Graphical abstract
Abbreviations: BGE, background electrolyte; BSA, bovine serum albumin; CM, CE mode; CPC, condensation particle counter; DMA, differential mobility analyzer; EM, electrophoretic mobility; ES, electrospray; FM, flushing mode; GEMMA, gas-phase electrophoretic mobility molecular analyzer; IgG, γ globulin; MW, molecular weight; NM, normal mode; TEM, transmission electron microscopy
Keywords: GEMMA, Ion mobility spectrometer, Scanning mobility particle sizer, Capillary electrophoresis, Electrospray, Desalting
Highlights
-
•
First demonstration of a hyphenated CE–GEMMA combination for proteins.
-
•
Feasibility of on-line CE–GEMMA in a commercial device at ambient pressure.
-
•
On-line combination of liquid phase and gas phase electrophoretic separation.
Abstract
Nanoparticle characterization is gaining importance in food technology, biotechnology, medicine, and pharmaceutical industry. An instrument to determine particle electrophoretic mobility (EM) diameters in the single-digit to double-digit nanometer range receiving increased attention is the gas-phase electrophoretic mobility molecular analyzer (GEMMA) separating electrophoretically single charged analytes in the gas-phase at ambient pressure. A fused-silica capillary is used for analyte transfer to the gas-phase by means of a nano electrospray (ES) unit. The potential of this capillary to separate analytes electrophoretically in the liquid phase due to different mobilities is, at measurement conditions recommended by the manufacturer, eliminated due to elevated pressure applied for sample introduction. Measurements are carried out upon constant feeding of analytes to the system. Under these conditions, aggregate formation is observed for samples including high amounts of non-volatile components or complex samples. This makes the EM determination of individual species sometimes difficult, if not impossible. With the current study we demonstrate that liquid phase electrophoretic separation of proteins (as exemplary analytes) occurs in the capillary (capillary zone electrophoresis, CE) of the nano ES unit of the GEMMA. This finding was consecutively applied for on-line desalting allowing EM diameter determination of analytes despite a high salt concentration within samples. The present study is to our knowledge the first report on the use of the GEMMA to determine EM diameters of analytes solubilized in the ES incompatible electrolyte solutions by the intended use of electrophoresis (in the liquid phase) during sample delivery. Results demonstrate the proof of concept of such an approach and additionally illustrate the high potential of a future on-line coupling of a capillary electrophoresis to a GEMMA instrument.
1. Introduction
Recent years saw a fast growing interest in nanoparticles, i.e., particles up to the range of a few 100 nm in diameter. However, research concerning the applicability of nanoparticles in various fields like biotechnology, medicine, pharmaceutical or food industry, and as well risk assessment of nanoparticle application relies on well-defined material (so-called certified reference material) for experimental work. Severe concerns relating to the safety of application of certain types of nanoparticle have currently been raised [1–6]. Typically, methods like transmission electron microscopy (TEM) are employed for the analysis of nanoparticle size distributions (as recently shown by Lin et al. [7]). Ion mobility mass spectrometry (IM-MS, as for instance reviewed in [8]) can be used to determine collision cross section values of biological macromolecules and protein assemblies. However, also gas-phase electrophoretic mobility molecular analysis (GEMMA) was introduced, which separates single charged analytes in the gas-phase at ambient pressure according to the analytes electrophoretic mobility (EM) diameters. In case of singly charged, spherical shaped analytes EM diameters correspond to particle diameters [9–12]. Recently, the GEMMA acronym is often being replaced by other terms like macro ion mobility spectrometer (macroIMS), LiquiScan-ES, nES-DMA or ES-SMPS spectrometer for the same instrument [13]. Nevertheless, for reasons of consistency with previous work we still employ the acronym GEMMA in this manuscript. Some very interesting instrumental characteristics like (i) fast analysis times, (ii) minimal sample pretreatment, (iii) low cost instrumentation (especially when compared to TEM instruments or mass spectrometers) and most importantly, (iv) the possibility of single, number based particle detection (i.e., determination of number concentrations) make the GEMMA very attractive, especially for the characterization of analytes in the nm size range (for a selection of papers concerning proteins [14,15], viruses [14,16,17], and polymer materials [18,19] refer to respective publications).
The GEMMA device [9] consists of three parts: (i) a charge reducing nano electrospray (nano ES) unit for aerosolization of analytes from an aqueous liquid solution and charge conditioning in a bipolar atmosphere induced by a 210Po α-radiation source, (ii) a nano differential mobility analyzer (nano DMA) as separation/sizing device, and (iii) an ultrafine condensation particle counter (CPC) as detector. In the nano ES unit, samples are electrosprayed from a cone tipped fused silica capillary in the cone–jet mode [20]. Additional forces influencing analyte migration though the nano ES capillary are (i) the applied high voltage along the capillary and (ii) the CO2/particle-free air sheath flow at the capillary tip. Subsequently, multiple charged, aerosolized droplets are dried and charge reduction occurs leading to a predictable equilibrium charge distribution of the nanoaerosol [21,22]. Particles are further transported via a sheath flow of air and CO2 (typically about 1 liter per minute, Lpm) into the nano DMA. There, particles are classified according to their EM diameter. An applied electric field with tunable strength between the electrodes of a cylindrical capacitor acts as orthogonal force to the high sheath flow applied between the electrodes to carry analytes through the instrument. It enables only nanoparticles of a certain (and narrow) EM diameter range to pass through the ion mobility analyzer. By variation of the applied field strength, a given EM diameter range can be scanned. (For a more detailed description of possible DMA setups refer to Flagan [23] and Intra and Tippayawong [24]). After passing the nano DMA, monodisperse analytes are transported to the CPC. There, particles are enlarged by condensation of supersaturated n-butanol or water vapor to optically detectable micrometer size for single particle detection upon passing of analytes through a focused laser beam [25]. In the past, GEMMA allowed already the determination of EM diameters for a number of analytes like large peptides and proteins [26,27], polysaccharides [19], glycoproteins [15], intact viruses [12,14,27,28], virus-like particles [29], vaccines [30], virus–antibody complexes [31], and gold particles [32–34], gelatin nanoparticles up to several 100 nm EM diameter [35] or carbon nanotubes [36]. Given a suitable, analyte specific calibration exists, the GEMMA derived EM diameter values can be converted to MW values, hence allowing MW determination of analytes even in a range which cannot be addressed by mass spectrometry.
Capillary electrophoresis (CE) separates analytes in solution (background electrolyte, BGE) upon application of an electric field. Analytes migrate with specific velocities through the capillary after equilibrium between an accelerating force (determined by the charge of an analyte and the electric field) and a retarding friction force (according to Stokes’ law) is reached. Analyte migration is described by the ratio between its velocity and the applied field strength (electrophoretic mobility, μi). Additionally, factors like the ionic strength of a given electrolyte solution and its pH play a role. For instance, BGEs with basic pH lead to movement of the solution bulk inside a fused silica capillary in direction of the cathode (electroosmosis) [37,38]. As the mobility of the electroosmotic flow (EOF) typically exceeds the electrophoretic net mobility (effective mobility) μieff of analytes, their overall movement is directed to the cathodic side of the capillary. In case the EOF mobility is known and the apparent mobility of an analyte is calculated from its migration behavior following standard protocols, μieff can be calculated by subtraction.
As the capillary employed for the nano ES unit of a conventional, commercially available GEMMA instrument fulfills all requirements of a standard CE setup, we investigated if electrophoretic separations can also be observed upon sample introduction to the GEMMA instrument. To our knowledge, until now, only the direct combination of a conventional CE instrument with a CPC detector (without a nano DMA in between) has been reported [39] and a non-commercially capillary isoelectric focusing instrumentation coupled to the GEMMA presented at a conference [40]. For standard measurements, electrophoretic separation of analytes in the nano ES capillary of a GEMMA instrument is not desired, instead analytes are continuously fed to the system. Therefore, electrophoretic effects in the liquid phase are suppressed by a relatively high pressure applied to the sample chamber (approximately 4 pounds per square inch differential, psid, which corresponds to approximately 0.3 bar along a 26 cm long capillary). However, this setup causes problems with samples either with complex composition or containing high amounts of salts (e.g. samples at physiological conditions). Note that the sample pretreatment by dilution in a volatile electrolyte solution is only practicable to a certain degree, because intended analytes would also become too diluted for analysis. With increasing concentration of non-volatile sample compounds, aerosolized droplets contain aggregates of sample components that make the determination of EM diameter values for actual analytes difficult, in the worst case even impossible. With the current study we therefore suggest to solve this problem by concentrating on the CE separation potential of a typical, commercially available GEMMA setup: by reduction of the applied pressure to the pressure chamber conditions in the nano ES capillary allowing electrophoresis can be obtained. This approach enables separation of analytes in the liquid phase of the nano ES source (i.e., in the capillary) prior to the determination of the analytes gas-phase electrophoretic mobilities coupling two electrophoresis based methods. It is therefore the aim of the current study (i) to present theoretical considerations considering CE separation of analytes in the capillary of the nano ES unit, (ii) to confirm these findings with experimental data obtained for two proteins as exemplary analytes to show the feasibility of this approach, and (iii) to demonstrate the applicability of our setup in the on-line desalting of a protein containing sample, which would otherwise only yield poorly interpretable spectra.
2. Materials and methods
2.1. Chemicals and reagents
Ammonium acetate (≥99.99%) and ammonium hydroxide (28.2% ammonia in water) were purchased from Sigma–Aldrich (St. Louis, MO, USA), sodium chloride (≥99.5%) as well as sodium hydroxide (≥99%) were obtained from Merck (Darmstadt, Germany). Ultra-high quality water was delivered from a Simplicity UV apparatus (Millipore, Molsheim, France) with 18.2 MΩ cm resistivity at 25 °C. Albumin (bovine, ≥96%, BSA, MW of 66 kDa according to manufacturing company, pI = 5.4 [41]) and γ globulin (bovine, ≥99%, IgG, MW 150 kDa according to manufacturing company, pI = 6.6 [41]) were purchased from Sigma–Aldrich as was dimethyl sulfoxide (DMSO, ≥99.9%). Benzoic acid (≥99.9%) was obtained from Fluka (Buchs, Switzerland).
2.2. Buffers
For CE and GEMMA analysis, ammonium acetate with identical ionic strength (I = 25 mmol L−1) but different pH values were employed: pH 7.4, 8.4, and pH 9.4 solutions were prepared. Ammonium hydroxide was used for pH adjustment of electrolytes. Solutions were filtered via >0.2 μm pore size syringe filters (sterile, surfactant free cellulose acetate membrane, Sartorius, Goettingen, Germany) prior application. Sodium hydroxide was at 1 mol L−1 concentration for flushing of the CE capillary between runs.
2.3. Sample preparation
Aqueous protein solutions of IgG and BSA (10 μmol L−1 each) were diluted tenfold in ammonium acetate (pH 7.4, 8.4, and 9.4, respectively) for the GEMMA experiments and twofold for the CE experiments. DMSO (1:8000 v/v final dilution) and benzoic acid (0.1 mmol L−1 final concentration) were applied in CE–UV samples as EOF marker and internal standard, respectively. Proteins were either combined in a mixed solution or present as single component samples as indicated within figures. For on-line desalting experiments, sodium chloride was added at 5 mmol L−1 final concentration to the GEMMA samples.
2.4. Instrumentation
The employed GEMMA system consisted of (i) a nano ES unit including a 210Po source (model 3480), (ii) a nano differential mobility analyzer (nano DMA, series 3080), and (iii) an ultrafine condensation particle counter (CPC, series 3025A). Samples were introduced into the nano ES unit via a 25 μm inner diameter and 26 cm long cone tipped fused silica capillary. The nano ES was operated with positive high voltage. All parts were from TSI Inc (Shoreview, MN, USA). For CE separations employing UV detection, an Agilent 3D CE (Waldbronn, Germany) was used. Electrophoresis was performed at 25 °C in positive polarity with a fused silica capillary of 50 μm inner diameter and Ltotal/effective = 60.2/51.7 cm from Polymicro (Phoenix, AZ, USA). Samples were introduced by application of 25 mbar pressure for 10 s. Analytes were separated by application of 20 kV leading to an electric field strength of 33.2 kV m−1. Detection was via assessment of UV absorption simultaneously at 200, 205, and 260 nm. Prior to each run, the capillary was equilibrated for 2 min with BGE and after each run it was washed for 1.5 min with sodium hydroxide solution followed by 2 min rinsing with water.
2.5. GEMMA analysis modes
The normal mode (NM) represents the conventional GEMMA setup used for analysis. For this, the sample is placed into the pressure chamber, and about 4 psid (approximately 0.3 bar) pressure and 2.6–2.8 kV are applied. This results typically in 300–500 nA current. The sheath flow in the nano ES unit is set to 1.1 Lpm (0.1 Lpm CO2, 1.0 Lpm air), the sheath flow in the nano DMA is at 13.5 Lpm. The scanning process (variation of the applied field strength) begins when a steady state is reached, i.e., when the sample is continuously fed to the system (about 5 min after the sample has initially been introduced to the GEMMA instrument). The scan range covers particles with the EM diameter between 2 nm and 60 nm, however, also a shorter scan range can be set. For every analysis a number of scans (n = 6) is taken with high repeatability and data from each scan is retrieved from the instrument. Subsequently (to correct for possibly occurring spikes), a resulting GEMMA spectrum is obtained as a median of these scans (individual scans are not depicted).
In the flushing mode (FM) the applied pressure in the nano ES unit is reduced to approx. 1 psid (approx. 70 mbar). Therefore, the impact of pressure gradient as driving force to move analytes through the capillary is greatly reduced. The voltage, sheath flow settings and the resulting current were as previously for NM. Furthermore, measurements are carried out not over a scan range but at a constant EM diameter value specific for a given analyte (determined via NM measurements at the peak apex of respective single charged analyte species – for BSA 7 nm, for IgG 9 nm EM diameter was set during FM, respectively). The time needed for analytes to pass through the capillary is determined. Measurements start immediately after immersion of the capillary end to the sample vial to allow for time determination of sample passage through the instrument and are terminated after a steady state (i.e., plateau of analyte detection) is reached.
For CE mode (CM) the same conditions (pressure, sheath flow, voltage) as in FM were applied. However, samples are injected to the system only for 2 s followed by the change of the sample tube to the respective electrolyte solution. This results in an analyte plug similar as for conventional CE setups. Additionally, in case of mixed samples (including BSA and IgG at 1 μmol L−1 concentration, each), no longer a constant EM diameter was regarded as for FM, but a size range between 6 nm and 12 nm EM diameter was scanned to follow the separation of analytes (scan time at 18 s, time for detector reset between scans at 3 s).
For FM and CM the capillary was filled with analyte free buffer prior to immersion of the sample.
3. Results and discussion
The results presented in the following sections demonstrate that under certain conditions electrophoretic separation of analytes can be achieved in the liquid phase within the capillary part of the nano ES unit of a conventional, commercially available GEMMA instrument. This was done theoretically calculating driving velocities in the nano ES capillary and their contribution to overall analyte migration. On the other hand, our theoretical considerations were verified experimentally. We monitored the separation of two proteins (BSA and IgG) as exemplary analytes in the nano ES capillary of the system. This finding opens up very interesting avenues like the on-line desalting of protein samples which we likewise demonstrated here. In the future, the CE separation of even nanoparticles prior to the GEMMA analysis by means of a standard GEMMA instrument seems to be easily feasible and an on-line hyphenation of a stand-alone CE to a GEMMA instrument appears highly interesting.
3.1. Theoretical considerations and experimental assessment of individual forces contributing to sample introduction to a GEMMA system via a nano ES process
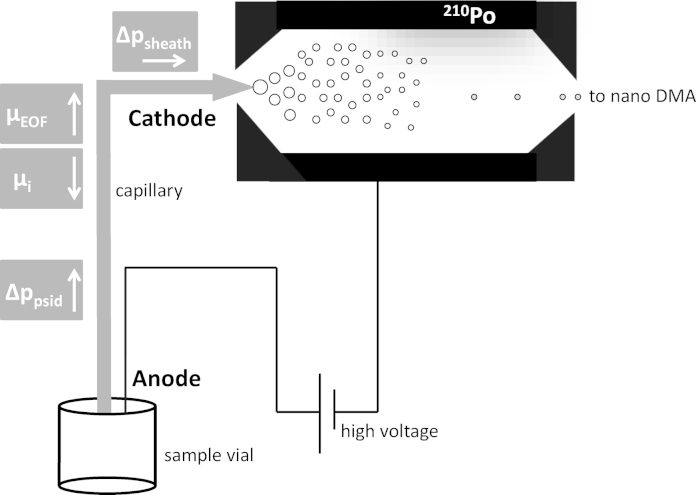
Samples are introduced into the nano ES unit of the GEMMA system via a fused silica cone tipped capillary. During sample introduction the movement of the analytes can be calculated by taking four processes into account: (i) pressure is applied to the pressure chamber of the instrument (Δppsid) to feed the sample containing electrolyte solution continuously to the nano ES capillary; (ii) upon application of electrolyte solutions in the basic pH range, electroosmosis occurs – the EOF (vEOF = E × μEOF) is directed to the cathode of the instrument, i.e., to the capillary tip (nano ES process operated with positive high voltage); (iii) the analytes electrophoretic net mobility μieff has to be regarded as well; (iv) finally, a mixed sheath flow of CO2 and air is applied at the capillary tip in order to transport droplets of the nano ES process to the nano DMA which may have a small impact (Δpsheath) on the pressure difference along the capillary. Fig. 1 gives an overview on respective contributions to analyte migration. Assessment of individual velocity contributions allows calculation of the overall time needed for analytes to pass through the GEMMA setup. The time needed for the analyte to pass through the nano DMA and to reach the CPC unit of the instrument is neglected as it is constant for all presented experiments. In doing so, the influence of Δppsid and Δpsheath on migration time of analytes can be calculated using Hagen–Poiseuille equation (capillary inner diameter, ID, d, 25 μm; in simplification the dynamic viscosity value of water, η, 1.002 mPa s, at 20 °C is used; length of capillary, L, 26 cm). The total movement of the analyte can be calculated with the following equation:
| (1) |
Fig. 1.
Schematic drawing of the nano ES unit of a GEMMA instrument: a sample vial is placed into the pressure chamber. Pressure and an electric field are applied leading to different forces acting on analyte particles upon sample introduction to the nano ES process. The directions of forces acting on particles in the liquid phase during sample introduction to the nano ES are indicated by arrows.
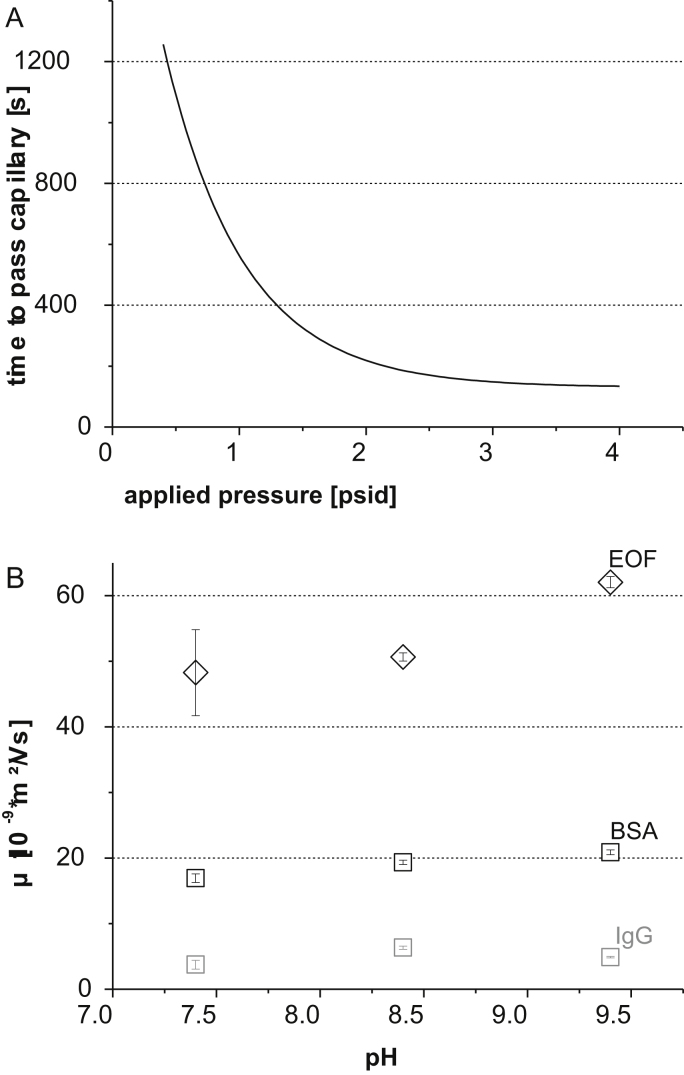
Calculated results for Δppsid are shown in Fig. 2(A); upon reduction of the applied pressure to the sample chamber, the time needed for analytes to pass through the nano ES capillary of the instrument is increased significantly.
Fig. 2.
Dependence of analyte migration time through the nano ES capillary on the applied pressure to the sample chamber (A): the migration time of analytes was calculated by Hagen–Poiseuille equation. Electrophoretic net mobilities of analytes under comparable conditions as during the nano ES process (B): the EOF mobility is increasing with increasing pH values, whereas the electrophoretic net mobilities of analytes do not change significantly.
Initially, Δpsheath was determined by measuring the time needed for water to pass an empty capillary by application of only this force at 2 Lpm. However, after 80 min still no solvent passing through the capillary was detected. Upon application of Hagen–Poiseuille equation the pressure difference to move the solvent through the capillary in 80 min was calculated. It was kept in mind that the time needed to fill an air filled capillary is only half the time needed to fill a previously solvent filled capillary (viscosity of air was neglected). Consequently, the sheath flow contribution was found to be smaller than 0.05 psid (approximately 3 mbar) and is hence negligible in comparison to Δppsid.
μEOF and μieff were determined by application of a conventional CE–UV setup. Samples containing 0.5 μmol L−1 BSA and IgG, respectively, in 25 mmol L−1 ammonium acetate at different pH values were analyzed. μEOF and μieff were calculated according to standard protocols from the migration of a neutral marker. Results are shown in Fig. 2(B) (average and standard deviation values for n = 4 measurements each are plotted). As expected, increasing pH values of ammonium acetate lead to an increase in the observed EOF, whereas net mobilities of analytes did not change significantly.
For the operation of the nano ES unit, a high electric field strength at the tip of the capillary is necessary to form a stable Taylor cone. This field is produced by a high voltage applied via a platin wire immersed in the sample solution and a grounded counter electrode. However, due to this design there is not only a voltage drop between the tip of the capillary and the counter electrode but also along the capillary. The electric field E along the capillary can be calculated as follows:
| (2) |
where I is the electric current measured by the nano ES unit, ID of the capillary, d, 2.5 × 10−5 m according to the manufacturing company and κ is the conductivity of the employed electrolyte solution. For a 25 mmol L−1 ammonium acetate solution at pH 9.4, κ was calculated to be 0.259 S m−1. As during the nano ES process an average current of 400 nA (see also experimental section) was recorded, E could be assessed in the range of 3.1 kV m−1. This is in the same order of magnitude as E values typically employed for CE separations on standard instruments.
Initial CE experiments were carried out at pH 7.4, 8.4, and 9.4, respectively. However, stable electrophoresis conditions were found only at slightly basic pH values; therefore, pH 9.4 was further applied for the GEMMA experiments. For this pH value, the time needed for BSA and IgG to migrate across the capillary considering Δppsid, μEOF, μieff was calculated as detailed above. The estimated time to pass the capillary was about 370 s for IgG and about 400 s for BSA (Δp = 1 psid, E = 3.1 kV m−1). The difference of migration times can be explained by different mobilities of respective proteins upon electrophoresis. Fig. 3 depicts corresponding spectra obtained in FM. The different time needed to pass a capillary for BSA (black) and IgG (grey) is clearly demonstrated. The migration time of IgG is about 30 s less than the migration time of BSA confirming theoretical calculations. However, it must be noted that the theoretically estimated migration times of analytes were approximately 80 s higher than measured values. A possible explanation for this observation might be found in uncertainties of the inner diameter of the used capillaries as well as in the pressure gauge of the instrument which is not designed for pressure determination with high precision. Differences between calculated and observed migration times can result from actual pressure values slightly deviating from read out values given by the instrument.
Fig. 3.
Analyte migration through the nano ES capillary in FM: BSA (black) and IgG (grey) samples (c = 1 μmol L−1 protein concentration, pH 9.4 ammonium acetate, respectively) were investigated. Measurements were performed at 1 psid (approximately 70 mbar) and 1.1 Lpm sheath flow at the capillary tip. Differences in BSA and IgG migration through the nano ES capillary are clearly detectable.
3.2. Instrumental feasibility
In order to separate analytes electrophoretically in the nano ES capillary of a standard GEMMA instrument we reduced Δppsid and Δpsheath to lowest possible values. However, initial experiments demonstrated (data not shown) that at least 0.4 psid (approximately 30 mbar) are necessary to transport analytes through the capillary as the signal intensity decreases with decreasing pressure applied to the sample at the capillary inlet. Likewise, if the sheath flow is further reduced, loss of the analyte signal in corresponding spectra is observed. The latter effect is most probably caused by insufficient transport of aerosolized particles from the capillary tip through the nano DMA to the detector unit of the instrument. A threshold of 0.3 Lpm sheath flow was determined.
3.3. GEMMA measurements in CE mode
The measurements of BSA and IgG solutions in ammonium acetate in CM were taken to confirm the results described already for FM (one protein per respective sample). The corresponding signals of analytes after injection to the GEMMA instrument are shown in Fig. 4 (n = 3 measurements). The difference between the migration time of IgG (grey) and BSA (black) is about 20 s and the peak width for both analytes about 30 s. These results again demonstrate the CE separation potential within the nano ES capillary of the commercial GEMMA instrument.
Fig. 4.
Analyte migration through the capillary in CM: BSA (black) and IgG (grey) samples were investigated under conditions as given in Fig. 3. Again, differences in analyte migration are detectable.
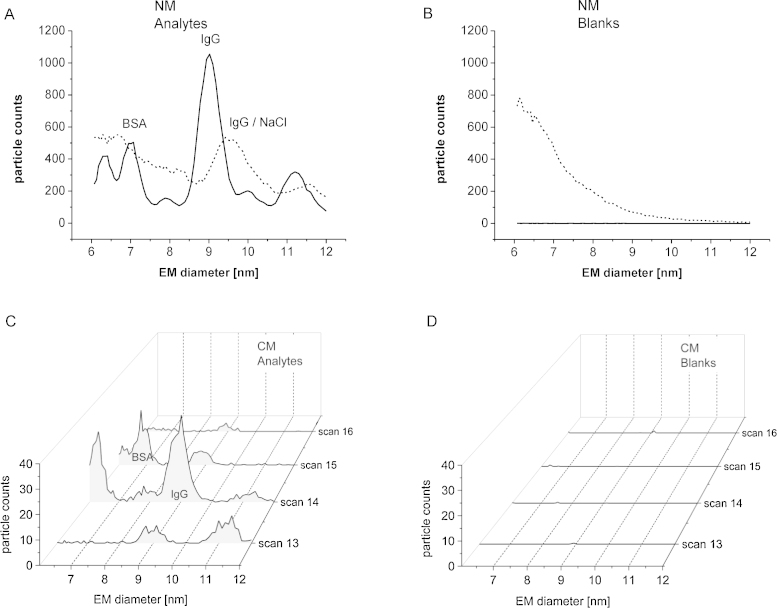
In a next step we mixed both proteins in a single sample and tried to resolve the two analytes via electrophoresis in the liquid phase in CM. Fig. 5 shows consecutive single GEMMA scans demonstrating the separation of the two proteins, BSA and IgG, from such a mixed sample. A peak corresponding to IgG with significant height can be found just in scan 15. BSA is detected only in scan 16. Small traces of IgG can be observed in scans 14 and 16, respectively. This can be explained by a broadly distributed IgG peak resulting from analyte molecules retained on the capillary wall or tip. A corresponding blank (data not shown) only gives a baseline signal. Nevertheless, it can be concluded that analytes injected from a single sample to a commercially available, conventional GEMMA instrument can indeed be separated by CE in the nano ES capillary. A true CE–GEMMA hyphenation is given in CM.
Fig. 5.
CE separation of BSA and IgG in CM: the sample contained both analytes, BSA and IgG at c = 1 μmol L−1 protein concentration and pH 9.4 ammonium acetate, respectively. Measurement conditions as in Fig. 3, median values from 6 individual measurements are shown. The IgG peak is detected in scan 15, BSA in scan 16. Hence, CE separation of analytes can be demonstrated.
3.4. On-line desalting in the nano ES capillary through electrophoretic analyte separation
Results from on-line desalting in the nano ES capillary of the GEMMA instrument (addition of 5 mmol L−1 sodium chloride to a mixed BSA/IgG sample in ammonium acetate) are depicted in Fig. 6, together with results for the same sample obtained in NM. GEMMA experiments in NM exhibit results shown in Fig. 6(A) (for samples) and Fig. 6(B) (for blanks), dashed lines, respectively. For comparison reasons also spectra recorded in the absence of sodium chloride (solid lines) are shown. Addition of sodium chloride leads to a massive increase in the background signal (Fig. 6(B)) due to salt aggregation in droplets generated by the nano ES process and their subsequent detection as residue particles. Concurrently, also aggregates between proteins and salt molecules are recorded. Whereas the IgG peak maximum shifts to higher EM diameter values by about 6% due to an unwanted salt crust formation around the protein, BSA can be no longer detected due to the increased background signal. Additionally, the IgG peak also increases in peak width as a result from heterogeneous protein/salt aggregates.
Fig. 6.
Comparison of the GEMMA spectra obtained for a BSA and IgG containing sample with and without sodium chloride addition in NM and CM: samples containing both analytes, BSA and IgG at c = 1 μmol L−1 (pH 9.4 ammonium acetate) were measured in NM (A); solid lines represent samples without salt addition, dashed lines depict the samples with sodium chloride addition (c = 5 mmol L−1). The salt addition also increases the background signal for a sample containing no protein, i.e., plain ammonium acetate (B). Additionally, BSA can no longer be detected in the presence of sodium chloride, the EM diameter of IgG is shifted to higher values and the width of the IgG peak increases significantly. Samples containing both analytes at c = 1 μmol L−1 and sodium chloride at c = 5 mmol L−1 were measured in CM as well (C) as were buffer blanks (D). The EM diameter of BSA and IgG correlates with the values determined from samples without sodium chloride addition measured in NM (see Fig. 5). Analytes were separated from the contaminating salt. Measurement conditions as in Fig. 3.
Taken together, these effects demonstrate well aggregation of proteinaceous analytes and sodium chloride for the GEMMA measurements: deposition of non-volatile salts on respective protein surfaces make the protein particles appear larger and more heterogeneous. However, GEMMA spectra in CM (analytes in Fig. 6(C), blanks in Fig. 6(D), respectively) demonstrate the feasibility of on-line desalting of samples. In contrast to spectra obtained in NM the EM diameter of BSA is again determined at 7 nm and for IgG at 9 nm. These values are in good accordance with values obtained for measurements of samples without salt addition (compare to Fig. 5). As was shown already in this previous figure, the proteins are again separated from each other.
It should be mentioned that 5 mmol L−1 sodium chloride is of course a value far from physiological buffer conditions. However, at the same time this concentration is such that it can be easily obtained via dilution of a sample in a volatile electrolyte solution, when analytes were originally dissolved in a physiological buffer. This is even the case for reasonable dilution factors still offering the possibility of analyte detection despite its lower concentration after dilution. Hence, the chosen sodium chloride concentration is an exemplary value, however, with significance for the analysis of future samples.
4. Conclusions
With the current work we demonstrate the electrophoretic separation of two proteins within the 26 cm nano ES capillary of a standard GEMMA device based on theoretical considerations describing the contribution of forces acting on analytes. Additionally, we verify our calculations experimentally. We found (i) BSA and IgG, two exemplary analytes, each in a respective sample, to migrate differently through the nano ES capillary and (ii) components of a mixed IgG/BSA sample to be electrophoretically separable prior to electrophoresis in the gas-phase (i.e., nano DMA). In order to obtain these results, the main force transferring samples from the sample vial to the capillary tip, the pressure applied to the pressure chamber for continuous sample introduction to the system, had to be reduced to the lowest acceptable value.
Our results demonstrate that an on-line CE–GEMMA (CE–ES–DMA) combination (electrophoresis in the liquid and subsequently in the gas-phase at atmospheric pressure) is even feasible with a commercial GEMMA instrument not intended for electrophoretic applications in the liquid phase. The fact that the instrument is not intended for CE lead to several technical difficulties like for instance unstable pressure settings or unnecessary long detector reset times (3 s). Therefore, at the moment only a rough pre-separation of samples was carried out (which, however, leads already to significantly improved results for the GEMMA measurements) as a kind of feasibility study. We believe that future instrumental advancements (like coupling of an independent commercial CE instrument (which much longer separation capillary) to a GEMMA) will open up new highly interesting avenues in nanoparticle, virus, virus-like particle, recombinant antibody and protein/protein complex/protein assemblies analysis.
Electrophoretic zone separation of sample constituents in the capillary of the nano ES unit furthermore allowed on-line desalting of samples. High concentrations of non-volatile sample components (salts, sugars, detergents, etc.) lead to heterogeneous aggregates between these constituents and analytes, which can be concluded from observed higher EM diameters and broader EM diameter distributions. In the worst case an increase in the baseline signal even thwarts the EM diameter determination of analytes. However, electrophoretic separation of these low molecular weight sample contaminations (from e.g. employed physiological buffers) from analyte particles in the liquid phase again allows the EM diameter determination of analytes despite an increased salt concentration. We therefore expand the GEMMA also to the analysis of complex samples or samples with higher salt concentrations, eliminating the impact of undesired components and salts from the GEMMA spectra. A logical continuation of presented work will deal with further CE aspects and their impact on sample introduction to the GEMMA system, for instance the addition of detergents to electrolyte solutions or electrophoretic sample stacking.
Author contributions
Idea and experimental design by MMD and VUW; experimental work by LK and VUW; guidance by MMD and VUW; physical background and calculations by PK and WWS; idea and supervision, instrumentation and funding by GA; all authors contributed to the manuscript writing.
Acknowledgement
This project was supported by Austrian Science Foundation (FWF), grant No. TRP29 to WWS and GA.
References
- 1.De Jong W.H., Borm P.J. Drug delivery and nanoparticles: applications and hazards. Int. J. Nanomed. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurer-Jones M.A., Bantz K.C., Love S.A., Marquis B.J., Haynes C.L. Toxicity of therapeutic nanoparticles. Nanomedicine. 2009;4:219–241. doi: 10.2217/17435889.4.2.219. [DOI] [PubMed] [Google Scholar]
- 3.Love S.A., Maurer-Jones M.A., Thompson J.W., Lin Y.S., Haynes C.L. Assessing nanoparticle toxicity. Ann. Rev. Anal. Chem. 2012;5:181–205. doi: 10.1146/annurev-anchem-062011-143134. [DOI] [PubMed] [Google Scholar]
- 4.Maurer-Jones M.A., Haynes C.L. Toward correlation in in vivo and in vitro nanotoxicology studies. J. Law Med. Ethics. 2012;40:795–801. doi: 10.1111/j.1748-720X.2012.00707.x. [DOI] [PubMed] [Google Scholar]
- 5.Jian F., Zhang Y., Wang J., Ba K., Mao R., Lai W., Lin Y. Toxicity of biodegradable nanoscale preparations. Curr. Drug Metab. 2012;13:440–446. doi: 10.2174/138920012800166517. [DOI] [PubMed] [Google Scholar]
- 6.Maurer-Jones M.A., Gunsolus I.L., Murphy C.J., Haynes C.L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 2013;85:3036–3049. doi: 10.1021/ac303636s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin K.S., Dehvari K., Liu Y.J., Kuo H., Hsu P.J. Synthesis and characterization of porous zero-valent iron nanoparticles for remediation of chromium-contaminated wastewater. J. Nanosci. Nanotechnol. 2013;13:2675–2681. doi: 10.1166/jnn.2013.7381. [DOI] [PubMed] [Google Scholar]
- 8.Jurneczko E., Barran P.E. How useful is ion mobility mass spectrometry for structural biology? The relationship between protein crystal structures and their collision cross sections in the gas phase. Analyst. 2011;136:20–28. doi: 10.1039/c0an00373e. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman S.L., Skogen J.W., Dorman F.D., Zarrin F., Lewis K.C. Macromolecule analysis based on electrophoretic mobility in air: globular proteins. Anal. Chem. 1996;68:1895–1904. doi: 10.1021/ac951128f. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman S.L., Kuchumov A.R., Kazakevich M., Vinogradov S.N. Analysis of a 3.6-MDa hexagonal bilayer hemoglobin from Lumbricus terrestris using a gas-phase electrophoretic mobility molecular analyzer. Anal. Biochem. 1998;259:195–202. doi: 10.1006/abio.1998.2644. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman S.L. Analysis of biomolecules using electrospray and nanoparticle methods: the gas-phase electrophoretic mobility molecular analyzer (GEMMA) J. Aerosol Sci. 1998;29:537–552. [Google Scholar]
- 12.Bacher G., Szymanski W.W., Kaufman S.L., Zoellner P., Blaas D., Allmaier G. Charge-reduced nano electrospray ionization combined with differential mobility analysis of peptides, proteins, glycoproteins, noncovalent protein complexes and viruses. J. Mass Spectrom. 2001;36:1038–1052. doi: 10.1002/jms.208. [DOI] [PubMed] [Google Scholar]
- 13.Kapellios E.A., Karamanou S., Sardis M.F., Aivaliotis M., Economou A., Pergantis S.A. Using nanoelectrospray ion mobility spectrometry (GEMMA) to determine the size and relative molecular mass of proteins and protein assemblies: a comparison with MALLS and QELS. Anal. Bioanal. Chem. 2011;399:2421–2433. doi: 10.1007/s00216-010-4634-3. [DOI] [PubMed] [Google Scholar]
- 14.Kaddis C.S., Lomeli S.H., Yin S., Berhane B., Apostol M.I., Kickhoefer V.A., Rome L.H., Loo J.A. Sizing large proteins and protein complexes by electrospray ionization mass spectrometry and ion mobility. J. Am. Soc. Mass Spectrom. 2007;18:1206–1216. doi: 10.1016/j.jasms.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemptner J., Marchetti-Deschmann M., Muller R., Ivens A., Turecek P., Schwarz H.P., Allmaier G. A comparison of nano-electrospray gas-phase electrophoretic mobility macromolecular analysis and matrix-assisted laser desorption/ionization linear time-of-flight mass spectrometry for the characterization of the recombinant coagulation glycoprotein von Willebrand factor. Rapid Commun. Mass Spectrom. 2010;24:761–767. doi: 10.1002/rcm.4440. [DOI] [PubMed] [Google Scholar]
- 16.Weiss V.U., Subirats X., Pickl-Herk A., Bilek G., Winkler W., Kumar M., Allmaier G., Blaas D., Kenndler E. Characterization of rhinovirus subviral A particles via capillary electrophoresis, electron microscopy and gas-phase electrophoretic mobility molecular analysis: part I. Electrophoresis. 2012;33:1833–1841. doi: 10.1002/elps.201100647. [DOI] [PubMed] [Google Scholar]
- 17.Kallinger P., Weiss V.U., Lehner A., Allmaier G., Szymanski W.W. Analysis and handling of bio-nanoparticles and environmental nanoparticles using electrostatic aerosol mobility. Particuology. 2013;11:14–19. [Google Scholar]
- 18.Kemptner J., Marchetti-Deschmann M., Siekmann J., Turecek P.L., Schwarz H.P., Allmaier G. GEMMA and MALDI-TOF MS of reactive PEGs for pharmaceutical applications. J. Pharm. Biomed. Anal. 2010;52:432–437. doi: 10.1016/j.jpba.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Malm L., Hellman U., Larsson G. Size determination of hyaluronan using a gas-phase electrophoretic mobility molecular analysis. Glycobiology. 2012;22:7–11. doi: 10.1093/glycob/cwr096. [DOI] [PubMed] [Google Scholar]
- 20.López-Herrera J.M., Ganán-Calvo A.M. A note on charged capillary jet breakup of conducting liquids: experimental validation of a viscous one-dimensional model. J. Fluid Mech. 2004;501:303–326. [Google Scholar]
- 21.Fuchs N.A. On the stationary charge distribution on aerosol particles in a bipolar ionic atmosphere. Geofis. Pura Appl. 1963;56:185–193. [Google Scholar]
- 22.Hoppel W.A., Frick G.M. Ion–aerosol attachment coefficients and the steady-state charge distribution on aerosols in a bipolar ion environment. Aerosol Sci. Technol. 1986;5:1–21. [Google Scholar]
- 23.Flagan R. History of electrical aerosol measurements. Aerosol Sci. Technol. 1998;28:301–380. [Google Scholar]
- 24.Intra P., Tippayawong N. An overview of differential mobility analyzers for size classification of nanometer-sized aerosol particles. Songklanakarin J. Sci. Technol. 2008;30:243–256. [Google Scholar]
- 25.Koropchak J.A., Sadain S., Yang X., Magnusson L.E., Heybroek M., Anisimov M., Kaufman S.L. Nanoparticle detection technology for chemical analysis. Anal. Chem. 1999;71:386A–394A. doi: 10.1021/ac990442x. [DOI] [PubMed] [Google Scholar]
- 26.Loo J.A., Berhane B., Kaddis C.S., Wooding K.M., Xie Y., Kaufman S.L., Chernushevich I.V. Electrospray ionization mass spectrometry and ion mobility analysis of the 20S proteasome complex. J. Am. Soc. Mass Spectrom. 2005;16:998–1008. doi: 10.1016/j.jasms.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 27.Allmaier G., Laschober C., Szymanski W.W. Nano ES GEMMA and PDMA, new tools for the analysis of nanobioparticles–protein complexes, lipoparticles, and viruses. J. Am. Soc. Mass Spectrom. 2008;19:1062–1068. doi: 10.1016/j.jasms.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Cole K.D., Pease L.F., 3rd, Tsai D.H., Singh T., Lute S., Brorson K.A., Wang L. Particle concentration measurement of virus samples using electrospray differential mobility analysis and quantitative amino acid analysis. J. Chromatogr. A. 2009;1216:5715–5722. doi: 10.1016/j.chroma.2009.05.083. [DOI] [PubMed] [Google Scholar]
- 29.Pease L.F., 3rd, Lipin D.I., Tsai D.H., Zachariah M.R., Lua L.H., Tarlov M.J., Middelberg A.P. Quantitative characterization of virus-like particles by asymmetrical flow field flow fractionation, electrospray differential mobility analysis, and transmission electron microscopy. Biotechnol. Bioeng. 2009;102:845–855. doi: 10.1002/bit.22085. [DOI] [PubMed] [Google Scholar]
- 30.Havlik M., Marchetti-Deschmann M., Friedbacher G., Messner P., Winkler W., Perez-Burgos L., Tauer C., Allmaier G. Development of a bio-analytical strategy for characterization of vaccine particles combining SEC and nanoES GEMMA. Analyst. 2014;139:1412–1419. doi: 10.1039/c3an01962d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laschober C., Wruss J., Blaas D., Szymanski W.W., Allmaier G. Gas-phase electrophoretic molecular mobility analysis of size and stoichiometry of complexes of a common cold virus with antibody and soluble receptor molecules. Anal. Chem. 2008;80:2261–2264. doi: 10.1021/ac702463z. [DOI] [PubMed] [Google Scholar]
- 32.Tsai D.H., Pease L.F., 3rd, Zangmeister R.A., Tarlov M.J., Zachariah M.R. Aggregation kinetics of colloidal particles measured by gas-phase differential mobility analysis. Langmuir. 2009;25:140–146. doi: 10.1021/la703164j. [DOI] [PubMed] [Google Scholar]
- 33.Pease L.F., 3rd, Tsai D.H., Hertz J.L., Zangmeister R.A., Zachariah M.R., Tarlov M.J. Packing and size determination of colloidal nanoclusters. Langmuir. 2010;26:11384–11390. doi: 10.1021/la100839t. [DOI] [PubMed] [Google Scholar]
- 34.Hinterwirth H., Wiedmer S.K., Moilanen M., Lehner A., Allmaier G., Waitz T., Lindner W., Laemmerhofer M. Comparative method evaluation for size and size-distribution analysis of gold nanoparticles. J. Sep. Sci. 2013;36:2952–2961. doi: 10.1002/jssc.201300460. [DOI] [PubMed] [Google Scholar]
- 35.Weiss V.U., Lehner A., Kerul L., Grombe R., Kratzmeier M., Marchetti-Deschmann M., Allmaier G. Characterization of crosslinked gelatin nanoparticles by electrophoretic techniques in the liquid and the gas phase. Electrophoresis. 2013;34:3267–3276. doi: 10.1002/elps.201300307. [DOI] [PubMed] [Google Scholar]
- 36.Pease L.F., 3rd, Tsai D.H., Fagan J.A., Bauer B.J., Zangmeister R.A., Tarlov M.J., Zachariah M.R. Length distribution of single-walled carbon nanotubes in aqueous suspension measured by electrospray differential mobility analysis. Small. 2009;5:2894–2901. doi: 10.1002/smll.200900928. [DOI] [PubMed] [Google Scholar]
- 37.Jouyban A., Kenndler E. Theoretical and empirical approaches to express the mobility of small ions in capillary electrophoresis. Electrophoresis. 2006;27:992–1005. doi: 10.1002/elps.200500696. [DOI] [PubMed] [Google Scholar]
- 38.Kleparnik K., Bocek P. Theoretical background for clinical and biomedical applications of electromigration techniques. J. Chromatogr. 1991;569:3–42. doi: 10.1016/0378-4347(91)80225-2. [DOI] [PubMed] [Google Scholar]
- 39.Lewis K.C., Jorgenson J.W., Kaufman S.L. Capillary zone electrophoresis with electrospray condensation particle counting detection. J. Capillary Electrophor. 1996;3:229–235. [PubMed] [Google Scholar]
- 40.Ma T., Fung Y. Hyphenating capillary isoelectric focusing with gas-phase electrophoretic mobility molecular analyzer for determination of proteins in human tear fluids. ECS Meeting Abstracts; MA2012-02; 2012. p. 2773. [Google Scholar]
- 41.Pihlasalo S., Auranen L., Hanninen P., Harma H. Method for estimation of protein isoelectric point. Anal. Chem. 2012;84:8253–8258. doi: 10.1021/ac301569b. [DOI] [PubMed] [Google Scholar]