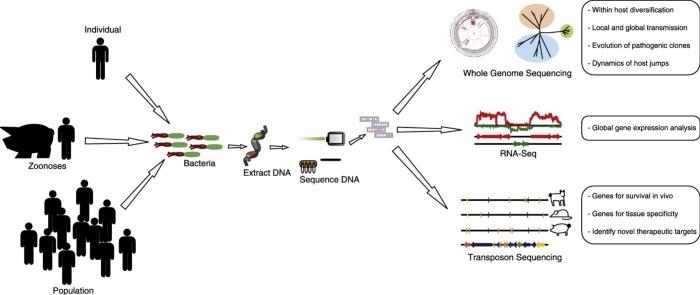
Graphical abstract
Highlights
-
•
High throughput sequencing (HTS) is revolutionizing research into bacterial pathogens.
-
•
HTS reveals that bacterial pathogens may undergo considerable diversification during infection.
-
•
HTS can allow tracing of outbreak origin and transmission.
-
•
HTS offers advantages over existing transcriptomic technologies for understanding global gene expression in bacteria.
-
•
Transposon mutagenesis and HTS is a powerful combination for identifying bacterial determinants required for in vivo survival.
Abstract
A revolution in sequencing technologies in recent years has led to dramatically increased throughput and reduced cost of bacterial genome sequencing. An increasing number of applications of the new technologies are providing broad insights into bacterial evolution, epidemiology, and pathogenesis. For example, the capacity to sequence large numbers of bacterial isolates is enabling high resolution phylogenetic analyses of bacterial populations leading to greatly enhanced understanding of the emergence, adaptation, and transmission of pathogenic clones. In addition, RNA-seq offers improved quantification and resolution for transcriptomic analysis, and the combination of high-throughput sequencing with transposon mutagenesis is a powerful approach for the identification of bacterial determinants required for survival in vivo. In this concise review we provide selected examples of how high throughput sequencing is being applied to understand the biology of bacterial pathogens, and discuss future technological advances likely to have a profound impact on the field.
Current Opinion in Microbiology 2014, 19:106–113
This review comes from a themed issue on Novel technologies in microbiology
Edited by Emmanuelle Charpentier and Luciano Marraffini
For a complete overview see the Issue and the Editorial
Available online 14th July 2014
http://dx.doi.org/10.1016/j.mib.2014.06.002
1369-5274/© 2014 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Introduction
The development of new technologies enabling rapid, inexpensive, and high-throughput DNA sequencing (HTS) that offer clear advantages over traditional Sanger sequencing has revolutionized the field of bacterial genomics [1•, 2]. Furthermore, the recent development of high-throughput ‘benchtop’ sequencers is empowering laboratories to sequence their bacteria of interest independently of specialist sequencing centres [2, 3]. An array of different technologies have been developed with the common feature that they parallelize the sequencing process, leading to the production of thousands or millions of sequence reads concurrently (for a review of the HTS technologies see [1•, 2]). HTS is being applied in a myriad of ways to address fundamental questions concerning the biology of infectious diseases. The high resolution offered by HTS allows the inference of transmission pathways during global pandemics and localized outbreaks, identification of molecular mechanisms underpinning the emergence of pathogenic clones, and the evolutionary analysis of bacterial populations during infection of individual patients. HTS also provides the potential for transcriptomic analyses with advantages over traditional hybridization approaches, including genome-wide coverage, accurate quantification, and single nucleotide resolution (for recent comprehensive reviews, see [4, 5•]). In addition, the combination of HTS with transposon mutagenesis leading to the development of approaches such as Tn-seq [6], transposon-directed insertion site sequencing (TraDIS) [7•], insertion sequencing (INseq) [8], and high-throughput insertion tracking by deep sequencing (HITS) [9] has facilitated the screening of libraries of hundreds of thousands of bacterial mutants to identify determinants required for survival during growth in vivo or in other specific growth conditions (for recent comprehensive reviews see [10, 11]).
In the current concise review, we will summarize selected recent studies that have applied HTS to answer important questions regarding the success of major bacterial pathogens. We provide an overview of some of the insights which can be derived from the application of these techniques.
Study of bacterial evolution during infection
The progression and outcome of infectious disease is determined by the dynamics of host–pathogen interactions, and recent studies employing HTS have offered novel insights into the evolution of bacterial pathogens during the course of colonization and infection [12•, 13, 14••, 15]. For example, an emerging theme in infectious disease research is the extent of genetic and phenotypic diversification that may occur among the infecting bacterial population within an individual host. In particular, Pseudomonas aeruginosa, Staphylococcus aureus, Mycobacterium abscessus, Mycobacterium tuberculosis, and Burkholderia dolosa have been demonstrated to undergo considerable diversification during infection, resulting in ‘clouds of diversity’ that originated from a single or closely related group of infecting bacteria [12•, 14••, 15, 16, 17•, 18]. During infection, random advantageous mutations may become fixed within sub-populations, due to selective pressures such as co-infection with other microorganisms, the host immune response, and antimicrobial chemotherapy [12•, 14••, 15, 17•, 18, 19]. Of note, cystic fibrosis (CF) patients are at particular risk of pulmonary infections, and a number of studies have utilized HTS to examine the genetic diversification of bacterial populations during long-term infection of CF patients [12•, 15, 18, 20]. For example, convergent evolution represented by independent mutations affecting O-antigen switching has been identified in chronic B. dolosa infections [15]. Furthermore, mutations influencing the smooth to rough morphotype transition of M. abscessus spp. during infection of the CF lung have been identified [20, 21], and distinct polymorphisms of loci influencing the alternative sigma factor (SigB) of S. aureus were identified in multiple sub-lineages of S. aureus within a single CF patient [18]. The study also revealed mutations underlying antibiotic resistance which occurred during infection as demonstrated in other studies of S. aureus chronic infection [22, 23]. Loss of virulence factor production has also been described during therapeutic Escherichia coli colonization of human patients with recurrent urinary tract infections [24••]. Numerous mutations associated with reduced virulence and adaptation to oxidative stresses, and the recurrence of mutations within individual patients strongly suggested that host-specific selective pressures influence microevolution during infection [24••]. In addition, a long term S. aureus carriage study identified an overall pattern of purifying selection within asymptomatically colonized hosts [13]. An enrichment of premature stop codons was observed in invasive bloodstream isolates when compared to carriage isolates from an individual who developed a fatal bacteraemia, implying that specific genetic changes in carriage isolates may be functionally important in pathogenesis [14••].
Differences in the composition of resident bacterial populations between healthy and disease states are increasingly being described, and deep sequencing meta-genomic methods can capture greater diversity from the microbiota in comparison to traditional methods relying on PCR amplification and Sanger sequencing [25]. For example, decreased microbial diversity in CF patients in comparison to healthy controls is associated with more severe inflammation [26] and distinct shifts in metabolic pathways have been identified [27]. Additionally, the effects of antimicrobial therapies on the gut microbiota have been investigated revealing increased phage mobilization [28, 29], and profound shifts in composition that persist after the cessation of therapy [30] (Figure 1).
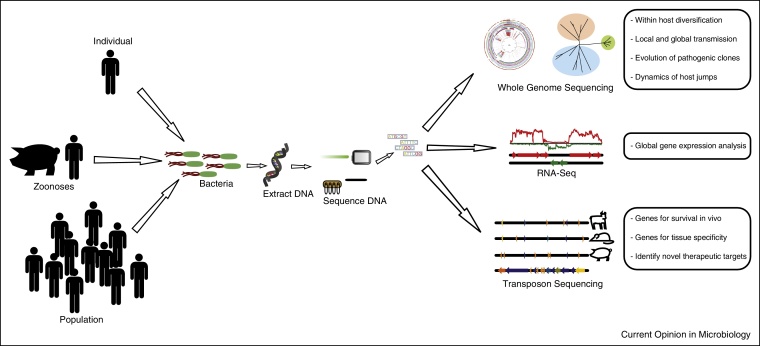
Figure 1.
Schematic diagram summarizing the applications of high throughput sequencing for studies of the epidemiology, evolution and pathogenesis of bacterial infections.
Identification of bacterial determinants required for pathogenesis
The application of HTS for measuring the bacterial transcriptome (RNA-seq) in different environmental conditions has considerable advantages over traditional hybridization-based techniques. The method requires isolation of RNA followed by reverse transcription to cDNA to allow library construction before HTS. Specifically, the inclusion of strain-specific genetic material, single base-pair resolution, and more accurate quantification of relative levels of gene expression are improvements on previous approaches using microarrays. Although RNA-seq technology has been relatively slow to be employed for bacterial transcriptomic studies due in part to technical challenges of contaminating rRNA, an increasing number of studies are now being published. For example, comparative gene expression analysis of the opportunistic human pathogen Aggregatibacter actinomycetemcomitans in vivo and in a biofilm model in vitro revealed differential expression of 14% of the transcriptome, providing new information relevant to the metabolic pathways important for infection [31]. Other selected studies have shown differential expression of master regulatory genes in Streptococcus pneumoniae [6], genes essential for the survival of Haemophilus influenzae outside of the host [32], and survival of Salmonella Typhi in bile [7•]. In addition, RNA-seq identified the extent of the S. Typhi ompR regulon [33] and revealed that the E. coli plasmid pO157_sal regulates the expression of chromosomal genes associated with the stress response, antibiotic resistance, and virulence [34].
A particularly powerful application is the combination of HTS with transposon mutagenesis. Briefly, libraries made up of bacteria each with single transposon insertions into non-essential genes are used as an innoculum for experimental infections or for culture in defined conditions. Subsequently, input and output populations are subjected to HTS allowing the relative quantification of mutants in each population. Accordingly, the complement of genes required for survival can be identified. For example, the application of transposon-directed insertion-site (TraDIS) sequencing to Salmonella enterica serovars S. Typhi and S. Typhimurium revealed a conserved core of 281 genes required for growth in both serovars [35]. Attenuation of O-antigen genes and ferric [Fe(III)] genes was observed in S. Typhimurium and S. Typhi respectively [35], which may partly reflect the distinct host tropisms of each serovar. A Tn-seq study of adaptation of S. pneumoniae to the nasopharynx and the lung indicated an array of genes associated with response to hydrogen peroxide which were important during lung infection, while genes associated with response to temperature and sucrose were involved in survival in the nasopharynx [36••]. In contrast, a study of the interactions of Moraxella catarrhalis with the host respiratory epithelia reported no difference in expression of adhesion factors between adhesive and planktonic states [37].
Finally, in a novel application of Tn-seq, the dynamics of H. influenzae and influenza A virus co-infection were examined in a mouse model of respiratory infection. Of note, H. influenzae genes associated with oxidative stress were required during co-infection with influenza A compared to H. influenzae infection alone [38•]. The use of HTS-transposon approaches is revealing much about the mechanisms of bacterial survival in vivo and in doing so may indicate novel therapeutic targets for controlling infection.
Identifying the source and transmission routes of disease outbreaks
Traditional bacterial typing methods are often of limited use for epidemiological investigations of infectious disease outbreaks due to their low level of discriminatory power [39]. The comparison of whole genome sequences can provide the ultimate level of nucleotide resolution between bacterial isolates and accordingly has the potential to identify transmission events within and between hospitals and in the community [3, 17•, 40•, 41, 42, 43•, 44••, 45, 46, 47]. Importantly, the application of rapid sequencing of suspected outbreak strains can inform both clinical practice and infection control procedures [17•]. The level of genetic variation observed among strains epidemiologically linked to an outbreak can help determine whether a point source or multiple strains are responsible [3, 40•, 44••, 48]. For example, based on multiple locus tandem repeats typing analysis, a clonal population of M. tuberculosis was implicated as the cause of a sustained outbreak of tuberculosis in Canada. However, in a seminal study by Gardy et al., integration of genome sequence information and social network analysis led to the discovery that the outbreak was caused by two circulating lineages sustained by crack cocaine users [44••]. In addition, HTS has been applied retrospectively to analyse S. aureus epidemics in hospitals revealing the dynamics of outbreaks confined to single hospital wards [3, 40•], and transmission between the hospital and community settings [17•]. The directionality of transmission, although often ambiguous, may sometimes be inferred by combining epidemiological data and analysis of bacterial population genome sequences [12•, 15]. For example, in a study of Burkholderia dolosa associated with chronic infections in a CF patient cohort, transmission events could be inferred from the phylogeny based on patterns of shared polymorphisms [15]. Also, a study of patients with M. abscessus subspecies massiliense pulmonary infections showed greater genetic diversity among isolates from a single patient than from different patients, suggestive of inter-patient transmission events [12•]. Recently, the development of novel bioinformatic algorithms has facilitated the identification of transmission events while accounting for the heterogeneity present in infecting bacterial populations [49].
Understanding the molecular basis for the emergence of pathogenic clones
The capacity to sequence large numbers of closely related isolates allows high-resolution phylogenies to be reconstructed, which may provide insights into the processes underlying the emergence and spread of pathogenic clones. Bacterial populations accumulate random mutations over time through inherent mutation rates specific for the organism and its ecology. Estimates of the mutation rate for a given bacterial population allow the construction of time-calibrated phylogenetic trees [50]. In particular, the application of Bayesian phylogenetic methods to sequences of closely related bacteria, can allow a time-scaled reconstruction of their evolutionary history and geographic dissemination, and may also reveal genetic events that correlate with the emergence of successful clones. The evolutionary history of an increasing array of bacterial pathogens has been examined using this approach including S. aureus, Shigella sonnei, S. Typhimurium and Vibrio cholerae [42, 43•, 51, 52, 53, 54, 55, 56]. In a concise review of this nature we can provide just a few recent examples. Methicillin-resistant S. aureus (MRSA) is an important cause of nosocomial infection in the United Kingdom, and recent studies have described genetic events preceding the emergence of the major hospital-adapted lineages EMRSA-15 and EMRSA-16, including polymorphisms associated with increased antimicrobial resistance and reduced virulence [42, 43•]. The reduction in virulence likely represents a fitness compensation in response to the increased energy costs associated with antibiotic resistance. In addition, a recent HTS study of invasive S. Typhimurium circulating in sub-Saharan Africa revealed the existence of two distinct epidemics that correlated strongly with peaks in HIV incidence in the region, suggesting that the increased number of immunocompromised hosts in sub-Saharan Africa has been a major contributor to the success of invasive S. Typhimurium [53]. Similarly, a study of S. Typhimurium DT104 from livestock and human hosts employed Bayesian phylogenetic methods with extended analysis of host-tropism using Markov jump methods to demonstrate that circulating DT104 strains represent independent epidemics. The data indicated that there had been limited cross-species transmission, contradicting a long-standing assumption regarding the zoonotic spread of S. Typhimurium [54].
In another HTS-based evolutionary study, it was demonstrated that the 7th cholera pandemic comprised of three independent waves, with the latter two being driven through acquisition of the SXT antibiotic resistance element by V. cholerae. This resulted in resistance to the commonly used anti-cholera therapies [52]. In the aftermath of the 2010 earthquake in Haiti, the country suffered from a prolonged outbreak of cholera. Phylogenetic reconstruction based on whole genome sequences of V. cholerae from affected patients identified several intercontinental transmission events originating from a source population in the Bay of Bengal [52]. Further analysis revealed that Haitian epidemic strains are related to strains circulating in Nepal, implicating movements of Nepali peacekeepers in the emergence of the epidemic [55] following a single introduction to Haiti [56].
The impact of human clinical interventions on the emergence of successful bacterial clones has been demonstrated for the S. pneumoniae PMEN1 lineage [57, 58]. Following introduction of a conjugate polysaccharide vaccine, capsule switching was observed [57] leading to the emergence of the vaccine escape serotype 19A. It was revealed that the geographic distribution of serotype 19A has rapidly expanded to replace vaccine-susceptible serotypes [58]. Further work based on HTS of 616 S. pneumoniae isolates identified variation in recombination rates between branches of the species’ phylogeny and also revealed an association between specific genetic determinants of S. pneumoniae and the age of the host. These data are consistent with adaptation of S. pneumoniae in response to the maturing host immune response [59••].
HTS also played a central role in the near real-time characterization of a high mortality outbreak of haemolytic-uraemic syndrome (HUS) in central Europe during 2011 [60, 61, 62], rapidly revealing that the increased virulence of the O104:H4 outbreak was likely due to acquisition of a Shiga-toxin encoding prophage [62], and that the strain was refractory to antibiotic therapy due to carriage of an extended spectrum β-lactamase [60].
The molecular basis of bacterial host switches
The high resolution phylogenetic analysis possible using HTS has enhanced our understanding of the capacity of bacterial pathogens to switch host species and adapt to survive and spread among novel host populations [51, 63, 64, 65, 66]. In particular, several studies have identified livestock as reservoirs for emerging bacterial strains capable of causing disease in humans [51, 63, 65]. For example, Price et al. examined the multi-host association of the ST398 clone of S. aureus by HTS providing evidence for the emergence of antibiotic resistant strains resulting from the use of antibiotics in the livestock industry [63]. Similarly, Spoor et al. demonstrated that cows are a potential reservoir of new strains of S. aureus with the capacity for pandemic spread in humans [51].
In addition to understanding the dynamics of cross-species transmission, HTS allows high-resolution analysis of genetic correlates of host specificity. For example, in one of the few genome-wide association studies (GWAS) of bacteria to be carried out, Shepphard et al. identified a genomic region encoding vitamin B5 biosynthesis components which is associated with adaptation of Campylobacter jejuni to the bovine host [67•]. Also, a S. Typhimurium ST4/74 transposon mutant pool was used to infect avian, bovine, and porcine hosts revealing host-specific gene repertoires, including genes associated with anaerobic growth in the avian host [68••]. Finally, HTS of isolates of the host-restricted DT2 lineage of S. Typhimurium and closely related strains suggested that adaptation of the DT2 lineage to the rock pigeon was not mediated by acquisition of novel genetic material, but rather by polymorphisms in the pre-existing genetic repertoire [69].
Future applications of HTS for understanding the biology of bacterial pathogens
The pace of development of sequencing technologies shows no sign of slowing, and platforms capable of single-molecule sequencing and ever increasing read lengths offer the possibility of highly accurate assemblies of individual pathogens within a microbial community [70]. While bacterial culture has previously been an essential step for the isolation of enough genomic DNA for whole genome sequencing, novel culture-free methodologies offer the ability to sequence un-culturable organisms. Such applications have relevance for investigating the cause of infectious diseases of unknown aetiology. Furthermore, the rapid diagnosis and in silico determination of sensitivity profiles of pathogens without the necessity for culture have obvious benefits for the treatment of clinical infections [71•, 72]. Furthermore, transcriptomic analysis of complex populations of bacteria within the microbiota will be theoretically feasible. To date, transcriptomic studies have focused on either the pathogen or the host. However, a comprehensive understanding of host–pathogen interactions would require simultaneous analysis of gene expression of both parties during infection. Dual RNA-seq offers the potential for transcriptomic analysis of both host and pathogen during the course of colonization and infection but there are technical difficulties to overcome before routine implementation of this technology is feasible, including removal of both bacterial and host rRNA, and large scale differences in relative amounts of RNA for host and pathogen [73]. Finally, the availability of genome sequences for large numbers of well-defined clinical isolates lends itself to the application of GWAS to bacteria, a method originally developed for human genetic association analysis which has the potential for unbiased identification of genetic determinants associated with a given phenotype [74]. For example molecular correlates of virulence or host-specificity may be determined through simultaneous analysis of genome data and the results from virulence assays [67•, 75, 76].
Overall the development of HTS technologies has revolutionized how we approach fundamental research into infectious diseases. Without doubt the new approaches will result in a very enhanced understanding of the biology of bacterial pathogens which will ultimately lead to improved infection control.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We are grateful to current and past members of the LBEP lab for their contributions to research on the evolutionary genomics of bacterial pathogens, and to the Biotechnology and Biological Sciences Research Council (UK) for ongoing funding.
References
- 1•.Loman N.J., Constantinidou C., Chan J.Z.M., Halachev M., Sergeant M., Penn C.W., Robinson E.R., Pallen M.J. High-throughput bacterial genome sequencing: an embarrassment of choice, a world of opportunity. Nat Rev Microbiol. 2012;10:599–606. doi: 10.1038/nrmicro2850. [DOI] [PubMed] [Google Scholar]; Comparison of high throughput sequencing platforms and their performance.
- 2.Loman N.J., Misra R.V., Dallman T.J., Constantinidou C., Gharbia S.E., Wain J., Pallen M.J. Performance comparison of benchtop high-throughput sequencing platforms. Nat Biotechnol. 2012;30:434–439. doi: 10.1038/nbt.2198. [DOI] [PubMed] [Google Scholar]
- 3.Eyre D.W., Golubchik T., Gordon N.C., Bowden R., Piazza P., Batty E.M., Ip C.L.C., Wilson D.J., Didelot X., O’Connor L. A pilot study of rapid benchtop sequencing of Staphylococcus aureus and Clostridium difficile for outbreak detection and surveillance. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filiatrault M.J. Progress in prokaryotic transcriptomics. Curr Opin Microbiol. 2011;14:579–586. doi: 10.1016/j.mib.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 5•.Croucher N.J., Thomson N.R. Studying bacterial transcriptomes using RNA-seq. Curr Opin Microbiol. 2010;13:619–624. doi: 10.1016/j.mib.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; A good review of RNA-seq technology.
- 6.Van Opijnen T., Bodi K.L., Camilli A. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods. 2009;6:767–772. doi: 10.1038/nmeth.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Langridge G.C., Phan M.D., Turner D.J., Perkins T.T., Parts L., Haase J., Charles I., Maskell D.J., Peters S.E., Dougan G. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res. 2009;19:2308–2316. doi: 10.1101/gr.097097.109. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first examples of transposon mutagenesis on a high-throughput scale.
- 8.Goodman A.L., McNulty N.P., Zhao Y., Leip D., Mitra R.D., Lozupone C.A., Knight R., Gordon J.I. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe. 2009;6:279–289. doi: 10.1016/j.chom.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gawronski J.D., Wong S.M.S., Giannoukos G., Ward D.V., Akerley B.J. Tracking insertion mutants within libraries by deep sequencing and a genome-wide screen for Haemophilus genes required in the lung. Proc Natl Acad Sci U S A. 2009;106:16422–16427. doi: 10.1073/pnas.0906627106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Opijnen T., Camilli A. Transposon insertion sequencing: a new tool for systems-level analysis of microorganisms. Nat Rev Microbiol. 2013;11 doi: 10.1038/nrmicro3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barquist L., Boinett C.J., Cain A.K. Approaches to querying bacterial genomes with transposon-insertion sequencing. RNA Biol. 2013;10:1161–1169. doi: 10.4161/rna.24765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Bryant J.M., Grogono D.M., Greaves D., Foweraker J., Roddick I., Inns T., Reacher M., Haworth C.S., Curran M.D., Harris S.R. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet. 2013;381:1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reveals how an understanding of intra-host and inter-host bacterial diversity can be used to infer transmission events.
- 13.Golubchik T., Batty E.M., Miller R.R., Farr H., Young B.C., Larner-Svensson H., Fung R., Godwin H., Knox K., Votintseva A. Within-host evolution of Staphylococcus aureus during asymptomatic carriage. PLoS ONE. 2013;8:e61319. doi: 10.1371/journal.pone.0061319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Young B.C., Golubchik T., Batty E.M., Fung R., Larner-Svensson H., Votintseva A.A., Miller R.R., Godwin H., Knox K., Everitt R.G. Evolutionary dynamics of Staphylococcus aureus during progression from carriage to disease. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1113219109. [DOI] [PMC free article] [PubMed] [Google Scholar]; Seminal paper highlighting the level of genetic diversity in colonising S. aureus isolates, and genetic changes associated with disease progression.
- 15.Lieberman T.D., Michel J.-B., Aingaran M., Potter-Bynoe G., Roux D., Davis M.R., Skurnik D., Leiby N., LiPuma J.J., Goldberg J.B. Parallel bacterial evolution within multiple patients identifies candidate pathogenicity genes. Nat Genet. 2011;43:1275–1280. doi: 10.1038/ng.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford C.B., Lin P.L., Chase M.R., Shah R.R., Iartchouk O., Galagan J., Mohaideen N., Ioerger T.R., Sacchettini J.C., Lipsitch M. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat Genet. 2011;43:482–486. doi: 10.1038/ng.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Harris S.R., Cartwright E.J., Török M.E., Holden M.T., Brown N.M., Ogilvy-Stuart A.L., Ellington M.J., Quail M.A., Bentley S.D., Parkhill J. Whole-genome sequencing for analysis of an outbreak of meticillin-resistant Staphylococcus aureus: a descriptive study. Lancet Infect Dis. 2013;13:130–136. doi: 10.1016/S1473-3099(12)70268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; A good example of how sequencing of bacterial genomes during a hospital outbreak can be used to inform infection control strategies.
- 18.McAdam P.R., Holmes A., Templeton K.E., Fitzgerald J.R. Adaptive evolution of Staphylococcus aureus during chronic endobronchial infection of a cystic fibrosis patient. PLoS ONE. 2011;6:e24301. doi: 10.1371/journal.pone.0024301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieberman T.D., Flett K.B., Yelin I., Martin T.R., McAdam A.J., Priebe G.P., Kishony R. Genetic variation of a bacterial pathogen within individuals with cystic fibrosis provides a record of selective pressures. Nat Genet. 2013;46(1):82–87. doi: 10.1038/ng.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreutzfeldt K.M., McAdam P.R., Claxton P., Holmes A., Seagar A.L., Laurenson I.F., Fitzgerald J.R. Molecular longitudinal tracking of Mycobacterium abscessus spp. during chronic infection of the human lung. PLoS ONE. 2013;8:e63237. doi: 10.1371/journal.pone.0063237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawlik A., Garnier G., Orgeur M., Tong P., Lohan A., Le Chevalier F., Sapriel G., Roux A.-L., Conlon K., Honoré N. Identification and characterization of the genetic changes responsible for the characteristic smooth-to-rough morphotype alterations of clinically persistent Mycobacterium abscessus. Mol Microbiol. 2013 doi: 10.1111/mmi.12387. [DOI] [PubMed] [Google Scholar]
- 22.Mwangi M.M., Wu S.W., Zhou Y., Sieradzki K., de Lencastre H., Richardson P., Bruce D., Rubin E., Myers E., Siggia E.D. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A. 2007;104:9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howden B.P., McEvoy C.R., Allen D.L., Chua K., Gao W., Harrison P.F., Bell J., Coombs G., Bennett-Wood V., Porter J.L. Evolution of multidrug resistance during Staphylococcus aureus infection involves mutation of the essential two component regulator WalKR. PLoS Pathog. 2011;7:e1002359. doi: 10.1371/journal.ppat.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24••.Zdziarski J., Brzuszkiewicz E., Wullt B., Liesegang H., Biran D., Voigt B., Grönberg-Hernandez J., Ragnarsdottir B., Hecker M., Ron E.Z. Host imprints on bacterial genomes — rapid, divergent evolution in individual patients. PLoS Pathog. 2010;6:e1001078. doi: 10.1371/journal.ppat.1001078. [DOI] [PMC free article] [PubMed] [Google Scholar]; A unique study demonstrating human host-specific adaptations acquired by an E. coli strain used for therapeutic management of recurrent urinary tract infections.
- 25.Salipante S.J., Sengupta D.J., Rosenthal C., Costa G., Spangler J., Sims E.H., Jacobs M.A., Miller S.I., Hoogestraat D.R., Cookson B.T. Rapid 16S rRNA next-generation sequencing of polymicrobial clinical samples for diagnosis of complex bacterial infections. PLoS ONE. 2013;8:e65226. doi: 10.1371/journal.pone.0065226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blainey P.C., Milla C.E., Cornfield D.N., Quake S.R. Quantitative analysis of the human airway microbial ecology reveals a pervasive signature for cystic fibrosis. Sci Transl Med. 2012;4:153ra130. doi: 10.1126/scitranslmed.3004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willner D., Furlan M., Haynes M., Schmieder R., Angly F.E., Silva J., Tammadoni S., Nosrat B., Conrad D., Rohwer F. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS ONE. 2009;4:e7370. doi: 10.1371/journal.pone.0007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modi S.R., Lee H.H., Spina C.S., Collins J.J. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature. 2013;499:219–222. doi: 10.1038/nature12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fancello L., Desnues C., Raoult D., Rolain J.M. Bacteriophages and diffusion of genes encoding antimicrobial resistance in cystic fibrosis sputum microbiota. J Antimicrob Chemother. 2011;66:2448–2454. doi: 10.1093/jac/dkr315. [DOI] [PubMed] [Google Scholar]
- 30.Dethlefsen L., Relman D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl):4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorth P., Trivedi U., Rumbaugh K., Whiteley M. Probing bacterial metabolism during infection using high-resolution transcriptomics. J Bacteriol. 2013;195:4991–4998. doi: 10.1128/JB.00875-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langereis J.D., Zomer A., Stunnenberg H.G., Burghout P., Hermans P.W.M. Nontypeable Haemophilus influenzae carbonic anhydrase is important for environmental and intracellular survival. J Bacteriol. 2013;195:2737–2746. doi: 10.1128/JB.01870-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perkins T.T., Davies M.R., Klemm E.J., Rowley G., Wileman T., James K., Keane T., Maskell D., Hinton J.C.D., Dougan G. ChIP-seq and transcriptome analysis of the OmpR regulon of Salmonella enterica serovars Typhi and Typhimurium reveals accessory genes implicated in host colonization. Mol Microbiol. 2013;87:526–538. doi: 10.1111/mmi.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao H.Q., Chen C., Xiong Y.W., Xu X.F., Lan R.T., Wang H.Y., Yao X.Y., Bai X.N., Liu X.T., Meng Q. Global transcriptional and phenotypic analyses of Escherichia coli O157:H7 strain Xuzhou21 and its pO157_Sal cured mutant. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0065466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barquist L., Langridge G.C., Turner D.J., Phan M.D., Turner A.K., Bateman A., Parkhill J., Wain J., Gardner P.P. A comparison of dense transposon insertion libraries in the Salmonella serovars Typhi and Typhimurium. Nucleic Acids Res. 2013;41:4549–4564. doi: 10.1093/nar/gkt148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Van Opijnen T., Camilli A. A fine scale phenotype–genotype virulence map of a bacterial pathogen. Genome Res. 2012;22:2541–2551. doi: 10.1101/gr.137430.112. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study uses transposon mutagenesis in more than 17 different environments to compare genes required for adaptation in different conditions.
- 37.De Vries S.P.W., Eleveld M.J., Hermans P.W.M., Bootsma H.J. Characterization of the molecular interplay between Moraxella catarrhalis and human respiratory tract epithelial cells. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0072193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Wong S.M., Bernui M., Shen H., Akerley B.J. Genome-wide fitness profiling reveals adaptations required by Haemophilus in coinfection with influenza A virus in the murine lung. Proc Natl Acad Sci U S A. 2013;110:15413–15418. doi: 10.1073/pnas.1311217110. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first application of Tn-seq for analysis of bacterial genes involved in co-infection.
- 39.Köser C.U., Ellington M.J., Cartwright E.J.P., Gillespie S.H., Brown N.M., Farrington M., Holden M.T.G., Dougan G., Bentley S.D., Parkhill J. Routine use of microbial whole genome sequencing in diagnostic and public health microbiology. PLoS Pathog. 2012;8:e1002824. doi: 10.1371/journal.ppat.1002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Köser C.U., Holden M.T.G., Ellington M.J., Cartwright E.J.P., Brown N.M., Ogilvy-Stuart A.L., Hsu L.Y., Chewapreecha C., Croucher N.J., Harris S.R. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med. 2012;366:2267–2275. doi: 10.1056/NEJMoa1109910. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first applications of HTS to tracing a hospital outbreak of infection.
- 41.Lewis T., Loman N.J., Bingle L., Jumaa P., Weinstock G.M., Mortiboy D., Pallen M.J. High-throughput whole-genome sequencing to dissect the epidemiology of Acinetobacter baumannii isolates from a hospital outbreak. J Hosp Infect. 2010;75:37–41. doi: 10.1016/j.jhin.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 42.McAdam P.R., Templeton K.E., Edwards G.F., Holden M.T.G., Feil E.J., Aanensen D.M., Bargawi H.J.A., Spratt B.G., Bentley S.D., Parkhill J. Molecular tracing of the emergence, adaptation, and transmission of hospital-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2012;109:1–6. doi: 10.1073/pnas.1202869109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Holden M.T.G., Hsu L.-Y., Kurt K., Weinert L.A., Mather A.E., Harris S.R., Strommenger B., Layer F., Witte W., de Lencastre H. A genomic portrait of the emergence, evolution, and global spread of a methicillin-resistant Staphylococcus aureus pandemic. Genome Res. 2013;23:653–664. doi: 10.1101/gr.147710.112. [DOI] [PMC free article] [PubMed] [Google Scholar]; Papers 42–43 highlighting the genetic changes preceding the global emergence of hospital associated MRSA.
- 44••.Gardy J.L., Johnston J.C., Ho Sui S.J., Cook V.J., Shah L., Brodkin E., Rempel S., Moore R., Zhao Y., Holt R. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011;364:730–739. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]; A landmark study combining genome sequences and epidemiological patient information to identify transmission of tuberculosis during a sustained outbreak.
- 45.Baker S., Holt K.E., Clements A.C.A., Karkey A., Arjyal A., Boni M.F., Dongol S., Hammond N., Koirala S., Duy P.T. Combined high-resolution genotyping and geospatial analysis reveals modes of endemic urban typhoid fever transmission. Open Biol. 2011;1:110008. doi: 10.1098/rsob.110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holt K.E., Thieu Nga T.V., Thanh D.P., Vinh H., Kim D.W., Vu Tra M.P., Campbell J.I., Hoang N.V.M., Vinh N.T., Minh P.V. Tracking the establishment of local endemic populations of an emergent enteric pathogen. Proc Natl Acad Sci U S A. 2013;110:17522–17527. doi: 10.1073/pnas.1308632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holt K.E., Baker S., Weill F.-X., Holmes E.C., Kitchen A., Yu J., Sangal V., Brown D.J., Coia J.E., Kim D.W. Shigella sonnei genome sequencing and phylogenetic analysis indicate recent global dissemination from Europe. Nat Genet. 2012;44:1056–1059. doi: 10.1038/ng.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reuter S., Harrison T.G., Köser C.U., Ellington M.J., Smith G.P., Parkhill J., Peacock S.J., Bentley S.D., Török M.E. A pilot study of rapid whole-genome sequencing for the investigation of a Legionella outbreak. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eyre D.W., Cule M.L., Griffiths D., Crook D.W., Peto T.E.A., Walker A.S., Wilson D.J. Detection of mixed infection from bacterial whole genome sequence data allows assessment of its role in Clostridium difficile transmission. PLoS Comput Biol. 2013;9:e1003059. doi: 10.1371/journal.pcbi.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gray R.R., Tatem A.J., Johnson J.A., Alekseyenko A.V., Pybus O.G., Suchard M.A., Salemi M. Testing spatiotemporal hypothesis of bacterial evolution using methicillin-resistant Staphylococcus aureus ST239 genome-wide data within a Bayesian framework. Mol Biol Evol. 2011;28:1593–1603. doi: 10.1093/molbev/msq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spoor L.E., McAdam P.R., Weinert L.A., Rambaut A., Hasman H., Aarestrup F.M., Kearns A.M., Larsen A.R., Skov R.L., Fitzgerald J.R. Livestock origin for a human pandemic clone of community-associated methicillin-resistant Staphylococcus aureus. MBio. 2013;4 doi: 10.1128/mBio.00356-13. e00356–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mutreja A., Kim D.W., Thomson N.R., Connor T.R., Lee J.H., Kariuki S., Croucher N.J., Choi S.Y., Harris S.R., Lebens M. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature. 2011 doi: 10.1038/nature10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okoro C.K., Kingsley R.A., Connor T.R., Harris S.R., Parry C.M., Al-Mashhadani M.N., Kariuki S., Msefula C.L., Gordon M.A., de Pinna E. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet. 2012 doi: 10.1038/ng.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mather A.E., Reid S.W.J., Maskell D.J., Parkhill J., Fookes M.C., Harris S.R., Brown D.J., Coia J.E., Mulvey M.R., Gilmour M.W. Distinguishable epidemics of multidrug-resistant Salmonella Typhimurium DT104 in different hosts. Science. 2013;341:1514–1517. doi: 10.1126/science.1240578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hendriksen R.S., Price L.B., Schupp J.M., Gillece J.D., Kaas R.S., Engelthaler D.M., Bortolaia V., Pearson T., Waters A.E., Prasad Upadhyay B. Population genetics of Vibrio cholerae from Nepal in 2010: evidence on the origin of the Haitian outbreak. MBio. 2011;2 doi: 10.1128/mBio.00157-11. e00157–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katz L.S., Petkau A., Beaulaurier J., Tyler S., Antonova E.S., Turnsek M.A., Guo Y., Wang S., Paxinos E.E., Orata F. Evolutionary dynamics of Vibrio cholerae O1 following a single-source introduction to Haiti. MBio. 2013;4 doi: 10.1128/mBio.00398-13. e00398–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Croucher N.J., Harris S.R., Fraser C., Quail M.A., Burton J., van der Linden M., McGee L., von Gottberg A., Song J.H., Ko K.S. Rapid pneumococcal evolution in response to clinical interventions. Science. 2011;331:430–434. doi: 10.1126/science.1198545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Golubchik T., Brueggemann A.B., Street T., Gertz R.E., Spencer C.C.A., Ho T., Giannoulatou E., Link-Gelles R., Harding R.M., Beall B. Pneumococcal genome sequencing tracks a vaccine escape variant formed through a multi-fragment recombination event. Nat Genet. 2012;44:352–355. doi: 10.1038/ng.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Croucher N.J., Finkelstein J.A., Pelton S.I., Mitchell P.K., Lee G.M., Parkhill J., Bentley S.D., Hanage W.P., Lipsitch M. Population genomics of post-vaccine changes in pneumococcal epidemiology. Nat Genet. 2013;45:656–663. doi: 10.1038/ng.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]; 3 excellent papers 57–59 highlighting the genetic changes in S. pneumoniae populations following the introduction of a vaccine.
- 60.Frank C., Werber D., Cramer J.P., Askar M., Faber M., an der Heiden M., Bernard H., Fruth A., Prager R., Spode A. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365:1771–1780. doi: 10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- 61.Rasko D.A., Webster D.R., Sahl J.W., Bashir A., Boisen N., Scheutz F., Paxinos E.E., Sebra R., Chin C.-S., Iliopoulos D. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med. 2011;365:709–717. doi: 10.1056/NEJMoa1106920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rohde H., Qin J., Cui Y., Li D., Loman N.J., Hentschke M., Chen W., Pu F., Peng Y., Li J. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N Engl J Med. 2011;365:718–724. doi: 10.1056/NEJMoa1107643. [DOI] [PubMed] [Google Scholar]
- 63.Price L.B., Stegger M., Hasman H., Aziz M., Larsen J., Andersen P.S., Pearson T., Waters A.E., Foster J.T., Schupp J. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. MBio. 2012;3 doi: 10.1128/mBio.00305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrison E.M., Paterson G.K., Holden M.T.G., Larsen J., Stegger M., Larsen A.R., Petersen A., Skov R.L., Christensen J.M., Bak Zeuthen A. Whole genome sequencing identifies zoonotic transmission of MRSA isolates with the novel mecA homologue mecC. EMBO Mol Med. 2013;5:509–515. doi: 10.1002/emmm.201202413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lebreton F., van Schaik W., McGuire A.M., Godfrey P., Griggs A., Mazumdar V., Corander J., Cheng L., Saif S., Young S. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. MBio. 2013;4 doi: 10.1128/mBio.00534-13. e00534–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lowder B.V., Guinane C.M., Ben Zakour N.L., Weinert L.A., Conway-Morris A., Cartwright R.A., Simpson A.J., Rambaut A., Nübel U., Fitzgerald J.R. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2009;106:19545–19550. doi: 10.1073/pnas.0909285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Sheppard S.K., Didelot X., Meric G., Torralbo A., Jolley K.A., Kelly D.J., Bentley S.D., Maiden M.C.J., Parkhill J., Falush D. Genome-wide association study identifies vitamin B5 biosynthesis as a host specificity factor in Campylobacter. Proc Natl Acad Sci U S A. 2013;110:11923–11927. doi: 10.1073/pnas.1305559110. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first applications of GWAS to bacteria: identification of genetic correlates of host specificity.
- 68••.Chaudhuri R.R., Morgan E., Peters S.E., Pleasance S.J., Hudson D.L., Davies H.M., Wang J.H., van Diemen P.M., Buckley A.M., Bowen A.J. Comprehensive assignment of roles for Salmonella Typhimurium genes in intestinal colonization of food-producing animals. PLoS Genet. 2013:9. doi: 10.1371/journal.pgen.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent study demonstrating the use of transposon mutagenesis in vivo to identify genes associated with survival in multiple host species.
- 69.Kingsley R.A., Kay S., Connor T., Barquist L., Sait L., Holt K.E., Sivaraman K., Wileman T., Goulding D., Clare S. Genome and transcriptome adaptation accompanying emergence of the definitive type 2 host-restricted Salmonella enterica serovar Typhimurium pathovar. MBio. 2013;4 doi: 10.1128/mBio.00565-13. e00565–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chin C.-S., Alexander D.H., Marks P., Klammer A.A., Drake J., Heiner C., Clum A., Copeland A., Huddleston J., Eichler E.E. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 71•.Loman N.J., Constantinidou C., Christner M., Rohde H., Chan J.Z.M., Quick J., Weir J.C., Quince C., Smith G.P., Betley J.R. A culture-independent sequence-based metagenomics approach to the investigation of an outbreak of Shiga-toxigenic Escherichia coli. JAMA. 2013;309:1502. doi: 10.1001/jama.2013.3231. [DOI] [PubMed] [Google Scholar]; Demonstration of the potential of metagenomic analysis of complex samples for investigating outbreaks in a culture-independent manner.
- 72.Seth-Smith H.M.B., Harris S.R., Skilton R.J., Radebe F.M., Golparian D., Shipitsyna E., Duy P.T., Scott P., Cutcliffe L.T., O’Neill C. Whole-genome sequences of Chlamydia trachomatis directly from clinical samples without culture. Genome Res. 2013 doi: 10.1101/gr.150037.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Westermann A.J., Gorski S.A., Vogel J. Dual RNA-seq of pathogen and host. Nat Rev Microbiol. 2012;10:618–630. doi: 10.1038/nrmicro2852. [DOI] [PubMed] [Google Scholar]
- 74.Falush D., Bowden R. Genome-wide association mapping in bacteria? Trends Microbiol. 2006;14:353–355. doi: 10.1016/j.tim.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Priest N.K., Rudkin J.K., Feil E.J., van den Elsen J.M.H., Cheung A., Peacock S.J., Laabei M., Lucks D.A., Recker M., Massey R.C. From genotype to phenotype: can systems biology be used to predict Staphylococcus aureus virulence? Nat Rev Microbiol. 2012;10:791–797. doi: 10.1038/nrmicro2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laabei M., Recker M., Rudkin J.K., Aldeljawi M., Gulay Z., Sloan T.J., Williams P., Endres J.L., Bayles K.W., Fey P.D. Predicting the virulence of MRSA from its genome sequence. Genome Res. 2014;24:839–849. doi: 10.1101/gr.165415.113. [DOI] [PMC free article] [PubMed] [Google Scholar]