Abstract
Gross chromosomal rearrangements and aneuploidy are among the most common somatic genomic abnormalities that occur during cancer initiation and progression, in particular in human solid tumor carcinogenesis. The loss of large chromosomal regions as consequence of gross rearrangements (e.g. deletions, monosomies, unbalanced translocations and mitotic recombination) have been traditionally associated with the existence of tumor suppressor genes within the areas affected by the loss of genetic material. The long arm of chromosome 16 was identified as being frequently associated with structural abnormalities in multiple neoplasias, that led us to focus attention on the detailed genetic dissection of this region resulting in the cloning of the putative tumor suppressor gene, WWOX (WW domain containing Oxidoreductase). Interestingly, the WWOX gene resides in the very same region as that of the common chromosomal fragile site 16D (FRA16D). The WWOX gene encodes a protein that contains two WW domains, involved in protein-protein interactions, and a short chain dehydrogenase (SDR) domain, possibly involved in sex-steroid metabolism. We have identified the WWOX WW domain ligand as the PPXY motif confirming the biochemical activity of this domain. WWOX normally resides in the Golgi and we will demonstrate that Golgi localization requires an intact SDR. Inactivation of the WWOX gene during tumorigenesis can occur by homozygous deletions and possibly mutation, however, aberrantly spliced forms of WWOX mRNA have been observed even when one allele is still intact. The aberrantly spliced mRNAs have deletions of the exons that encode the SDR and these WWOX protein isoforms display abnormal intracellular localization to the nucleus possibly functioning as dominant negative inhibitors of full length WWOX. Thus, generation of aberrant transcripts of WWOX may represent a novel mechanism to functionally inactivate WWOX without genomic alteration of the remaining allele. In this article we will review the cloning and identification of WWOX as the target of FRA16D. In addition, we will discuss the possible biochemical functions of WWOX and present evidence that ectopic WWOX expression inhibits tumor growth.
Chromosome 16q arm LOH in cancer led to the cloning of WWOX
For several years our laboratory has been interested in defining the progression of chromosomal and allelotypic abnormalities in human breast cancer (Aldaz et al., 1995; Brenner and Aldaz, 1995; Charpentier and Aldaz, 2002; Chen et al., 1996). In early studies with human samples, in order to better understand the timing for presentation of allelic losses in breast carcinogenesis, we performed a comparative allelotypic study of pre-invasive stages of breast carcinoma versus invasive ductal carcinomas. We observed that chromosome 7p, 16q and 17p17q were frequently affected by LOH and allelic imbalances at pre-invasive stages of breast cancer progression (Aldaz et al., 1995). Other studies at the time and later also reached very similar conclusions in particular implicating chromosome arms 16q and 17p17q as early anomalies (Lakhani et al., 1995; Radford et al., 1995; Fujii et al., 1996; O’Connell et al., 1998). Losses affecting 16q and ch17 were observed early in ductal hyperplastic stages of pre-malignant tumor progression (Lakhani et al., 1995). All these studies also helped to settle the notion that atypical hyperplasias and ductal carcinoma in situ were true precursor lesions of invasive breast cancer, as previously proposed by Page and Dupont (1990). The information gained from these allelotypic studies was more recently further confirmed with other methodologies such as comparative genomic hybridization (CGH) (Lu et al., 1998; Buerger et al., 1999; Gong et al., 2001).
Interestingly, by means of conventional cytogenetics the long arm of chromosome 16 had already been suggested several years ago as a site for the occurrence of non-random primary structural abnormalities in breast cancer (Dutrillaux et al., 1990; Pandis et al., 1992). In particular, 16q was shown to participate systematically in nonrandom translocations with chromosome 1 and to show frequent deletions (Pandis et al., 1992). In order to refine the loci of interest we generated a high-resolution deletion map of ch16q in ductal carcinoma in situ, utilizing microdissected material from paraffin embedded samples (Chen et al., 1996). We identified three distinctive areas with high LOH. Two areas were previously identified by other groups 16q21 and 16q24.2→qter (Cleton-Jansen et al., 1994). However, the third was the most frequently affected area in our study spanning microsatellite markers D16S515–D16S504 with the highest incidence of LOH at D16S518 and we estimated this subregion lay in 16q23.3→q24.1 spanning approximately 2–3 Mb (Chen et al., 1996).
For the most proximal region, 16q22.1, it was proposed that the target of the losses could be the gene CADH1 (E-cadherin), a cellular adhesion molecule. However, only lobular breast carcinomas showed mutations affecting this gene and no evidence of mutations or homozygous losses were reported for the more common ductal carcinomas (Berx et al., 1995). Several other studies ruled out various candidate genes mapping more distally in ch16q as possible tumor suppressor genes (Crawford et al., 1999; Savino et al., 1999).
Further stimulating the search of the target gene(s) for LOH, was the fact that 16q and in particular the same sub-region we identified (16q23.3→q24.1) were also reported as affected by LOH in numerous other epithelial neoplasias such as: prostate cancer (Cher et al., 1995; Suzuki et al., 1996; Elo et al., 1999; Li et al., 1999), ovarian cancer (Iwabuchi et al., 1995; Kawakami et al., 1999), hepatocellular carcinomas (Nishida et al., 1992) and in Wilm’s tumor (Skotnicka-Klonowicz et al., 2000). Interestingly, in many of these tumor types the LOH of this specific region of 16q associated with a worse prognosis and lower survival (Elo et al., 1999; Li et al., 1999; Skotnicka-Klonowicz et al., 2000).
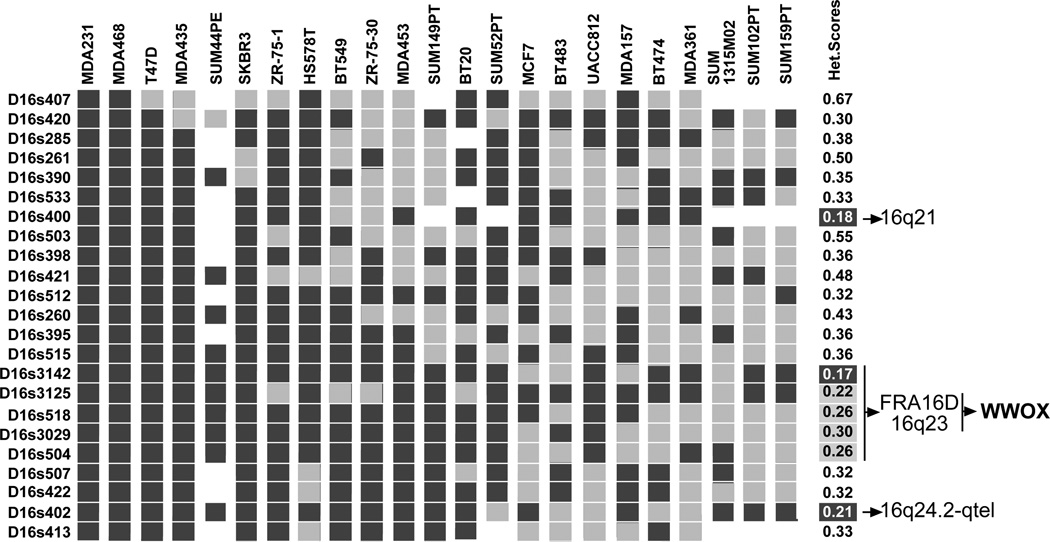
In our laboratory, we then launched a classical positional cloning project attempting to identify the gene(s) targeted by the high frequency of LOH observed at 16q23.3→q24.1 (the most highly affected area in our view). In one of our first studies, we performed a 16q allelotypic analysis using highly polymorphic markers (10.70 heterozygosity scores) on a panel of breast cancer lines attempting to identify regions of homozygous loss that could help us narrow down the region of interest (Bednarek et al., 2000). We basically confirmed the observations of our previous study identifying three distinctive regions in which the heterozygosity scores for various adjacent markers was drastically reduced from >10.70 to <0.17–0.21 indicating that very likely those regions represented areas of hemizygous loss rather than homozygosity. However, we failed to identify regions of homozygous loss. Nevertheless, we observed that many of the cell lines appeared to display hemizygosity affecting loss of the complete or most of the 16q arm (Fig. 1). We observed similar anomalies in the analysis of normal-tumor matched DCIS samples (Chen et al., 1996). This type of aberration is typical and usually the consequence of mitotic recombination events or loss of a complete chromosome arm as was suggested to occur frequently in 16q and possibly the main reason for LOH in this autosome (Gupta et al., 1997).
Fig. 1.
High resolution allelotype of chromosome 16 in breast cancer cell lines using highly polymorphic microsatellite markers. Markers arranged in mapping and linkage order as previously described (Chen et al., 1996). Dark gray blocks indicate that a single allele was observed at the corresponding locus (i.e. hemi or homozygosity), light gray blocks indicate heterozygosity is preserved. Blank blocks were not determined. Numbers at right represent the heterozygosity scores calculated at each specific locus from analyzing this breast cancer panel. Several breast cancer lines showed hemizygosity affecting all or most of the chromosome 16q arm. Areas of high LOH mapped to 16q21, 16q23 and 16q24.2. The most commonly affected area (16q23) was determined to be FRA16D and to contain the WWOX gene. The SUM breast cancer cell lines were a generous gift from Dr. Stephen Ethier (Department of Radiation Oncology, The University of Michigan Health System).
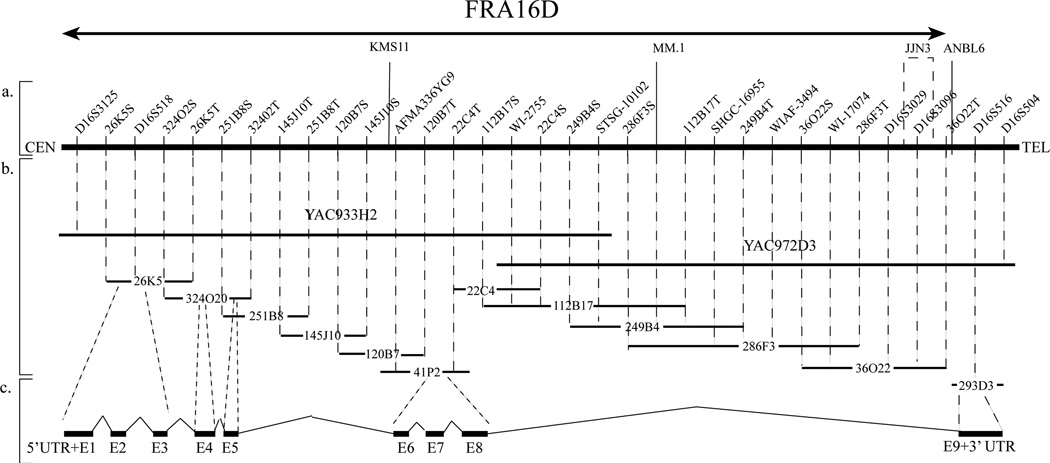
We identified only later that a breast cancer cell strain (1739) (obtained from the SW Medical Center, Dallas, gift of Dr. Lauren Gollahon) displayed a homozygous loss between STS markers 249B4S and D16S3029 (Bednarek et al., 2000). We used shotgun sequencing of this region and sequenced approximately 400,000 bp, including a continuous sequence of ~100,000 bp reported to GenBank (accession no. AF179633). We also followed a second approach, a solution hybridization cDNA capture method (Futreal et al., 1994). After sequencing and analyzing over 35 candidate cDNAs plus all candidate ESTs mapping to the region of interest we identified only one cDNA with evidence of exon-intron structure. We proceeded then to clone the complete cDNA and characterize the candidate gene (Bednarek et al., 2000) (Fig. 2).
Fig. 2.
Physical map of the 16q23.3 →q24.1 chromosome region containing WWOX. (a) STS markers are ordered from centromere (left) to telomere (right). The relative distance between STS markers is arbitrary. The nomenclature used for the STSs derived from the ends of specific BACs indicates first the BAC clone address followed by S or T (i.e. Sp6 or T7 vector sequencing primers). KMS11, MM.1, JJN3 and ANBL6 represent the location of four multiple myeloma translocation breakpoints t(14;16). (b) The isolated BACs and YACs used for this physical map are represented by horizontal solid black lanes (Bednarek et al., 2000). The size of the clones are not to scale. (average size of a BAC 150 kb). (c) Black rectangular boxes represent WWOX exons as numbered. The approximate position of each exon in each corresponding BAC is illustrated.
The WWOX gene encodes a 414 amino acid protein that was predicted to contain two distinct protein domains identified using the PROSITE database. The WWOX amino terminal 88 amino acids show high sequence conservation to the WW domain family of proteins and the carboxy terminal 326 amino acids are homologous to the short-chain dehydrogenase/ reductase (SDR) superfamily (Fig. 3). Therefore, we named the gene WWOX for WW domain containing Oxidoreductase.
Fig. 3.
WWOX amino acid sequence. WW domains are boxed and conserved tryptophans and prolines shown in bold. Note that in the second WW domain one tryptophan is replaced by a tyrosine (conservative change). The short chain dehydrogenase/reductase domain is underlined and the conserved motif YXXXK and S, characteristic of a substrate binding site are highlighted by black boxes. The motif GXXXGXG, typical of a coenzyme binding site (NADH or NADPH) is shown in bold italics. Leucine 291 is highlighted and the mutation to proline observed in an esophageal tumor is shown below the wildtype amino acid sequence.
WWOX, target of the second most common chromosomal fragile site, FRA16D
Mammalian chromosomes display regions of fragility where some are spontaneous and directly evident and others are disclosed upon treatment with specific chemicals or under specific culture conditions. These non-random regions prone to breakage are known as chromosomal fragile sites. They are specific loci susceptible to the spontaneous or induced occurrence of gaps, breaks, rearrangement and usually they are highly recombinogenic. There are two known classes of chromosomal fragile sites “rare” and “common”. The “rare” or heritable fragile sites are transmitted in a Mendelian codominant fashion, e.g. the FRAXA, and FRAXE associated with the mental retardation fragile X syndrome or FRA11B associated with Jacobsen syndrome. Rare fragile sites occur in less than 5% of the population and the mechanistic basis of their occurrence is usually associated with expansions of trinucleotide and minisatellite repeats. It was shown that they are not an in vitro artifact since they were clearly demonstrated to also occur in vivo (reviewed in Smith et al., 1998; Sutherland et al., 1998; Richards 2001). The “common” or constitutive chromosomal fragile sites are found in all individuals and are basically a constant chromosome feature. Most of these common sites become evident by exposure of cells to inhibitors of DNA replication such as aphidicolin (Glover et al., 1984). This compound inhibits DNA polymerases alpha and delta. A minority of common fragile sites can also be induced by exposure to 5-azacytidine or BrdU. The expression of common fragile sites can be further enhanced by exposure to caffeine. Approximately 80 common or constitutive chromosomal fragile sites have been described. However, by far the most frequent common fragile sites are predominantly FRA3B at 3p14.2 and FRA16D (16q23.3→q24.1) and with much less frequency FRA6E (6q26), FRA7H (7q32) and FRAXB (Xp22) (Smith et al., 1998).
The WWOX cloned gene spanned a huge genomic region of 1,113,822 bp in size (according to the human genome sequence data base) in the region 16q23.3→q24.1. It is composed of nine exons (Fig. 2). Interestingly even though the coding region itself was not large, introns 5 and 8 were very large. Intron 8 alone spans 779,639 bp. In addition, we detected from our sequencing and physical map of the region that both introns 5 and 8 harbored the sequences of previously reported translocation breakpoints in multiple myeloma. Within intron 8 we identified the translocation breakpoints MM.1, JJN3 and ANBL6 according to reported GenBank sequences (Fig. 2). These three multiple myeloma translocation breakpoints (and a fourth one KMS11 in intron 5) participated in translocations t(14;16)(q32;q23) (Chesi et al., 1998). Intron 8 also displayed repetitive sequences of various family types and we detected the presence of the sequence of a pseudogene for ribosomal protein S3 (Bednarek et al., 2000). These various unusual genomic characteristics plus the true physical mapping generated, led us to speculate that perhaps we had cloned the gene spanning the FRA16D. In fact, studies on the same region, based in part on the identification of STS markers of LOH, previously reported by us (Chen et al., 1996), were almost simultaneously reported confirming that this region was indeed that of FRA16D (Mangelsdorf et al., 2000; Paige et al., 2000). In those reports, however, they could not identify any particular gene but they observed that various tumor lines (ovary, lung, colon and gastric carcinoma) displayed homozygous deletions and instability in that specific area. Soon after our original report (Bednarek et al., 2000), Reid et al. (2000) cloned the same WWOX gene and named it FOR (fragile site FRA16D oxidoreductase). We also independently confirmed that WWOX mapped to FRA16D by hybridizing the YAC clone containing most of the coding region of WWOX to metaphase plates of cells treated with aphidicolin (Fig. 4). A more recent detailed physical map of the region by Krummel et al. (2000) also agreed with the conclusion that this region is indeed that of the FRA16D.
Fig. 4.
WWOX is the target FRA16D. Left panel: Representative normal human G-Banded metaphase plate of cells treated with aphidicolin in order to expose constitutive fragile sites. Arrows point to both chromosomes 16 with chromatid breaks at the 16q23 region (FRA16D). Right panel: same metaphase after chromosome in situ hybridization using as probe YAC933H2 (see Fig. 2). Red dots represent the region of hybridization right on top of the FRA16D region.
WWOX as a cancer-associated gene
A potential role for chromosomal fragile sites in cancer was suggested several years ago by Yunis and Soreng (1984). Since then the issue has been fairly controversial. The chromosomal regions spanned by common fragile sites have demonstrated associations with cancer. FRA3B occurs within a region deleted in multiple cancers, including balanced translocations affecting this locus in familial renal cancer t(3:8) (p14.2;q24). The gene spanning FRA3B was cloned and named FHIT (Fragile Histidine Triad protein) (Ohta et al., 1996). FRA6E occurs within a region frequently affected in ovarian and gastric cancer and FRA7H shows frequent loss in prostate, breast and pancreatic cancers (Smith et al., 1998).
It is hypothesized that the fragile sites predispose the chromosomes to breakage and since they contain highly recombinogenic sequences generate rearrangement and this constitutes the basis of the common alterations found in these various regions in cancer (Smith et al., 1998). A corollary of this hypothesis would be that such common fragility would affect the function of genes encoded in those chromosome regions and as a consequence alteration or inactivation of such genes may be of importance in cancer development or progression.
However, the main question that remains to be answered is: Are abnormalities affecting common fragile sites and their resident genes “Cause or Consequence” of tumor progression? One could argue, as was criticized for the case of FHIT, that abnormalities affecting this gene could be likely the consequence of FHIT residing in a region commonly affected by chromosomal rearrangements, rather than being the inactivation of such gene related to the origin of the cancer (Le Beau et al., 1998). Genes like FHIT, and for that matter, all the other genes residing in common fragile site regions, could just simply be innocent bystanders.
It is interesting to note that the findings of genomic and transcriptional abnormalities affecting WWOX the FRA16D gene, as discussed in previous and following sections are very similar to findings observed with FHIT, the FRA3B gene. Both genes span huge genomic regions, both genes are affected by translocations and homozygous deletions in particular affecting intron regions. In most cases of hemizygous deletion point mutations were usually not found in the remaining allele. Both genes expressed multiple aberrantly spliced forms in tumors not found in normal samples.
However, compelling evidence has accumulated indicating that FHIT also behaves as a suppressor gene when ectopically expressed. Several cases of homozygous deletions were demonstrated in various cancer lines. Fhit KO mice are more susceptible to cancer than wild type mice. Fhit protein is reduced or lost in many cancer types. (reviewed in Huebner and Croce, 2001; Richards 2001).
We hypothesize contrary to the bystander notion, that it is precisely the location of WWOX within a fragile site region that makes it a prime candidate to be affected by genomic rearrangements during carcinogenesis and tumor progression. It is also possible that the role in facilitating tumor progression is even more important than its putative role in the initiation of neoplasia. We can also not ignore a potential role for WWOX haploinsufficiency in the carcinogenesis process.
It is also interesting to note that there is a remarkable sequence conservation at the human and mouse orthologous common fragile site regions both for human FRA3B/FHIT with mouse Fra14A2/Fhit (Shiraishi et al., 2001) and more recently reported also for human FRA16D/WWOX and mouse Fra8ei/Wwox (Krummel et al., 2002). It is really intriguing that the sequence conservation in both cases is not limited only to the coding regions but it is found throughout very long intron regions as well. There has to be some evolutionary explanation (perhaps regulatory sequences) for preserving homology so well in non-coding DNA sequences.
WWOX, a potential tumor suppressor gene?
We determined that the area spanned by WWOX is affected by hemizygous loss in the vast majority of breast cancer cell lines and primary tumors and detected one case of homozygous deletion (Bednarek et al., 2000). As also mentioned, others have reported homozygous deletions in various cancer lines in the region spanned by WWOX. However, as was the case with the deletion that we found in breast cancer most deletions fell in intron regions of the gene. Initially, we were cautious not to call WWOX a tumor suppressor gene because when we investigated the cell lines that were hemizygous for WWOX we failed to detect any mutation in the coding exons of the remaining allele. We detected a series of polymorphism in the WWOX coding region in those lines that were heterozygous e.g. MCF7 cells. We, however, observed variable levels of expression across breast cancer lines with some lines not expressing WWOX and others overexpressing WWOX as detected by Northern and RT-PCR analyses (Bednarek et al., 2000).
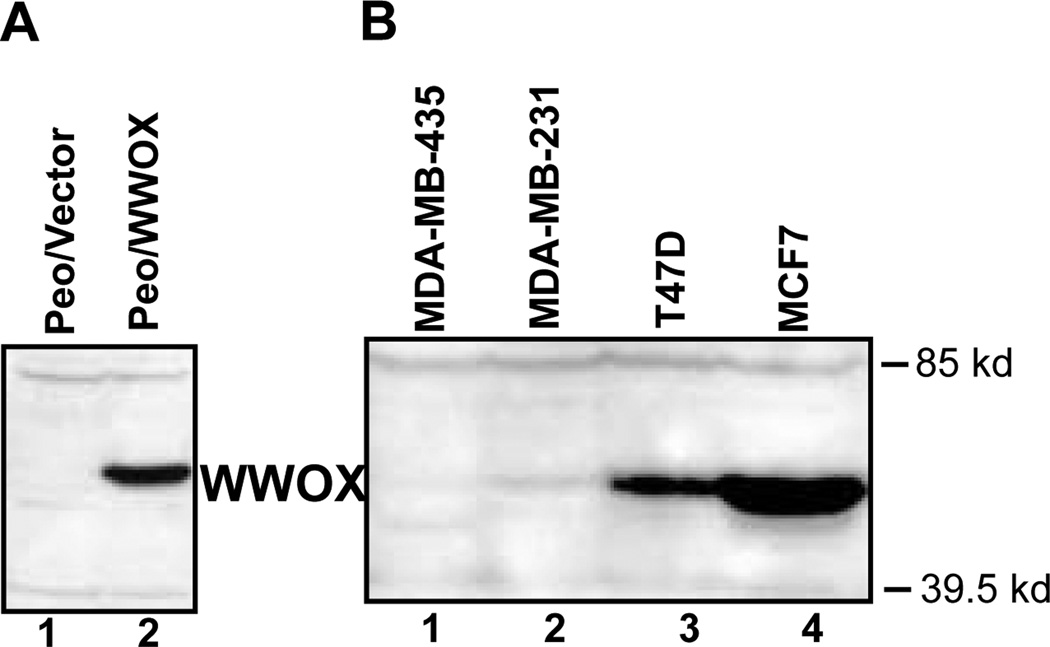
We have recently generated WWOX specific antiserum to be used to determine WWOX protein levels in normal and cancer cells. The antiserum was raised using a GST fusion to WWOX amino acid residues 12–94 containing both of the WW domains. Specific immunodetection of the WWOX protein was demonstrated by Western blotting of total cellular extracts from the ovarian cancer cell lines, Peo/vector and Peo/WWOX. (Fig. 5a). The ovarian cancer cell line, Peo, has a homozygous deletion of the WWOX gene and does not produce full-length WWOX (the original Peo cell line with homozygous WWOX deletion was kindly provided by Drs. Hanni Gabra and Adam Paige, Imperial Cancer Research Fund, Edinburgh, UK) (Paige et al., 2001). Peo cells were stably transfected with a WWOX expression vector creating the cell line Peo/WWOX to generate a positive control for WWOX protein expression. A protein of ~46 kDa, the predicted size of WWOX, was clearly evident in the extracts from Peo/WWOX positive control cell extracts that was absent in the Peo/vector negative control cell extracts.
Fig. 5.
WWOX protein expression in cancer cells. (A) Western blot analysis was used to determine the specificity of the WWOX antiserum. 50 ug of total cell protein from the cell lines, Peo/vector, (lane 1) and Peo/WWOX (lane 2). was separated by 10 % SDS-PAGE and transferred to PVDF membrane. WWOX proteins were immunodetected using the anti-WWOX anti-serum (1:1000) and detected using chemiluminescent detection (ECLplus, Amersham). Clearly, an ~46 kDa protein was detected in Peo/WWOX cell extracts that is undetectable in Peo/vector, having homozygous WWOX deletion, cell extracts. (B) We determined WWOX protein expression in the breast cancer cell lines MCF7, T47D, MDA-MB-231 and MDA-MB-435 by Western blotting using the WWOX specific antiserum. 50 µg of total protein from each cell line was analyzed as described in (a). MCF7 and T47D were observed to have very prominent band at ~46 kDa while MDA-MB-231 and MDA-MB-435 had a barely visible to undetectable band. The band observed in MCF7 and T47D was shown to comigrate with the exogenously expressed WWOX band observed in Peo/WWOX.
We are currently using this antiserum to measure WWOX protein levels in cancer cell lines and in normal tissues. Figure 5b shows the results of a Western analysis of total protein extracts from breast cancer cell lines. The WWOX protein levels correlated quite well with our previous Northern and real-time PCR analyses of WWOX gene expression in these cell lines (Bednarek et al., 2000, 2001). Interestingly, WWOX expression appears to be inversely correlated with the aggressiveness of these cell lines as determined by xenograph tumor growth assays. We detected very low or basically undetectable WWOX expression in the highly tumorigenic cell lines, MDA-MB-231 and MDA-MB-435 compared to the less aggressive cell lines MCF7 and T47D. It is also intriguing that high WWOX protein levels correlated with estrogen receptor (ER) status in these cell lines as well. MCF7 and T47D having high levels of WWOX are ER positive while MDA-MB-231 and MDA-MB-435 having very low levels of WWOX are ER negative. We are currently measuring WWOX protein levels in a panel of primary breast tumors to determine whether WWOX expression correlates with prognostic factors such as ER status.
It seems paradoxical that a putative tumor suppressor would be expressed at higher levels in some cancer cell lines when compared to normal cells as we observed (Bednarek et al., 2001). In some cases tumor suppressor genes have been observed to be highly expressed in tumors under certain circumstances (e.g. p16). Presently it is not clear what the functional significance of varying levels of WWOX expression may represent.
It was interesting that WWOX mRNA and protein expression was undetectable in the highly tumorigenic breast cancer cell line MDA-MB-435 even though it is hemizygous for the WWOX allele. We demonstrated that the lack of expression was not the result of a methylation phenomenon, since expression did not increase upon treatment with 5-aza-2′-deoxycytidine, an inhibitor of CpG methylation. Furthermore, we also did not find any signs of methylation in the CpG island around the promoter and first exon of WWOX when investigated by bisulfite DNA sequencing on the various cell lines (Bednarek et al., 2001). Therefore loss of WWOX expression in cancer cells is not due to transcriptional silencing by DNA methylation but is likely the result of alterations in transcription factor activity that regulates the WWOX promoter. We have recently cloned the WWOX promoter sequence to determine the mechanisms of WWOX gene transcriptional regulation.
Supporting a role of WWOX in affecting cancer growth other investigators detected homozygous losses in the same region, and more recently, but more importantly several of these deletions were confirmed to be affecting WWOX exons in ovarian and other cancer cell lines (Paige et al., 2001). Such genomic deletions were effectively knocking out the enzymatic portions of the WWOX coding region. More recently, it was observed that WWOX’s LOH was complemented with a mutation in a single case of esophageal carcinoma (Kuroki et al., 2002). The mutation resulted in a Leucine to Proline substitution at amino acid 291. Although the consequence of this mutation on WWOX function is not known it is interesting to note that this amino acid is in close proximity to the predicted active site of the SDR and may affect catalytic activity (see Fig. 3).
The mouse homologue of WWOX was cloned and a further link to cancer was suggested since, it was reported that Wwox could play a role in apoptosis and may interact with p53 and it was proposed that Wwox may enhance the cytotoxicity of TNF (Chang et al., 2001). However, the nature of such putative interaction with p53 was not clearly demonstrated and requires further investigation.
It is clear that the WWOX gene is a frequent target for genomic instability affecting WWOX gene expression in multiple cancers making it a potential suppressor of tumor growth. To test this hypothesis we determined the effect of WWOX expression on in vivo tumor growth of the highly tumorigenic breast cancer cell lines, MDA-MB-231 and MDA-MB-435, (Bednarek et al., 2001) which express barely detectable and undetectable levels of WWOX, respectively (Fig. 5B). Cell lines stably expressing WWOX (Fig. 6, top panel) were injected into the mammary fat pad of nude mice and observed for 35 days. Figure 6 shows the dramatic effect on tumor growth of the WWOX expressing cell lines compared to the vector control cell lines. This is the strongest reported evidence demonstrating that WWOX behaves as a tumor suppressor. In addition, we demonstrated that ectopic WWOX expression strongly inhibited anchorage-independent growth in soft agar of breast cancer lines MDA-MB-435 and T47D (Bednarek et al., 2001). Mouse models having genetically modified WWOX alleles will more clearly define the role WWOX may play in tumorigenesis.
Fig. 6.
WWOX tumor growth inhibition. Top panel: The breast cancer cell lines MDA MB 231 and MDA MB 435 were infected with the recombinant retroviruses expressing a WWOX cDNA or having no insert (Bednarek et al., 2001). Pools of stably expressing cell lines were selected for G418 resistance and analyzed for WWOX expression by western blotting. Cell lines infected with empty vector (MDA MB 231/vector and MDA MB 435/vector, lanes 1 and 3, respectively) had low to undetectable endogenous WWOX expression as observed previously (see Fig. 5). Cell lines infected with WWOX expressing vector (MDA MB 231/WWOX and MDA MB 435/ WWOX, lanes 2 and 4, respectively) had high levels of WWOX expression. Bottom panel: Tumor growth of each cell line was observed by injecting 1 × 106 cells into the thoracic mammary fat pad of BALB/c athymic mice (Bednarek et al., 2001). The average tumor volume after 35 days of tumor growth is shown (± SEM). Tumor volume of the stably expressing WWOX cell lines is plotted relative to the tumor volume of the empty vector transfected cell lines. Average tumor volumes were; MDA MB 231/vector, 302 ± 71 mm3; MDA MB 231/WWOX, 103 ± 11mm3; MDA MB 435/vector, 664 ± 239 mm3; MDA MB 435/WWOX, 81 ± 48 mm3. In addition, it was observed that WWOX expressing cell lines had differences in tumor latency and kinetics of tumor growth (Bednarek et al., 2001).
WWOX aberrant transcripts
Several WWOX transcripts have been detected in normal and cancer cells (Fig. 7). The full length WWOX mRNA (transcript variant 1; Fig. 7 and Fig. 2) is the only transcript expressed in normal tissues and encodes a full length WWOX protein containing the WW domains and the SDR domain. In tumors the most commonly expressed transcripts are full length WWOX mRNA and four aberrantly spliced mRNAs (transcript variants 2–4). The transcript variants 2–4 will be referred to as aberrantly spliced transcripts based on the observations that they are only expressed in cancer tissues and have not been observed in normal tissues. In our initial studies we detected the common occurrence of aberrantly spliced WWOX transcripts with deletions of exons 6–8 and 5–8 in various carcinoma cell lines, multiple myeloma cell lines and primary breast tumors (transcript variants 3 and 4 in Fig. 7). Subsequent studies have reported the existence of additional aberrant transcripts (transcript variants 2 and 5) (Driouch et al., 2002; Ried et al., 2000). More importantly, these aberrant mRNA forms were not detected in normal tissues (Ried et al., 2000; Bednarek et al., 2001; Paige et al., 2001; Driouch et al., 2002). Furthermore, by using primers specific to the novel exon-exon junctions generated by the aberrant splicing, we determined that approximately 33% of primary breast carcinomas expressed the aberrant delta6–8 WWOX mRNA form (transcript variant 3, Bednarek et al., 2001). Other groups have found independently the common expression of aberrant WWOX spliced forms in cancer samples, in particular in breast, ovarian and esophageal cancers (Croce et al., 1999; Paige et al., 2001; Driouch et al., 2002; Kuroki et al., 2002). It is of particular interest that in breast cancer we and others have observed aberrantly spliced transcripts produced in cells that do not have genomic deletions of WWOX exons. Thus WWOX aberrant forms can be generated by alternative splicing of WWOX mRNA as well as by exon deletions of the WWOX allele in cancer.
Fig. 7.
Schematic representation of WWOX aberrantly spliced mRNAs. The WWOX gene is represented schematically at the top. Numbered boxes indicate exons encoded by the WWOX gene. Exons shaded grey are included in the full length WWOX transcript (transcript variant 1) that is expressed in normal and tumor cells. The alternative exons 6´, 9´ and 10 observed in aberrantly spliced transcripts are shown as unshaded boxes. Aberrantly spliced transcripts, transcript variants 2–5, are shown below the full length WWOX transcript, and have been observed only in cancer cell lines and cancer tissues. The transcript variants shown are numbered according to NCBI Ref-Seq. GenBank accession numbers AF211943, variant 1; AF211943, AF227526, variant 2; AF395123, variant 3; AF395124, variant 4; AF2119443, AF227528, variant 5. The black areas indicate the protein coding regions for the WW domains and the SDR catalytic domain. Transcript variants 4 and 5 have out-of-frame deletions while transcript variant 3 has an in-frame deletion. N-normal tissue; T-tumor tissue or cancer cell lines; An indicates polyadenylation. Transcript variants 1, 3 and 4 (Bednarek et al., 2001); transcript variants 2 and 5 (Ried et al., 2000).
The possibility exists that WWOX aberrantly spliced transcripts are nonfunctional mRNAs. Splicing abnormalities occur frequently in cancer, perhaps due to increased cellular proliferation or other unknown abnormalities in the splicing machinery. So it is not uncommon to find spliced variants or abnormally spliced genes in tumors cells, e.g. estrogen receptor. Furthermore, one could hypothesize that the putative splicing abnormalities would more often affect genes that have very long transcripts such as WWOX. It remains to be determined whether the aberrantly spliced WWOX transcripts are utilized to produce their encoded protein isoforms. We are currently analyzing WWOX protein expression in several tumor types, and since the anti-WWOX antibody recognizes epitopes within the WW domains it will also detect proteins encoded by the aberrantly spliced mRNAs if expressed.
An additional WWOX mRNA (transcript 2) has been described that has the first eight exons spliced to two alternate exons (9´ and 10, Ried et al., 2000). This transcript codes for a protein similar to full length WWOX encoded by transcript variant 1 containing the WW domains and an intact SDR domain but with a different carboxy terminal amino acid sequence. We have positively identified transcript variant 1 as the mRNA encoding full length WWOX protein in MCF-7 breast cancer cells by virtue of the difference in the carboxy terminal amino acid sequence using MALDI-TOF mass spectroscopy peptide sequencing of immunoprecipitated WWOX protein (Ludes-Meyers et al, unpublished observation).
In summary, the data shows that the WWOX allele is a target for disruption in multiple tumor types and WWOX expression can be altered by various mechanisms including homozygous deletion of exons, transcriptional dysregulation and aberrant splicing.
Biochemical features of the WWOX protein
WWOX is a 414 amino acid protein. We named this gene WWOX because it contains two WW-domains at the amino terminus coupled to a region with high homology to the short-chain dehydrogenase/reductase family of enzymes (SDR) (Fig. 3). WW motifs are known to be involved in protein-protein interactions. Protein-protein interactions are essential in transmission of signal-transduction information. There are a small number of modular protein-protein recognition domains. Proline-rich motifs are often targeted for protein-protein interactions, probably due to the unique structural properties of these regions. Domains that recognize proline-rich motifs include SH3, EVH1 and WW domains. The highly compact WW domains (35–45aa in length) are characterized by the presence of a pair of conserved tryptophans (i.e.W). These two “signature” W residues are spaced approximately 20–22 aa apart and play a fundamental role in the structure and function of the domain. WW domains recognize proline-containing sequences, and they have a very diverse sequence preference. Based on such binding preference there are basically four groups of WW domains. Two are major and more common, Groups I and II and two less common, Groups III and IV. Group I binds the minimal core consensus Pro–Pro–X–Tyr (PPXY). Examples of WW domain containing proteins from Group I include YAP65, dystrophin and NEDD4. The proteins containing the specific proline-rich motifs constitute specific ligands, for instance the ligand of the WW domain of dystrophin is beta-distroglycan which contains the (PPXY) motif. Group II WW domain containing proteins include the formin binding proteins and FE65 that typically bind the Pro–Pro–Leu–Pro (PPLP) motif. Group III WW domains select polyproline motifs flanked by Arg or Lys and Group IV WW domains have preference for phospho–Ser–Pro or phospho–Thr–Pro containing ligands. An example of this last group of WW domain containing proteins is Pin-1. This protein can regulate early mitotic events by interaction with various mitosis-specific phosphoproteins such as CdC25C phosphatase (reviewed in Sudol and Hunter, 2000). We are in the process of determining the in vitro and in vivo biochemical activity of the WWOX WW domains. In preliminary studies we have observed that the first WW domain specifically interacts with the PPXY ligand defining this WWOX domain as a Group I WW domain (Ludes-Meyers et al., manuscript in preparation). These studies confirm that the WWOX WW domains have specific ligand binding activity and studies are ongoing to identify in vivo protein partners involved in WWOX tumor growth inhibition.
As reported, the central portion of WWOX contains very high homology to the SDR family of enzymes. Based on the high expression of WWOX in hormonally regulated tissues (human testis, prostate and ovary) and its amino acid sequence homology to specific oxidoreductases, we postulated that WWOX may be an enzyme involved in sex-steroid metabolism (Bednarek et al., 2000). According to Kallberg et al., based on structural analyses of human SDRs and comparison across species, they detected the occurrence of 63 different SDR enzymes in humans, reduced to truly 58 after elimination of possible isozymes (Kallberg et al., 2002). Of these, 46 enzymes are of the classical type and 17 of the extended type (these are related to mostly sugar metabolism). WWOX structure is the archetypal representative of one of four separate clusters of classical SDRs (Kallberg et al., 2002). The other three clusters are represented by human Hep27, FVT1 and 17beta-HSD3. To date only the molecular function of 17beta-HSD3 has been defined (involved in tissue-specific testosterone synthesis). Interestingly, the other three clusters represented by WWOX, Hep27 and FVT1 all demonstrated some link to cancer. Hep27 was cloned from a hepatocellular carcinoma and is a nuclear protein related to growth control (Gabrielli et al., 1995). FVT1 was identified as a target for translocation in non-Hodgkin lymphomas showing overexpression in certain T cell malignancies and indicating some role in tumorigenic processes (Rimokh et al., 1993). As mentioned, we hypothesized that WWOX is related to sex-steroid metabolism and according to Kallberg and co-workers this is further supported not only by its tissue distribution and structural features but also by two other human SDRs found in WWOX’s cluster, CGI-82 and PAN2. CGI-82 is abundantly expressed in prostate (Lin et al., 2001) and PAN2 displays sequence similarities to other hydroxysteroid dehydrogenases (Kallberg et al., 2002).
To date the only known link to a biological function of WWOX is its subcellular localization to the Golgi apparatus. This was demonstrated by transient expression of a GFP-fusion to full length WWOX followed by visualization of green fluorescence using confocal microscopy. Cells expressing the GFP-WWOX fusion protein clearly had perinuclear green fluorescence that colocalized only with a Golgi specific marker but not with other organelle specific markers (e.g. Mitotracker). Interestingly, GFP-fusions with proteins encoded by two aberrantly spliced transcripts, delta 6–8 and delta 5–8, showed exclusively nuclear fluorescence (Bednarek et al., 2001). A common feature of the predicted proteins encoded by WWOX delta forms is deletion of the predicted enzymatic domain of the full-length protein while retaining the WW domains. Interestingly, a nuclear localization signal has been identified in the mouse Wwox protein to reside between the two WW domains (Chang et al., 2001). Hypothetically, these proteins will be inactive enzymatically and could act as dominant-negative competitors of the normal WWOX protein.
The WWOX SDR is required for localization to the Golgi
The observation that full length WWOX resides in the Golgi apparatus, while proteins encoded by aberrantly spliced transcripts localize to the nucleus, suggests that amino acids within the SDR domain are involved in directing WWOX to the Golgi. Chang et al. (2001) reported a 65 amino acid residue sequence as a mitochondrial targeting signal located between Wwox amino acids 209–273. In previous studies we observed colocalization of GFP-WWOX with a Golgi specific marker (Golgi 58 K protein) but did not observe colocalization of GFP-WWOX with a mitochondria-specific dye (MitoTracker Red CMXRos). It is not clear why there is a discrepancy between these two observations. To more precisely define the WWOX Golgi targeting sequences we have used site directed mutagenesis to make deletions of the SDR domain while keeping the WW domains intact (Fig. 8). Mutant delta5–7 has the protein coding sequences of exons 5, 6 and 7 deleted resulting in an in-frame fusion of exons 1–4 to exons 8 and 9. Mutant delta8 has exon 8 deleted resulting in an in-frame fusion of exons 1–7 to exon 9. Cells expressing either of the mutants show similar diffuse cytoplasmic and nuclear fluorescence with striking exclusion from the nucleoli (Fig. 8). Clearly proper subcellular localization is affected by disruption of the SDR domain. Analysis of single amino acid changes within the full length WWOX protein will be required to clearly define the amino acids required for proper subcellular localization. We have also generated a GFP fusion with the SDR domain alone by deleting the WW domains. Surprisingly this mutant was observed to have perinuclear fluorescence similar to full length WWOX (J.H. Ludes-Meyers, data not shown). Although these studies do not precisely define the amino acid residues required for WWOX subcellular localization we conclude that the SDR domain is necessary and sufficient for Golgi localization.
Fig. 8.
The WWOX catalytic domain is sufficient for Golgi localization. To determine the domains required for WWOX Golgi localization we generated deletion mutants of WWOX in the context of the WWOX-GFP fusion protein. These mutants were transiently expressed in the immortal mammary epithelial cell line, MCF10F, and visualized using confocal microscopy. The WWOX protein and the deletion mutants are shown schematically (top panel). Two mutants were analyzed. Δ5–7 has an in-frame fusion between the coding sequence of exon 4 and exon 8 deleting the coding sequences of exons 5,6 and 7. Δ8 has an in-frame fusion between the coding sequence of exon 7 and exon 9 deleting exon 8. The catalytic active site is contained within the exon 8 coding sequence (see Fig. 7a). The confocal images show cross sections through the cytoplasmic and nuclear regions of the cells. NLS, putative nuclear localization signal.
Since the WW domains are dispensable for Golgi localization it is reasonable to postulate that they are involved in tumor growth inhibition through specific protein-protein interactions in the Golgi. Recently, a Drosophila gene salvador was cloned that encodes a protein containing two WW domains and is involved in cell cycle regulation and apoptosis. Most importantly, the human ortholog of salvador (SAV1) was observed to be mutated in three cancer cell lines (Tapon et al., 2002). This provides another example, together with WWOX, of a WW domain containing protein that has been linked to cancer.
Conclusions
In summary, we have cloned a candidate cancer gene mapping to region 16q23.3→q24.1. This gene is the target of FRA16D and codes for the protein WWOX. WWOX contains two WW domains and a short-chain dehydrogenase/reductase enzyme domain that is probably involved in sex-steroid hormone metabolism.
We hypothesize that the normal cellular function of WWOX may be affected by various different mechanisms at the genomic and transcriptional level. The single allele losses observed in various tumor types and in particular breast cancer, by itself may be of importance and perhaps sufficient in leading to phenotypic abnormalities, as was observed in cases of tumor suppression inactivation due to haploinsufficiency. Our data indicate that WWOX behaves as a potent suppressor of tumor growth and suggest that abnormalities affecting this gene at the genomic and transcriptional level could be relevant in carcinogenesis. The development of mouse strains with targeted inactivation of the Wwox allele will help define the role that WWOX may play in tumorigenesis. In addition, WWOX specific reagents are now available to determine the clinical relevance of WWOX abnormalities at the genomic and transcription levels.
References
- Aldaz CM, Chen T, Sahin A, Cunningham J, Bondy M. Comparative allelotype of in situ and invasive human breast cancer: high frequency of microsatellite instability in lobular breast carcinomas. Cancer Res. 1995;55:3976–3981. [PubMed] [Google Scholar]
- Bednarek AK, Keck-Waggoner CL, Daniel RL, Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ, Aldaz CM. WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res. 2001;61:8068–8073. [PubMed] [Google Scholar]
- Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3 → q24.1, a region frequently affected in breast cancer. Cancer Res. 2000;60:2140–2145. [PubMed] [Google Scholar]
- Berx G, Cleton-Jansen A, Nollet F, de Leeuw W, van de Vijver M, Cornelisse C, van Roy F. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995;14:6107–6115. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner AJ, Aldaz CM. Chromosome 9p allelic loss and p16/CDKN2 in breast cancer and evidence of p16 inactivation in immortal breast epithelial cells. Cancer Res. 1995;55:2892–2895. [PubMed] [Google Scholar]
- Buerger H, Otterbach F, Simon R, Poremba C, Diallo R, Decker T, Riethdorf L, Brinkschmidt C, Dock-horn-Dworniczak B, Boecker W. Comparative genomic hybridization of ductal carcinoma in situ of the breast-evidence of multiple genetic pathways. J Pathol. 1999;187:396–402. doi: 10.1002/(SICI)1096-9896(199903)187:4<396::AID-PATH286>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chang NS, Pratt N, Heath J, Schultz L, Sleve D, Carey GB, Zevotek N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J biol Chem. 2001;276:3361–3370. doi: 10.1074/jbc.M007140200. [DOI] [PubMed] [Google Scholar]
- Charpentier A, Aldaz CM. The molecular basis of breast carcinogenesis. In: Coleman WB, Tsongalis GJ, editors. Molecular Basis of Human Cancer: Genomic Instability and Molecular Mutation in Neoplastic Transformation. Totowa: Humana Press; 2002. pp. 347–363. [Google Scholar]
- Chen T, Sahin A, Aldaz C. Deletion map of chromosome 16q in ductal carcinoma in situ of the breast: refining a putative tumor suppressor gene region. Cancer Res. 1996;56:5605–5609. [PubMed] [Google Scholar]
- Cher ML, Ito T, Weidner N, Carroll PR, Jensen RH. Mapping of regions of physical deletion on chromosome 16q in prostate cancer cells by fluorescence in situ hybridization (FISH) J Urol. 1995;153:249–254. doi: 10.1097/00005392-199501000-00086. [DOI] [PubMed] [Google Scholar]
- Chesi M, Bergsagel PL, Shonukan OO, Martelli ML, Brents LA, Chen T, Schrock E, Ried T, Kuehl WM. Frequent dysregulation of the c-maf proto-oncogene at 16q23 by translocation to an Ig locus in multiple myeloma. Blood. 1998;91:4457–4463. [PubMed] [Google Scholar]
- Cleton-Jansen A, Moerland E, Kuipers-Dijkshoorn N, Callen D, Sutherland G, Hansen B, Devilee P, Cornelisse C. At least two different regions are involved in allelic imbalance on chromosome arm 16q in breast cancer. Genes Chrom Cancer. 1994;9:101–107. doi: 10.1002/gcc.2870090205. [DOI] [PubMed] [Google Scholar]
- Crawford J, Ianzano L, Savino M, Whitmore S, Cleton-Jansen AM, Settasatian C, d’apolito M, Seshadri R, Pronk JC, Auerbach AD, Verlander PC, Mathew CG, Tipping AJ, Doggett NA, Zelante L, Callen DF, Savoia A. The PISSLRE gene: structure, exon skipping, and exclusion as tumor suppressor in breast cancer. Genomics. 1999;56:90–97. doi: 10.1006/geno.1998.5676. [DOI] [PubMed] [Google Scholar]
- Croce CM, Sozzi G, Huebner K. Role of FHIT in human cancer. J Clin Oncol. 1999;17:1618–1624. doi: 10.1200/JCO.1999.17.5.1618. [DOI] [PubMed] [Google Scholar]
- Driouch K, Prydz H, Monese R, Johansen H, Lidereau R, Frengen E. Alternative transcripts of the candidate tumor suppressor gene, WWOX, are expressed at high levels in human breast tumors. Oncogene. 2002;21:1832–1840. doi: 10.1038/sj.onc.1205273. [DOI] [PubMed] [Google Scholar]
- Dutrillaux B, Gerbault-Seureau M, Zafrani B. Characterization of chromosomal anomalies in human breast cancer. A comparison of 30 paradiploid cases with few chromosome changes. Cancer Genet Cytogenet. 1990;49:203–217. doi: 10.1016/0165-4608(90)90143-x. [DOI] [PubMed] [Google Scholar]
- Elo JP, Harkonen P, Kyllonen AP, Lukkarinen O, Vihko P. Three independently deleted regions at chromosome arm 16q in human prostate cancer: allelic loss at 16q24.1 → q24.2 is associated with aggressive behaviour of the disease, recurrent growth, poor differentiation of the tumour and poor prognosis for the patient. Br J Cancer. 1999;79:156–160. doi: 10.1038/sj.bjc.6690025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Szumel R, Marsh C, Zhou W, Gabrielson E. Genetic progression, histological grade, and allelic loss in ductal carcinoma in situ of the breast. Cancer Res. 1996;56:5260–5265. [PubMed] [Google Scholar]
- Futreal PA, Cochran C, Rosenthal J, Miki Y, Swenson J, Hobbs M, Bennett LM, Haugen-Strano A, Marks J, Barrett JC, et al. Isolation of a diverged homeo-box gene, MOX1, from the BRCA1 region on 17q21 by solution hybrid capture. Hum molec Genet. 1994;3:1359–1364. doi: 10.1093/hmg/3.8.1359. [DOI] [PubMed] [Google Scholar]
- Gabrielli F, Donadel G, Bensi G, Heguy A, Melli M. A nuclear protein, synthesized in growth-arrested human hepatoblastoma cells, is a novel member of the short-chain alcohol dehydrogenase family. Eur J Biochem. 1995;232:473–477. doi: 10.1111/j.1432-1033.1995.473zz.x. [DOI] [PubMed] [Google Scholar]
- Glover TW, Berger C, Coyle J, Echo B. DNA polymerase alpha inhibition by aphidicolin induces gap and breaks at common fragile sites in human chromosomes. Hum Genet. 1984;67:136–142. doi: 10.1007/BF00272988. [DOI] [PubMed] [Google Scholar]
- Gong G, DeVries S, Chew KL, Cha I, Ljung BM, Waldman FM. Genetic changes in paired atypical and usual ductal hyperplasia of the breast by comparative genomic hybridization. Clin Cancer Res. 2001;7:2410–2414. [PubMed] [Google Scholar]
- Gupta PK, Sahota A, Boyadjiev SA, Bye S, Shao C, O’Neill JP, Hunter TC, Albertini RJ, Stambrook PJ, Tischfield JA. High frequency in vivo loss of heterozygosity is primarily a consequence of mitotic recombination. Cancer Res. 1997;57:1188–1193. [PubMed] [Google Scholar]
- Huebner K, Croce CM. FRA3B and other common fragile sites: the weakest links. Nature Rev Cancer. 2001;1:214–221. doi: 10.1038/35106058. [DOI] [PubMed] [Google Scholar]
- Iwabuchi H, Sakamoto M, Sakunaga H, Ma YY, Carcangiu ML, Pinkel D, Yang-Feng TL, Gray JW. Genetic analysis of benign, low-grade, and high-grade ovarian tumors. Cancer Res. 1995;55:6172–6180. [PubMed] [Google Scholar]
- Kallberg Y, Oppermann U, Jornvall H, Persson B. Short-chain dehydrogenase/reductase (SDR) relationships: A large family with eight clusters common to human, animal, and plant genomes. Protein Sci. 2002;11:636–641. doi: 10.1110/ps.26902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M, Staub J, Cliby W, Hartmann L, Smith DI, Shridhar V. Involvement of H-cadherin (CDH13) on 16q in the region of frequent deletion in ovarian cancer. Int J Oncol. 1999;15:715–720. doi: 10.3892/ijo.15.4.715. [DOI] [PubMed] [Google Scholar]
- Krummel KA, Denison SR, Calhoun E, Phillips LA, Smith DI. The common fragile site FRA16D and its associated gene WWOX are highly conserved in the mouse at Fra8E1. Genes Chrom Cancer. 2002;34:154–167. doi: 10.1002/gcc.10047. [DOI] [PubMed] [Google Scholar]
- Krummel KA, Roberts LR, Kawakami M, Glover TW, Smith DI. The characterization of the common fragile site FRA16D and its involvement in multiple myeloma translocations. Genomics. 2000;69:37–46. doi: 10.1006/geno.2000.6321. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Trapasso F, Shiraishi T, Alder H, Mimori K, Mori M, Croce CM. Genetic alterations of the tumor suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer Res. 2002;62:2258–2260. [PubMed] [Google Scholar]
- Lakhani SR, Collins N, Sloane JP, Stratton MR. Loss of heterozygosity in lobular carcinoma in situ of the breast. J clin Pathol: Mol Pathol. 1995;48:M74–M78. doi: 10.1136/mp.48.2.m74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Beau MM, Drabkin H, Glover TW, Gemmill R, Rassool FV, McKeithan TW, Smith DI. An FHIT tumor suppressor gene? Genes Chrom Cancer. 1998;21:281–289. doi: 10.1002/(sici)1098-2264(199804)21:4<281::aid-gcc1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Li C, Berx G, Larsson C, Auer G, Aspenblad U, Pan Y, Sundelin B, Ekman P, Nordenskjold M, van Roy F, Bergerheim US. Distinct deleted regions on chromosome segment 16q23 →q24 associated with metastases in prostate cancer. Genes Chrom Cancer. 1999;24:175–182. [PubMed] [Google Scholar]
- Lin B, White JT, Ferguson C, Wang S, Vessella R, Bumgarner R, True LD, Hood L, Nelson PS. Prostate short-chain dehydrogenase reductase 1 (PSDR1): a new member of the short-chain steroid dehydrogenase/reductase family highly expressed in normal and neoplastic prostate epithelium. Cancer Res. 2001;61:1611–1618. [PubMed] [Google Scholar]
- Lu YJ, Osin P, Lakhani SR, Di Palma S, Gusterson BA, Shipley JM. Comparative genomic hybridization analysis of lobular carcinoma in situ and atypical lobular hyperplasia and potential roles for gains and losses of genetic material in breast neoplasia. Cancer Res. 1998;58:4721–4727. [PubMed] [Google Scholar]
- Mangelsdorf M, Ried K, Woollatt E, Dayan S, Eyre H, Finnis M, Hobson L, Nancarrow J, Venter D, Baker E, Richards RI. Chromosomal fragile site FRA16D and DNA instability in cancer. Cancer Res. 2000;60:1683–1689. [PubMed] [Google Scholar]
- Nishida N, Fukuda Y, Kokuryu H, Sadamoto T, Isowa G, Honda K, Yamaoka Y, Ikenaga M, Imura H, Ishizaki K. Accumulation of allelic loss on arms of chromosomes 13q, 16q and 17p in the advanced stages of human hepatocellular carcinoma. Int J Cancer. 1992;51:862–868. doi: 10.1002/ijc.2910510605. [DOI] [PubMed] [Google Scholar]
- O’Connell P, Pekkel V, Fuqua SA, Osborne CK, Clark GM, Allred DC. Analysis of loss of heterozygosity in 399 premalignant breast lesions at 15 genetic loci. J natl Cancer Inst. 1998;90:697–703. doi: 10.1093/jnci/90.9.697. [DOI] [PubMed] [Google Scholar]
- Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, et al. The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell. 1996;84:587–597. doi: 10.1016/s0092-8674(00)81034-x. [DOI] [PubMed] [Google Scholar]
- Page DL, Dupont WD. Anatomic markers of human premalignancy and risk of breast cancer. Cancer. 1990;66:1326–1335. doi: 10.1002/1097-0142(19900915)66:14+<1326::aid-cncr2820661405>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Paige AJ, Taylor KJ, Stewart A, Sgouros JG, Gabra H, Sellar GC, Smyth JF, Porteous DJ, Watson JE. A 700-kb physical map of a region of 16q23.2 homozygously deleted in multiple cancers and spanning the common fragile site FRA16D. Cancer Res. 2000;60:1690–1697. [PubMed] [Google Scholar]
- Paige AJ, Taylor KJ, Taylor C, Hillier SG, Farrington S, Scott D, Porteous DJ, Smyth JF, Gabra H, Watson JE. WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc natl Acad Sci, USA. 2001;98:11417–11422. doi: 10.1073/pnas.191175898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandis N, Heim S, Bardi G, Idvall I, Mandahl N, Mitelman F. Whole-arm t(1;16) and i(1q) as sole anomalies identify gain of 1q as a primary chromosomal abnormality in breast cancer. Genes Chrom Cancer. 1992;5:235–238. doi: 10.1002/gcc.2870050310. [DOI] [PubMed] [Google Scholar]
- Radford D, Fair K, Phillips N, Ritter J, Steinbrueck T, Holt M, Donis-Keller H. Allelotyping of ductal carcinoma in situ of the breast: deletion of loci on 8p, 13q, 16q, 17p and 17q. Cancer Res. 1995;55:3399–3405. [PubMed] [Google Scholar]
- Richards RI. Fragile and unstable chromosomes in cancer: causes and consequences. Trends Genet. 2001;17:339–345. doi: 10.1016/s0168-9525(01)02303-4. [DOI] [PubMed] [Google Scholar]
- Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A, Venter D, Baker E, Richards RI. Common chromosomal fragile site FRA16D sequence: identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum molec Genet. 2000;9:1651–1663. doi: 10.1093/hmg/9.11.1651. [DOI] [PubMed] [Google Scholar]
- Rimokh R, Gadoux M, Bertheas MF, Berger F, Garoscio M, Deleage G, Germain D, Magaud JP. FVT-1, a novel human transcription unit affected by variant translocation t(2;18)(p11;q21) of follicular lymphoma. Blood. 1993;81:136–142. [PubMed] [Google Scholar]
- Savino M, d’Apolito M, Centra M, van Beerendonk HM, Cleton-Jansen AM, Whitmore SA, Crawford J, Callen DF, Zelante L, Savoia A. Characterization of copine VII, a new member of the copine family, and its exclusion as a candidate in sporadic breast cancers with loss of heterozygosity at 16q24.3. Genomics. 1999;61:219–226. doi: 10.1006/geno.1999.5958. [DOI] [PubMed] [Google Scholar]
- Shiraishi T, Druck T, Mimori K, Flomenberg J, Berk L, Alder H, Miller W, Huebner K, Croce CM. Sequence conservation at human and mouse orthologous common fragile regions, FRA3B/FHIT and Fra14A2/Fhit. Proc natl Acad Sci, USA. 2001;98:5722–5727. doi: 10.1073/pnas.091095898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotnicka-Klonowicz G, Rieske P, Bartkowiak J, Szymik-Kantorowicz S, Daszkiewicz P, Debiec-Rychter M. 16q heterozygosity loss in Wilms’ tumour in children and its clinical importance. Eur J Surg Oncol. 2000;26:61–66. doi: 10.1053/ejso.1999.0742. [DOI] [PubMed] [Google Scholar]
- Smith DI, Huang H, Wang L. Common fragile sites and cancer (Review) Int J Oncol. 1998;12:187–196. [PubMed] [Google Scholar]
- Sudol M, Hunter T. NeW wrinkles for an old domain. Cell. 2000;103:1001–1004. doi: 10.1016/s0092-8674(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Baker E, Richards RI. Fragile sites still breaking. Trends Genet. 1998;14:501–506. doi: 10.1016/s0168-9525(98)01628-x. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Komiya A, Emi M, Kuramochi H, Shiraishi T, Yatani R, Shimazaki J. Three distinct commonly deleted regions of chromosome arm 16q in human primary and metastatic prostate cancers. Genes Chrom Cancer. 1996;17:225–233. doi: 10.1002/(SICI)1098-2264(199612)17:4<225::AID-GCC4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber DA, Hariharan IK. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Yunis JJ, Soreng AL. Constitutive fragile sites and cancer. Science. 1984;226:1199–1204. doi: 10.1126/science.6239375. [DOI] [PubMed] [Google Scholar]