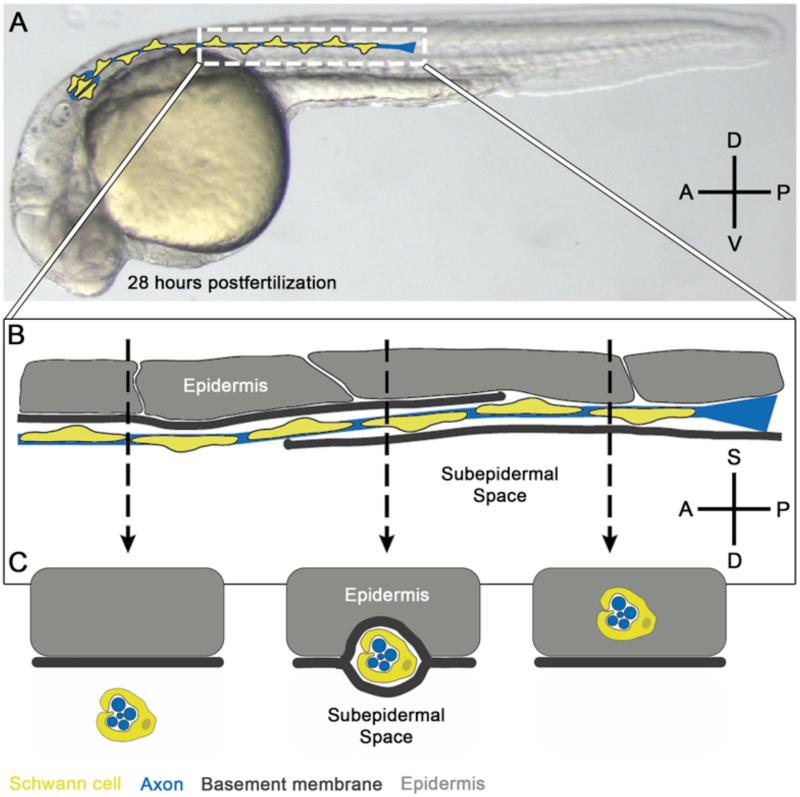
A new role for Schwann cells in the repositioning of a peripheral nerve has recently been described in zebrafish (Raphael et al., 2010). The posterior lateral line nerve innervates sensory organs that sense changes in water currents and, in larvae and adults resides just below the basement membrane of the epidermis (Ghysen and Dambly-Chaudiere, 2007; Raphael et al., 2010; Winklbauer, 1989). Recent work revealed that the nerve (both axons and Schwann cells) initially grows within the epidermis, and then rapidly transitions across the epidermal basement membrane to its mature location in the subepidermal space (Figure 2; Raphael et al., 2010). Schwann cells are required for this process, as mutants lacking Schwann cells have the nerve improperly located within the epidermis, and transplantation of wildtype Schwann cells into these mutants is sufficient to restore the nerve to its correct position. Significant defects arise when the posterior lateral line nerve is mislocalized in the epidermis, including defasciculation of the nerve and mispositioning of the nerve along the dorsal-ventral axis. This is apparently the result of the nerve being pulled by the ventrally migrating sensory organs that it innervates. In wildtype animals, the epidermal basement membrane separates the main body of the nerve from the sensory organs; in mutants lacking Schwann cells, however, the nerve remains improperly located within the epidermis in close contact with its target sensory organs. This anatomical organization, with a sensory organ within an epidermal layer and the main nerve fascicle is located below a basement membrane, also occurs in many sensory tissues including the tongue, skin, nasal epithelium, and vestibular organ (Boulais and Misery, 2008; Fernandez et al., 1990; Nedelec et al., 2005; Northcutt, 2004; Oakley and Witt, 2004; Purcell and Perachio, 1997; Si et al., 2003), suggesting that this may be a conserved method of protecting axons that do not easily regenerate from the remodeling or frequent turnover of their targets.
Figure 2. Schwann cells are required for repositioning a peripheral nerve.
(A) The posterior lateral line nerve in cartoon superimposed on a 28 hours postfertilization zebrafish embryo. Schwann cells (yellow) co-migrate with axons (blue; Gilmour et al., 2002). Anterior (A) is to the left, posterior (P) to the right, dorsal (D) up, ventral down (v). (B) Zoom of dashed region in (A), showing the transition of the posterior lateral line nerve across the epidermal basement membrane (dark grey) from the epidermis (light grey) into the subepidermal space (unlabeled), with anterior-most portions of the nerve transitioning prior to posterior portions closer to the outgrowing axonal growth cones (Raphael et al., 2010). Dashed lines indicate cross sections shown in (C). (C) Cross sections through the posterior lateral line nerve showing the position of the nerve with respect to the basement membrane (Raphael et al., 2010). Anterior (far left) the nerve has transitioned across the basement membrane and is embedded in the subepidermal space. Middle, the nerve is transitioning, with basement membrane both superficial and medial to the nerve. Posterior (far right) the nerve is still within the epidermis, superficial to the basement membrane. In (C) superficial is up (S), deep (D) down, anterior left, posterior right. (A, B) adapted from Raphael et al., 2010.
Oligodendrocytes
While this review has focused primarily on Schwann cell development, advances have also been made in the understanding of oligodendrocyte development. Forward genetic screens have uncovered new genes required during oligodendrocyte development and myelination, while detailed timelapse imaging studies have elucidated the complex behavior of oligodendrocytes in vivo (Kirby et al., 2006; Larson et al., 2010; Lyons et al., 2009; Parichy and Turner, 2003; Parichy et al., 2003; Pogoda et al., 2006; Takada et al., 2010). For example, two genes required for oligodendrocyte development that came out of forward genetic screens are kif1b and tuba8l3a – a kinesin molecular motor and a tubulin gene (Larson et al., 2010; Lyons et al., 2009; Parichy and Turner, 2003; Parichy et al., 2003). Both mutants have defects in the localization of myelin specific mRNAs in glial processes. Furthermore, kif1b mutant oligodendrocytes have inappropriate, myelin-like membrane compaction in proximal processes and around the cell body, supporting the possibility that myelin mRNA localization within oligodendrocyte distal processes prevents ectopic membrane compaction in other parts of the cell (Lyons et al. 2009). In vivo timelapse imaging studies have also revealed new interactions between oligodendrocytes – oligodendrocyte precursor cells (OPCs) actively repel each other through contact inhibition (Kirby et al., 2006), presumably to ensure the proper spacing of oligodendrocytes throughout the CNS. Many questions about oligodendrocyte development remain unanswered, including the axonal signals that regulate oligodendrocytes, the cell-cell contact signals that OPCs use to repulse each other, and the cues that coordinate the organization of the cytoskeleton to make myelin. Future studies combining genetics with in vivo imaging studies in zebrafish will address these questions.
Conclusions and Future Directions
The zebrafish is a genetically tractable vertebrate model organism that is now being used to study the development of myelinating glial cells. Many of the genes required for myelination are conserved from zebrafish to mammals. Recent studies in zebrafish have uncovered new roles for previously known myelin genes and have found new genes that regulate Schwann cell and oligodendrocyte development. In vivo imaging is being used to study the behavior of developing Schwann cells and oligodendrocytes; advances in imaging techniques may soon allow for the study of glial cells in adult zebrafish (Blackburn et al., 2011). Future studies will combine these powerful techniques to do high throughput screening using in vivo imaging – automated in vivo screening was used in a small molecule screen described in Box 1 (Buckley et al., 2010). Improvements in sequencing techniques could be used as a screening method as well – mutations could be chemically induced and then the genome of the mutagenized fish would be sequenced looking for mutations in every single gene. This would rapidly generate many thousands of mutants and once mutations were found they could then be phenotypically assayed. Zebrafish will continue to contribute important information to the myelin field, as new forward genetic and small molecule screens uncover more myelin genes and pathways and in vivo imaging continues to improve.
Box 1. Experimental advantages of the zebrafish model system.
The zebrafish model system offers several experimental advantages that facilitate the investigation of myelination and other aspects of vertebrate biology.
Forward Genetic Screens
Forward genetic screens in zebrafish are a standard tool in the field, using either chemicals or insertional mutagenesis (Amsterdam and Hopkins, 2006). Two forward genetic screens for myelin mutants have been reported – a screen for defects in the expression of mbp mRNA that led to the discovery of the erbb2, erbb3, and gpr126 mutants discussed in the main text and a “shelf” screen, where known mutants with neural defects were similarly screened, that found four mutants, neckless, motionless, iguana, and doc, required for myelination (Kazakova et al., 2006; Pogoda et al., 2006). Forward genetic screens are unbiased in that they are not looking for a specific gene, but rather are looking for a specific phenotype that defines gene function. Additionally, because current screens have not yet reached saturation, future screening for myelin mutants will yield new genes (Pogoda et al., 2006). Finally, with improvements in the zebrafish genome and rapid advances in sequencing technologies, positional cloning of mutated genes has become much easier and faster in recent years.
Reverse Genetics: Morpholinos, TILLING, and Zinc Fingers
Zebrafish also offers several approaches to study the function of a particular gene of interest, such as a human disease gene, in a developmental context. Overexpression of genes can be achieved by simply injecting RNA into the embryo or by generating transgenic fish with the Tol2 vector system (Kawakami, 2007; Xu et al., 2008). Knockdown of a gene can be performed with antisense morpholino oligonucleotides that block either mRNA splicing or translation (Shestopalov and Chen, 2010). Two methods are available to generate a mutation in a gene of interest. TILLING (targeting induced local lesions in genomes) works by rapidly screening the sequences of many chemically generated mutations to find a mutation in the gene of interest (Stemple 2004). For example, the mutation in zebrafish krox20 was generated using TILLING (Monk et al., 2009). By contrast, zinc finger mutagenesis targets a gene of interest using zinc finger nucleases that generate targeted double stranded breaks; loss-of-function mutations often result from imprecise repair of these breaks (Doyon et al., 2008; Meng et al., 2008).
Small Molecules
Small molecules have been used extensively in zebrafish to activate or inactivate different signaling pathways (e.g. AG1478 and forskolin described in main text; Lyons et al., 2005; Monk et al., 2009; Raphael et al., 2011). Additionally, small molecule screens have been conducted to look for compounds with certain activities in vivo – these screens have uncovered compounds that rescue a cardiac defect, protect hair cells, and have behavioral effects, among others (Ou et al., 2009; Peterson et al., 2004; Rihel et al., 2010). A recent small molecule screen combined in vivo imaging of oligodendrocytes precursor cells with high-throughput screening; several compounds were found that affected the number of olig2:EGPF expressing oligodendrocyte precursor cells, or the amount of mbp expression in the spinal cord (Buckley et al., 2010). Small molecule screens for compounds that ameliorate myelin-related mutant phenotypes could be an approach toward strategies for therapeutic remyelination.
In vivo imaging
Several examples in the main text demonstrate power of in vivo imaging for studying glial development in zebrafish. Future studies will take advantage of in vivo imaging to generate better probes to watch the localization of molecules during myelination and to follow the behavior of individual cells (Yoo et al., 2010). A recent study used “brainbow” to label different neurons in the zebrafish brain with different combinations of red, green, and blue fluorescent proteins – this technique could adapted to address questions in myelination and many other areas (Pan et al., 2011). Additionally, new microscopic techniques, such as “Selective Plane Illumination Microscopy” (SPIM), are allowing for even more detailed in vivo imaging studies (Arrenberg et al., 2010; Huisken and Stainier, 2009). New imaging methods and transgenic reporter systems with continue to exploit the optical clarity of the zebrafish embryo.
Acknowledgements
We thank Julie Perlin, Kelly Monk, and David Lyons for helpful comments on the manuscript. ARR is supported by a Stanford Graduate Fellowship and an NIH training grant. W.S.T. is supported by NIH grant NS050223.
References
- Aguayo AJ, Bray GM. Experimental pathology of unmyelianted fibers. In: Dyck PJ, Thomas PK, Lambert EH, editors. Peripheral Neuropathy. W. B. Saunders Co.; Philadelphia: 1975. [Google Scholar]
- Aguayo AJ, Bray GM, Terry LC, Sweezey E. Three dimensional analysis of unmyelinated fibers in normal and pathologic autonomic nerves. J Neuropathol Exp Neurol. 1976;35:136–151. doi: 10.1097/00005072-197603000-00002. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Hopkins N. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 2006;22:473–478. doi: 10.1016/j.tig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Arrenberg AB, Stainier DY, Baier H, Huisken J. Optogenetic control of cardiac function. Science. 2010;330:971–974. doi: 10.1126/science.1195929. [DOI] [PubMed] [Google Scholar]
- Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave K-A, Franklin RJM, Meijer D, Brakebusch C, Suter U, Relvas JB. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J Cell Biol. 2007;177:1051–1061. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger P, Niemann A, Suter U. Schwann cells and the pathogenesis of inherited motor and sensory neuropathies (Charcot-Marie-Tooth disease) Glia. 2006;54:243–257. doi: 10.1002/glia.20386. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Nave KA. Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia. 2008;56:1491–1497. doi: 10.1002/glia.20753. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir TK, Fredriksson R, Hoglund PJ, Gloriam DE, Lagerstrom MC, Schioth HB. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics. 2004;84:23–33. doi: 10.1016/j.ygeno.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Blackburn JS, Liu S, Raimondi AR, Ignatius MS, Salthouse CD, Langenau DM. High-throughput imaging of adult fluorescent zebrafish with an LED fluorescence macroscope. Nat Protoc. 2011;6:229–241. doi: 10.1038/nprot.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulais N, Misery L. The epidermis: a sensory tissue. Eur J Dermatol. 2008;18:119–127. doi: 10.1684/ejd.2008.0348. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes & development. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CE, Marguerie A, Roach AG, Goldsmith P, Fleming A, Alderton WK, Franklin RJ. Drug reprofiling using zebrafish identifies novel compounds with potential pro-myelination effects. Neuropharmacology. 2010;59:149–159. doi: 10.1016/j.neuropharm.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Bunge RP. Glial cells and the central myelin sheath. Physiol Rev. 1968;48:197–251. doi: 10.1152/physrev.1968.48.1.197. [DOI] [PubMed] [Google Scholar]
- Chen S, Velardez MO, Warot X, Yu Z-X, Miller SJ, Cros D, Corfas G. Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-L, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol. 2003;163:889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Cotter L, Ozcelik M, Jacob C, Pereira JA, Locher V, Baumann R, Relvas JB, Suter U, Tricaud N. Dlg1-PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve overmyelination. Science. 2010;328:1415–1418. doi: 10.1126/science.1187735. [DOI] [PubMed] [Google Scholar]
- Coulpier F, Decker L, Funalot B, Vallat JM, Garcia-Bragado F, Charnay P, Topilko P. CNS/PNS boundary transgression by central glia in the absence of Schwann cells or Krox20/Egr2 function. J Neurosci. 2010;30:5958–5967. doi: 10.1523/JNEUROSCI.0017-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulpier F, Decker L, Funalot B, Vallat JM, Garcia-Bragado F, Charnay P, Topilko P. Krox20 inactivation in the PNS leads to CNS/PNS boundary transgression by central glia. Revue neurologique. 2011;167:51–56. doi: 10.1016/j.neurol.2010.07.043. [DOI] [PubMed] [Google Scholar]
- Darbas A, Jaegle M, Walbeehm E, van den Burg H, Driegen S, Broos L, Uyl M, Visser P, Grosveld F, Meijer D. Cell autonomy of the mouse claw paw mutation. Dev Biol. 2004;272:470–482. doi: 10.1016/j.ydbio.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Dong Z, Sinanan A, Parkinson D, Parmantier E, Mirsky R, Jessen KR. Schwann cell development in embryonic mouse nerves. J Neurosci Res. 1999;56:334–348. doi: 10.1002/(SICI)1097-4547(19990515)56:4<334::AID-JNR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, Amora R, Hocking TD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Amacher SL. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- Feltri ML, Graus Porta D, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, Mueller U, Wrabetz L. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156:199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol. 1990;63:767–780. doi: 10.1152/jn.1990.63.4.767. [DOI] [PubMed] [Google Scholar]
- Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm ER, Bösl MR, Meijer D, Wegner M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol. 2010;189:701–712. doi: 10.1083/jcb.200912142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraher JP. The transitional zone and CNS regeneration. J Anat. 1999;194:161–182. doi: 10.1046/j.1469-7580.1999.19420161.x. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R, Gloriam DE, Hoglund PJ, Lagerstrom MC, Schioth HB. There exist at least 30 human G-protein-coupled receptors with long Ser/Thr-rich N-termini. Biochemical and biophysical research communications. 2003;301:725–734. doi: 10.1016/s0006-291x(03)00026-3. [DOI] [PubMed] [Google Scholar]
- Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a schwann cell. Bioessays. 2000a;22:987–996. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C. A dual role of erbB2 in myelination and in expansion of the schwann cell precursor pool. J Cell Biol. 2000b;148:1035–1046. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geren BB, Raskind J. Development of the Fine Structure of the Myelin Sheath in Sciatic Nerves of Chick Embryos. Proc Natl Acad Sci U S A. 1953;39:880–884. doi: 10.1073/pnas.39.8.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudiere C. The lateral line microcosmos. Genes Dev. 2007;21:2118–2130. doi: 10.1101/gad.1568407. [DOI] [PubMed] [Google Scholar]
- Gilmour DT, Maischein HM, Nusslein-Volhard C. Migration and function of a glial subtype in the vertebrate peripheral nervous system. Neuron. 2002;34:577–588. doi: 10.1016/s0896-6273(02)00683-9. [DOI] [PubMed] [Google Scholar]
- Goebbels S, Oltrogge JH, Kemper R, Heilmann I, Bormuth I, Wolfer S, Wichert SP, Möbius W, Liu X, Lappe-Siefke C, Rossner MJ, Groszer M, Suter U, Frahm J, Boretius S, Nave K-A. Elevated phosphatidylinositol 3,4,5-trisphosphate in glia triggers cell-autonomous membrane wrapping and myelination. J Neurosci. 2010;30:8953–8964. doi: 10.1523/JNEUROSCI.0219-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Raible DW, Piotrowski T. Regulation of latent sensory hair cell precursors by glia in the zebrafish lateral line. Neuron. 2005;45:69–80. doi: 10.1016/j.neuron.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Grossmann KS, Wende H, Paul FE, Cheret C, Garratt AN, Zurborg S, Feinberg K, Besser D, Schulz H, Peles E, Selbach M, Birchmeier W, Birchmeier C. The tyrosine phosphatase Shp2 (PTPN11) directs Neuregulin-1/ErbB signaling throughout Schwann cell development. Proc Natl Acad Sci USA. 2009;106:16704–16709. doi: 10.1073/pnas.0904336106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M, Komiyama NH, Nave K-A, Grant SG, Sherman DL, Brophy PJ. FAK is required for axonal sorting by Schwann cells. J Cell Biol. 2007;176:277–282. doi: 10.1083/jcb.200609021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn AF, Chang Y, Webster HD. Development of myelinated nerve fibers in the sixth cranial nerve of the rat: a quantitative electron microscope study. J Comp Neurol. 1987;260:491–500. doi: 10.1002/cne.902600403. [DOI] [PubMed] [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Current biology : CB. 2007;17:R29–35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Henry EW, Eicher EM, Sidman RL. The mouse mutation claw paw: forelimb deformity and delayed myelination throughout the peripheral nervous system. J Hered. 1991;82:287–294. doi: 10.1093/oxfordjournals.jhered.a111088. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL. The Ionic Basis of Nervous Conduction. Science. 1964;145:1148–1154. doi: 10.1126/science.145.3637.1148. [DOI] [PubMed] [Google Scholar]
- Huisken J, Stainier DY. Selective plane illumination microscopy techniques in developmental biology. Development. 2009;136:1963–1975. doi: 10.1242/dev.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K, Moolenaar WH. G protein-coupled receptors: the inside story. BioEssays : news and reviews in molecular, cellular and developmental biology. 2010;32:13–16. doi: 10.1002/bies.200900153. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Brennan A, Morgan L, Mirsky R, Kent A, Hashimoto Y, Gavrilovic J. The Schwann cell precursor and its fate: a study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron. 1994;12:509–527. doi: 10.1016/0896-6273(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R, Morgan L. Role of cyclic AMP and proliferation controls in Schwann cell differentiation. Ann N Y Acad Sci. 1991;633:78–89. doi: 10.1111/j.1749-6632.1991.tb15597.x. [DOI] [PubMed] [Google Scholar]
- Kao SC, Wu H, Xie J, Chang CP, Ranish JA, Graef IA, Crabtree GR. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science. 2009;323:651–654. doi: 10.1126/science.1166562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K. Tol2: a versatile gene transfer vector in vertebrates. Genome biology. 2007;8(Suppl 1):S7. doi: 10.1186/gb-2007-8-s1-s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakova N, Li H, Mora A, Jessen KR, Mirsky R, Richardson WD, Smith HK. A screen for mutations in zebrafish that affect myelin gene expression in Schwann cells and oligodendrocytes. Dev Biol. 2006;297:1–13. doi: 10.1016/j.ydbio.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci. 2006;9:1506–1511. doi: 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- Kucenas S, Wang WD, Knapik EW, Appel B. A selective glial barrier at motor axon exit points prevents oligodendrocyte migration from the spinal cord. J Neurosci. 2009;29:15187–15194. doi: 10.1523/JNEUROSCI.4193-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson TA, Gordon TN, Lau HE, Parichy DM. Defective adult oligodendrocyte and Schwann cell development, pigment pattern, and craniofacial morphology in puma mutant zebrafish having an alpha tubulin mutation. Dev Biol. 2010;346:296–309. doi: 10.1016/j.ydbio.2010.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DA, Naylor SG, Scholze A, Talbot WS. Kif1b is essential for mRNA localization in oligodendrocytes and development of myelinated axons. Nat Genet. 2009;41:854–858. doi: 10.1038/ng.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DA, Pogoda H-M, Voas MG, Woods IG, Diamond B, Nix R, Arana N, Jacobs J, Talbot WS. erbb3 and erbb2 are essential for schwann cell migration and myelination in zebrafish. Curr Biol. 2005;15:513–524. doi: 10.1016/j.cub.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Martin JR, Webster HD. Mitotic Schwann cells in developing nerve: their changes in shape, fine structure, and axon relationships. Dev Biol. 1973;32:417–431. doi: 10.1016/0012-1606(73)90251-0. [DOI] [PubMed] [Google Scholar]
- McQualter JL, Bernard CC. Multiple sclerosis: a battle between destruction and repair. J Neurochem. 2007;100:295–306. doi: 10.1111/j.1471-4159.2006.04232.x. [DOI] [PubMed] [Google Scholar]
- Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave K-A. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monuki ES, Weinmaster G, Kuhn R, Lemke G. SCIP: a glial POU domain gene regulated by cyclic AMP. Neuron. 1989;3:783–793. doi: 10.1016/0896-6273(89)90247-x. [DOI] [PubMed] [Google Scholar]
- Morrissey TK, Levi AD, Nuijens A, Sliwkowski MX, Bunge RP. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc Natl Acad Sci U S A. 1995;92:1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K-A, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Nedelec S, Dubacq C, Trembleau A. Morphological and molecular features of the mammalian olfactory sensory neuron axons: What makes these axons so special? J Neurocytol. 2005;34:49–64. doi: 10.1007/s11068-005-5047-7. [DOI] [PubMed] [Google Scholar]
- Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin Cell Dev Biol. 2010;21:922–928. doi: 10.1016/j.semcdb.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino J, Saunders TL, Sagane K, Morrison SJ. Lgi4 promotes the proliferation and differentiation of glial lineage cells throughout the developing peripheral nervous system. J Neurosci. 2010;30:15228–15240. doi: 10.1523/JNEUROSCI.2286-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari A, Zambroni D, Quattrini A, Court FA, D'Urso A, Recchia A, Tybulewicz VLJ, Wrabetz L, Feltri ML. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177:1063–1075. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt RG. Taste buds: development and evolution. Brain Behav Evol. 2004;64:198–206. doi: 10.1159/000079747. [DOI] [PubMed] [Google Scholar]
- Oakley B, Witt M. Building sensory receptors on the tongue. J Neurocytol. 2004;33:631–646. doi: 10.1007/s11068-005-3332-0. [DOI] [PubMed] [Google Scholar]
- Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, Nakamura K, Tanaka S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci. 2004;24:6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Cunningham LL, Francis SP, Brandon CS, Simon JA, Raible DW, Rubel EW. Identification of FDA-approved drugs and bioactives that protect hair cells in the zebrafish (Danio rerio) lateral line and mouse (Mus musculus) utricle. J Assoc Res Otolaryngol. 2009;10:191–203. doi: 10.1007/s10162-009-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E, Abello G, Jaegle M, van Berge L, Hamer D, Kegel L, Driegen S, Sagane K, Bermingham JR, Jr., Meijer D. Adam22 is a major neuronal receptor for Lgi4-mediated Schwann cell signaling. J Neurosci. 2010;30:3857–3864. doi: 10.1523/JNEUROSCI.6287-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YA, Livet J, Sanes JR, Lichtman JW, Schier AF. Multicolor brainbow imaging in zebrafish. Cold Spring Harb Protoc. 2011;2011 doi: 10.1101/pdb.prot5546. pdb prot5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parichy DM, Turner JM. Zebrafish puma mutant decouples pigment pattern and somatic metamorphosis. Dev Biol. 2003;256:242–257. doi: 10.1016/s0012-1606(03)00015-0. [DOI] [PubMed] [Google Scholar]
- Parichy DM, Turner JM, Parker NB. Essential role for puma in development of postembryonic neural crest-derived cell lineages in zebrafish. Dev Biol. 2003;256:221–241. doi: 10.1016/s0012-1606(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Pereira JA, Benninger Y, Baumann R, Gonçalves AF, Ozçelik M, Thurnherr T, Tricaud N, Meijer D, Fässler R, Suter U, Relvas JB. Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J Cell Biol. 2009;185:147–161. doi: 10.1083/jcb.200809008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. Observations on the Connexions between Myelin Sheaths and Glial Cells in the Optic Nerves of Young Rats. Journal of anatomy. 1964;98:125–134. [PMC free article] [PubMed] [Google Scholar]
- Peters A, Muir AR. The relationship between axons and Schwann cells during development of peripheral nerves in the rat. Q J Exp Physiol Cogn Med Sci. 1959;44:117–130. doi: 10.1113/expphysiol.1959.sp001366. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Shaw SY, Peterson TA, Milan DJ, Zhong TP, Schreiber SL, MacRae CA, Fishman MC. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol. 2004;22:595–599. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- Pogoda HM, Sternheim N, Lyons DA, Diamond B, Hawkins TA, Woods IG, Bhatt DH, Franzini-Armstrong C, Dominguez C, Arana N, Jacobs J, Nix R, Fetcho JR, Talbot WS. A genetic screen identifies genes essential for development of myelinated axons in zebrafish. Dev Biol. 2006;298:118–131. doi: 10.1016/j.ydbio.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Purcell IM, Perachio AA. Three-dimensional analysis of vestibular efferent neurons innervating semicircular canals of the gerbil. J Neurophysiol. 1997;78:3234–3248. doi: 10.1152/jn.1997.78.6.3234. [DOI] [PubMed] [Google Scholar]
- Raphael AR, Lyons DA, Talbot WS. ErbB signaling has a role in radial sorting independent of Schwann cell number. Glia. 2011 doi: 10.1002/glia.21175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael AR, Lyons DA, Talbot WS. In submission.
- Raphael AR, Perlin JR, Talbot WS. Schwann cells reposition a peripheral nerve to isolate it from postembryonic remodeling of its targets. Development. 2010;137:3643–3649. doi: 10.1242/dev.057521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, Haggarty SJ, Kokel D, Rubin LL, Peterson RT, Schier AF. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science. 2010;327:348–351. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner M. Snapshot: neural crest. Cell. 2010;143:486–486. doi: 10.1016/j.cell.2010.10.025. e481. [DOI] [PubMed] [Google Scholar]
- Schreiner S, Cossais F, Fischer K, Scholz S, Bosl MR, Holtmann B, Sendtner M, Wegner M. Hypomorphic Sox10 alleles reveal novel protein functions and unravel developmental differences in glial lineages. Development. 2007;134:3271–3281. doi: 10.1242/dev.003350. [DOI] [PubMed] [Google Scholar]
- Shestopalov IA, Chen JK. Oligonucleotide-based tools for studying zebrafish development. Zebrafish. 2010;7:31–40. doi: 10.1089/zeb.2010.0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si X, Zakir MM, Dickman JD. Afferent innervation of the utricular macula in pigeons. J Neurophysiol. 2003;89:1660–1677. doi: 10.1152/jn.00690.2002. [DOI] [PubMed] [Google Scholar]
- Stampfli R. Saltatory conduction in nerve. Physiol Rev. 1954;34:101–112. doi: 10.1152/physrev.1954.34.1.101. [DOI] [PubMed] [Google Scholar]
- Svaren J, Meijer D. The molecular machinery of myelin gene transcription in Schwann cells. Glia. 2008;56:1541–1551. doi: 10.1002/glia.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed N, Reddy K, Yang DP, Taveggia C, Salzer JL, Maurel P, Kim HA. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J Neurosci. 2010;30:6122–6131. doi: 10.1523/JNEUROSCI.1681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada N, Kucenas S, Appel B. Sox10 is necessary for oligodendrocyte survival following axon wrapping. Glia. 2010;58:996–1006. doi: 10.1002/glia.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki I. Physiologic properties of the myelin sheath and of the node of Ranvier. Prog Neurobiol. 1959;4:159–172. [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voas MG, Glenn TD, Raphael AR, Talbot WS. Schwann cells inhibit ectopic clustering of axonal sodium channels. J Neurosci. 2009;29:14408–14414. doi: 10.1523/JNEUROSCI.0841-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster HD, Martin R, O'Connell MF. The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study. Dev Biol. 1973;32:401–416. doi: 10.1016/0012-1606(73)90250-9. [DOI] [PubMed] [Google Scholar]
- Winklbauer R. Development of the lateral line system in Xenopus. Prog Neurobiol. 1989;32:181–206. doi: 10.1016/0301-0082(89)90016-6. [DOI] [PubMed] [Google Scholar]
- Woldeyesus MT, Britsch S, Riethmacher D, Xu L, Sonnenberg-Riethmacher E, Abou-Rebyeh F, Harvey R, Caroni P, Birchmeier C. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 1999;13:2538–2548. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Stemple D, Joubin K. Microinjection and cell transplantation in zebrafish embryos. Methods in molecular biology. 2008;461:513–520. doi: 10.1007/978-1-60327-483-8_35. [DOI] [PubMed] [Google Scholar]
- Yoo SK, Deng Q, Cavnar PJ, Wu YI, Hahn KM, Huttenlocher A. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Developmental cell. 2010;18:226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W-M, Chen Z-L, North AJ, Strickland S. Laminin is required for Schwann cell morphogenesis. J Cell Sci. 2009;122:929–936. doi: 10.1242/jcs.033928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL. Schwann cell-specific ablation of laminin gamma1 causes apoptosis and prevents proliferation. J Neurosci. 2005;25:4463–4472. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanazzi G, Einheber S, Westreich R, Hannocks MJ, Bedell-Hogan D, Marchionni MA, Salzer JL. Glial growth factor/neuregulin inhibits Schwann cell myelination and induces demyelination. J Cell Biol. 2001;152:1289–1299. doi: 10.1083/jcb.152.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]