Abstract
Flavopiridol is a broad cyclin-dependent kinase inhibitor (CDKI) that induces apoptosis of malignant lymphocytes in vitro and in murine lymphoma models. We conducted a phase I dose-escalation study to determine the maximum tolerated dose (MTD) for single-agent flavopiridol administered on a pharmacokinetically derived hybrid dosing schedule to patients with relapsed and refractory non-Hodgkin’s lymphoma. Dose was escalated independently in one of four cohorts: indolent B-cell (cohort 1), mantle cell (cohort 2), intermediate grade B-cell including transformed lymphoma (cohort 3), and T-/NK-cell excluding primary cutaneous disease (cohort 4). Forty-six patients were accrued. Grade 3 or 4 leukopenia was observed in the majority of patients (60%), but infection was infrequent. Common non-hematologic toxicties included diarrhea and fatigue. Biochemical tumor lysis was observed in only 2 patients, and no patients required hemodialysis for its management. Dose escalation was completed in two cohorts (indolent and aggressive B-cell). Dose-limiting toxicities were not observed, and the MTD was not reached in either cohort at the highest dose tested (50 mg/m2 bolus + 50 mg/m2 continuous infusion weekly for 4 consecutive weeks of a 6 week cycle). Clinical benefit was observed in 26% of 43 patients evaluable for response, including 14% with partial responses (2 mantle cell, 3 indolent B-cell, and 1 diffuse large B-cell). The single-agent activity of this first-generation CDKI suggests that other agents in this class merit further study in lymphoid malignancies, both alone and in combination.
Keywords: flavopiridol, non-Hodgkin’s lymphoma, cyclin-dependent kinase inhibitors, phase 1 trial, pharmacokinetics
INTRODUCTION
Notwithstanding significant advances in the treatment of non-Hodgkin’s lymphoma (NHL) over the last two decades, a significant proportion of patients will inevitably relapse after initial therapy. While stem cell transplantation can be curative in a fraction of these patients, treatment options are limited for those patients ineligible for transplant, relapsing after transplant, or otherwise refractory to extant agents. There remains a need for new agents with novel mechanisms of action.
Flavopiridol is a broad cyclin-dependent kinase inhibitor that induces apoptosis of malignant lymphocytes in vitro and in murine lymphoma models at concentrations that are attainable clinically.1–4 Flavopiridol has been administered to lymphoma patients in the clinic according to various schedules, including daily IV bolus dosing and 72-hour continuous IV infusion.5,6 Activity in these initial studies proved disappointing, likely because the tested schedules were modeled after in vitro studies using fetal bovine serum, to which flavopiridol binding is notably lower than human serum.7 We developed a pharmacokinetically derived dosing schedule in which flavopiridol is administered by 30-minute intravenous bolus followed by a 4-hour continuous intravenous infusion.8 When given in this manner to patients with refractory chronic lymphocytic leukemia (CLL), flavopiridol was highly active, even against high-risk disease. 9,10
PATIENTS AND METHODS
Patients
Patients were enrolled on this National Cancer Institute (NCI)-sponsored clinical study following approval by The Ohio State University Institutional Review Board. All patients provided written informed consent. Patients with a confirmed diagnosis of non-Hodgkin’s lymphoma NHL were accrued to one of four cohorts defined by World Health Organization (WHO) criteria: indolent B-cell (cohort 1), mantle cell (cohort 2), intermediate grade B-cell including transformed lymphoma (cohort 3), and T-/NK-cell excluding primary cutaneous disease (cohort 4). All patients were required to have received at least one prior therapy. Patients with indolent B-cell NHL must have received at least 2 prior regimens, one of which must have included rituximab. Patients with diffuse large B-cell NHL could not be candidates for aggressive salvage chemotherapy and/or potentially curative autologous stem cell transplant. Further enrollment requirements included: age ≥ 18 years, Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2, creatinine ≤1.5 mg/dL (or estimated creatinine clearance ≥ 70 mL/min), transaminases ≤ 3 times the upper limit of normal (ULN), bilirubin ≤2 times ULN, hemoglobin ≥9 g/dL, absolute neutrophil count (ANC) ≥1,500/mm3, and platelets ≥50,000/mm3 unless attributable to marrow involvement by the patient’s underlying lymphoma. Patients could not have received therapy within 4 weeks of enrollment. Pregnant women and patients with HIV infection were excluded.
Treatment plan
This phase I trial was a nonrandomized, dose-escalation study to determine the maximum tolerated dose (MTD) for single-agent flavopiridol administered on a hybrid dosing schedule. Flavopiridol was given as a 30-minute loading dose followed by a 4-hour continuous infusion for 4 consecutive weeks every 6 weeks (1 cycle of therapy) for a maximum of 6 cycles. Dose escalation proceeded according to a 3 + 3 design within each disease cohort, enrolling 3 to 6 patients at each of 3 dose levels. The total dose of flavopiridol at dose level 1 was 60 mg/m2 (30 mg/m2 bolus + 30 mg/m2 continuous infusion); at dose level 2, 80 mg/m2 (30 mg/m2 bolus + 50 mg/m2 continuous infusion); and at dose level 3, 100 mg/m2 (50 mg/m2 bolus + 50 mg/m2 continuous infusion). As hyperacute tumor lysis syndrome (TLS) has been reported with this schedule when used to treat CLL, the first flavopiridol infusion was delivered in hospital supported by vigorous IV hydration (for at least 10 hours pre- and post-treatment) and with careful monitoring for and aggressive management of hyperkalemia according to an established protocol previously described.9 Patients were subsequently transitioned to outpatient treatment on day 8 of therapy.
Stipulated supportive care consisted of allopurinol 300 mg daily, calcium acetate 1334 mg prior to initiation of flavopiridol bolus, and ondansetron 16 mg orally 30–60 minutes before each flavopiridol treatment. Patients with intolerance to allopurinol or deemed to be at high risk of tumor lysis could receive prophylactic rasburicase (4.5 mg IV) 2 hours prior to flavopiridol administration at the discretion of their treating physician. Corticosteroid administration for cytokine release syndrome prophylaxis was likewise left to the discretion of treating physicians, but all patients ultimately received 20 mg dexamethasone IV prior to flavopirdol during the first cycle of treatment, and the majority regularly received at least 10 mg dexamethasone pre-medication with subsequent infusions. Prophylactic antimicrobials and colony stimulating factors were not required but were also permitted at the discretion of the treating physician.
Assessment of toxicity and response
NCI Common Toxicity Criteria for Adverse Events (version 3.0) were used to define and grade toxicity. Patients were assessed for clinical response after two, four, and six cycles with laboratory studies, physical exam, and CT scans. Response was evaluated using modified NCI-sponsored Working Group Lymphoma Response Criteria.11
Dose-limiting toxicity
Patients in each individual disease group were independently evaluated for dose limiting toxicity (DLT) during the first cycle of treatment. DLT included 1) any grade 3–4 non-hematologic toxicity that did not resolve or decrease to grade 1–2 within 2 weeks, or 2) any grade 4 hematologic toxicity that caused more than a one week delay in treatment. The maximum tolerated dose (MTD) was defined as that dose beneath the dose at which 2 or more of 6 patients experienced DLT.
Pharmacokinetics
Whole blood samples were collected for pharmacokinetic (PK) analysis during the first (day 1) and fourth (day 22) treatments during cycle 1. Samples were collected on both occasions prior to dosing and at 0.5, 1, 3, 4.5, 6, 8 and 24 hours after the start of the bolus infusion. Blood samples were collected in sodium heparin tubes, and plasma was immediately separated and stored at −70°C for later analysis. Flavopiridol quantification in plasma samples was achieved using a validated liquid chromatography-tandem mass spectrometry assay as previously described12. Plasma flavopiridol concentration-time data were analyzed using standard non-compartmental methods in WinNonlin Professional v 5.2.1 (Pharsight, Mountain View, CA).
Statistical analyses of PK parameters were performed on all enrolled patients with evaluable PK data. Paired t-tests evaluated differences in AUC0-∞ and Cmax between days 1 and 22 of cycle 1. Cycle 1, day 1 data were used to test associations between PK parameters and toxicities or response among assessable patients. These associations were tested using one-way analysis of variance (ANOVA) and 2-sample t-tests (i.e. Cmax), or the nonparametric Kruskal-Wallis test (i.e. AUC0-∞). Data are described with means ± standard deviations and/or medians with ranges. These data were analyzed using SigmaPlot v11 (Systat Software Inc.).
Statistical considerations
No formal hypothesis testing was planned. Descriptive statistics were provided for the primary end points of safety and tolerability. The evaluable population included all patients completing one cycle of therapy or discontinuing therapy during the first cycle secondary to toxicity. Median duration of response calculated from the date of best response until the earliest time of either disease progression or death, was estimated among responding patients using the Kaplan-Meier method.
RESULTS
Patients
Between April 2006 and September 2010, a total of 46 patients were accrued to the study and received at least one dose of flavopiridol. Accrual ceased in advance of study completion when support from the study sponsor was withdrawn. Eleven of the 46 patients discontinued therapy during cycle 1 for reasons other than toxicity: disease progression (7 patients), death from cardiac events unrelated to treatment (1 patient) and withdrawal of informed consent (3 patients). Characteristics of the remaining 35 patients evaluable for toxicity are detailed in Table 1. In general, these patients were heavily pre-treated (median prior therapies = 3, including 4 patients who relapsed after radioimmunotherapy and 4 patients post-autologous stem cell transplant) and had high-risk disease (bulky disease 46%, marrow involvement 43%).
Table 1.
Patient Characteristics
| Characteristic | All Patients N = 46 |
Not Assessable N = 11 |
Assessable N = 35 |
|---|---|---|---|
| No. of Females (%) | 20 (43) | 4 (36) | 16 (46) |
| Median Age, years (range) | 66 (34–84) | 65 (34–77) | 67 (49–84) |
| Performance Status, no. (%) | |||
| 0 | 8 (17) | 1 (9) | 7 (20) |
| 1 | 34 (74) | 8 (73) | 26 (74) |
| 2 | 4 (9) | 2 (18) | 2 (6) |
| Diagnosis , no. (%) | |||
| Indolent B-Cell NHL | 15 (33) | 3 (27) | 12 (34) |
| Intermediate Grade B-NHL | 17 (37) | 6 (55) | 11 (31) |
| Mantle Cell Lymphoma | 7 (15) | 1 (9) | 6 (17) |
| T/NK-Cell NHL | 7 (15) | 1 (9) | 6 (17) |
| Median LDH, (range) | 211 (105–1874) | 274 (117–1874) | 187 (105–418) |
| Bulky Disease ≥ 5cm, no. (%) | 25 (54) | 9 (82) | 16 (46) |
| Bone Marrow Involvement, no. (%) | 22 (48) | 7 (64) | 15 (43) |
| Median No. of Prior Treatments (range) | 3 (1–8) | 3 (1–7) | 3 (1–8) |
Delivery of Therapy
Patients received a median of 2 cycles of flavopiridol (range 0–6, see Table 2). Five patients (14%) completed all 6 cycles of planned therapy. Among patients completing at least 1 cycle of therapy, the most common reason for treatment discontinuation was disease progression (20 patients). Five patients discontinued therapy secondary to adverse events, 1 patient proceeded to transplant, 1 patient died as a result of cardiac arrhythmia unrelated to the primary malignancy or flavopiridol, 1 patient was removed by his treating physician to begin alternative therapy, and 1 patient withdrew informed consent. On additional patient, who had demonstrated stable disease after 3 cycles of treatment for subcutaneous panniculitis-like T-cell histology, discontinued therapy after diagnosis of an EBV-positive primary central nervous system B-cell NHL.
Table 2.
Common treatment-related toxicities (N= 35 patients)
| Toxicity, n (%) | Grade 2 | Grade 3 | Grade 4 | All |
|---|---|---|---|---|
| Hematologic | ||||
| Leukopenia | 4 (11) | 17 (49) | 9 (26) | 30 (86) |
| Neutropenia | 3 (9) | 8 (23) | 23 (66) | 34 (97) |
| Lymphopenia | 3 (9) | 9 (26) | 13 (37) | 25 (71) |
| Anemia | 7 (20) | 15 (43) | 0 (0) | 22 (63) |
| Thrombocytopenia | 4 (11) | 4 (11) | 5 (14) | 13 (37) |
| Infection | ||||
| Documented Infection | 2 (6) | 7 (20) | 0 (0) | 9 (26) |
| Neutropenic Fever | 1 (3) | 3 (9) | 0 (0) | 4 (11) |
| Non-Hematologic | ||||
| Increased ALT/AST | 3 (9) | 7 (20) | 2 (6) | 12 (34) |
| Hyberbilirubinemia | 5 (14) | 1 (3) | 0 (0) | 6 (17) |
| Nausea | 10 (29) | 2 (6) | 0 (0) | 12 (34) |
| Vomiting | 8 (23) | 2 (6) | 0 (0) | 10 (29) |
| Anorexia | 7 (20) | 1 (3) | 0 (0) | 8 (23) |
| Diarrhea | 9 (26) | 18 (51) | 0 (0) | 27 (77) |
| Tumor Lysis Syndrome | 0 (0) | 2 (6) | 0 (0) | 2 (6) |
| Cytokine Release Syndrome | 6 (17) | 2 (6) | 0 (0) | 8 (23) |
| Fatigue | 9 (26) | 12 (34) | 0 (0) | 21 (60) |
Toxicity
Flavopiridol treatment was generally well tolerated. Common grade ≥2 toxicities are listed in Table 3. Hematologic toxicity was common, primarily leukopenia, which occurred in 86% of patients. However, while grade 3 or 4 neutropenia was reported for 23% and 66% of patients, respectively, no grade 4 infectious toxicity was observed. Seven patients (20%) experienced documented grade 3 infection: 2 pneumonia, 2 colitis, 1 catheter-associated bacteremia, 1 severe otitis media, and 1 unspecified. Two of the aforementioned patients, one with colitis and one with unspecified infection, were also treated for grade 3 neutropenic fever in addition to one other patient without documented infection. Flavopiridol does not cause significant T-cell lymphopenia10,13, and opportunistic infections were not observed.
Table 3.
Response to Therapy (N= 35)
| Dose Level | Response | Diagnoses |
|---|---|---|
|
1 (30 + 30) |
PR | Mantle cell (1), Follicular (1) |
| SD | Richter’s transformation (1), Anaplastic large cell (1), Panniculitis-like subcutaneous T-cell (1) |
|
|
2 (30 + 50) |
PR | Mantle cell (1), Follicular (1) |
| SD | Peripheral T-cell NOS (1) | |
|
3 (50 + 50) |
PR | Diffuse large B-cell (1), Small lymphocytic (1) |
| SD | Diffuse large B-cell (1) |
Non-hematologic toxicity was generally manageable. The most common grade 3 non-hematologic toxicities were diarrhea (18 patients, 51%) and fatigue (12 patients, 34%). Most patients experiencing diarrhea had also received potassium binding resins and sorbitol, so it was impossible to determine the extent to which diarrhea (typically occurring on the treatment and post-treatment day) was related to study drug. Reversible grade 3 elevation of hepatic transaminases was observed in 7 patients (20%). Grade 3 tumor lysis syndrome, characterized chiefly by elevation of serum potassium and phosphorous, occurred in 2 patients: one responding patient with mantle cell lymphoma (treated at dose level 2) and a second patient with Richter’s transformation of CLL (treated at dose level 1). In both cases tumor lysis occurred during the first exposure to flavopiridol, did not recur with subsequent administrations of the drug, and responded to medical intervention (such as oral potassium binding resin, diuretics) without need for hemodialysis.
Planned dose escalation was complete in two cohorts (indolent B-cell NHL, intermediate grade B-cell NHL) when the trial was closed, although one patient treated at the starting dose level in the intermediate grade cohort was later deemed ineligible for toxicity assessment since she did not successfully receive all four doses of cycle one. No dose-limiting toxicities were observed among the patients treated. A maximum tolerated dose was not reached in the two cohorts (indolent and intermediate grade B-cell NHL) fully accrued at the maximum planned dose of 100 mg/m2 (50 mg/m2 bolus + 50 mg/m2 continuous infusion).
Response
Forty-three patients were evaluated for response, including 7 patients who progressed before completing 1 cycle of therapy. Responses were observed at all dose levels (Table 4). Excluding 3 patients who withdrew consent before response could be assessed, 11 patients of 43 patient (26%, 95% CI: 14%-41%) achieved disease control, either stable disease (SD) or partial response (PR). Six patients (14%) achieved partial response (PR) after a median 6 cycles of treatment (range 2–6): 2 mantle cell, 3 indolent B-cell (2 follicular and 1 small lymphocytic), and 1 diffuse large B-cell (DLBCL). An additional 5 patients (12%) achieved stable disease after median 3 cycles (range 1–6): 1 DLBCL, 1 Richter’s transformation of CLL, and 3 T-cell histologies (anaplastic large cell, panniculitis-like subcutaneous, peripheral T-cell NOS). Of the 11 patients with PR or SD, 5 remained progression-free with a median follow-up of 1.9 months beyond the date of best response. The estimated median duration of response was approximately 6 months (179 days).
Table 4.
Treatment Delivery
| Dose Level |
||||
|---|---|---|---|---|
| Level 1 (30 + 30) |
Level 2 (30 + 50) |
Level 3 (50 + 50) |
All | |
| Disposition During Treatment | ||||
| No. registered | 13 | 19 | 14 | 46 |
| No. evaluable for toxicity | 11 | 12 | 12 | 35 |
| Diagnosis , no. | ||||
| Indolent B-Cell NHL | 3 | 3 | 6 | 12 |
| Intermediate Grade B-NHL | 2 | 3 | 6 | 11 |
| Mantle Cell Lymphoma | 3 | 3 | 0 | 6 |
| T/NK-Cell NHL | 3 | 3 | 0 | 6 |
| Median Cycles Received (range) | 3 (1–6) | 2 (0–6) | 2 (0–6) | 2 (0–6) |
| Median doses received (range) | 12 (4–24) | 8 (2–24) | 8 (3–24) | 8 (2–24) |
| Reason for Discontinuing Therapy | ||||
| Completed all planned therapy | 3 (27) | 1 (8) | 1 (8) | 5 (14) |
| Adverse Event | 0 (0) | 2 (17) | 3 (25) | 5 (14) |
| Disease Progression | 6 (55) | 7 (58) | 7 (58) | 20 (57) |
| Death | 0 (0) | 1 (8) | 0 (0) | 1 (3) |
| Proceeded to transplant | 0 (0) | 1 (8) | 0 (0) | 1 (3) |
| Other | 2 (18) | 0 (0) | 1 (8) | 3 (9) |
Treatment enabled 5 patients with PR or SD to receive a stem cell transplant. Three patients underwent allogeneic transplantation, including 1 patient with transformed lymphoma who achieved PR, 1 responding patient with mantle cell, and 1 DLBCL with SD. Two patients underwent autologous transplantation: 1 patient with peripheral T-cell NHL and stable disease and 1 patient with follicular NHL who achieved PR. Two patients who received allogeneic transplants died in remission secondary to transplant-related complications, but the third (transformed follicular) is alive and in continuous complete remission almost 5 years later. One autologous transplant patient relapsed 6 months after that procedure and underwent subsequent allogeneic procedure, since which he remains in remission. The second autologous transplant patient continues in remission from follicular lymphoma but has since developed therapy-related MDS.
Pharmacokinetics
A total of 71 PK profiles comprising 484 plasma flavopiridol concentrations were determined for 45 of 46 patients following treatment on days 1 and/or 22 of cycle 1. PK samples were not collected for one patient, and 7 PK profiles for 5 patients were omitted due to ≥ 25% AUC(0-∞) extrapolation. Among those with PK data on both days 1 and 22 (n=24), the mean AUC0-∞ was 10.404 ± 5.923 and 11.949 ± 5.109 hr*µM, respectively, and the mean Cmax was 1.954 ± 0.886 and 2.071 ± 0.820 µM, respectively. Differences in AUC0-∞ and Cmax for day 22 versus day 1 were not significant (P=0.38 and P=0.75), where the average difference in AUC0-∞ was 0.943 hr*µ, (95% CI −1.230 to 3.115 hr*µM) and the average difference in Cmax was 0.0631 µM (95% CI: −0.348 to 0.474 µM).
We observed no significant difference in AUC0-∞ among the 3 dose levels (P=0.36; Figure 1), although the average Cmax of dose level 3 (2.436 ± 1.149 µM) was higher than the Cmax of dose levels 1 and 2 combined (1.714 ± 0.624 µM) (P=0.09 not assuming equal variances; Figure 1). There were no large differences in total body clearance, apparent volume of distribution during terminal phase and terminal phase elimination half-life of flavopiridol among the 3 dose levels or among the four disease subgroups. Overall, pharmacokinetics appeared similar to that reported for flavopiridol in other hematologic malignancies8,9,14.
Figure 1.
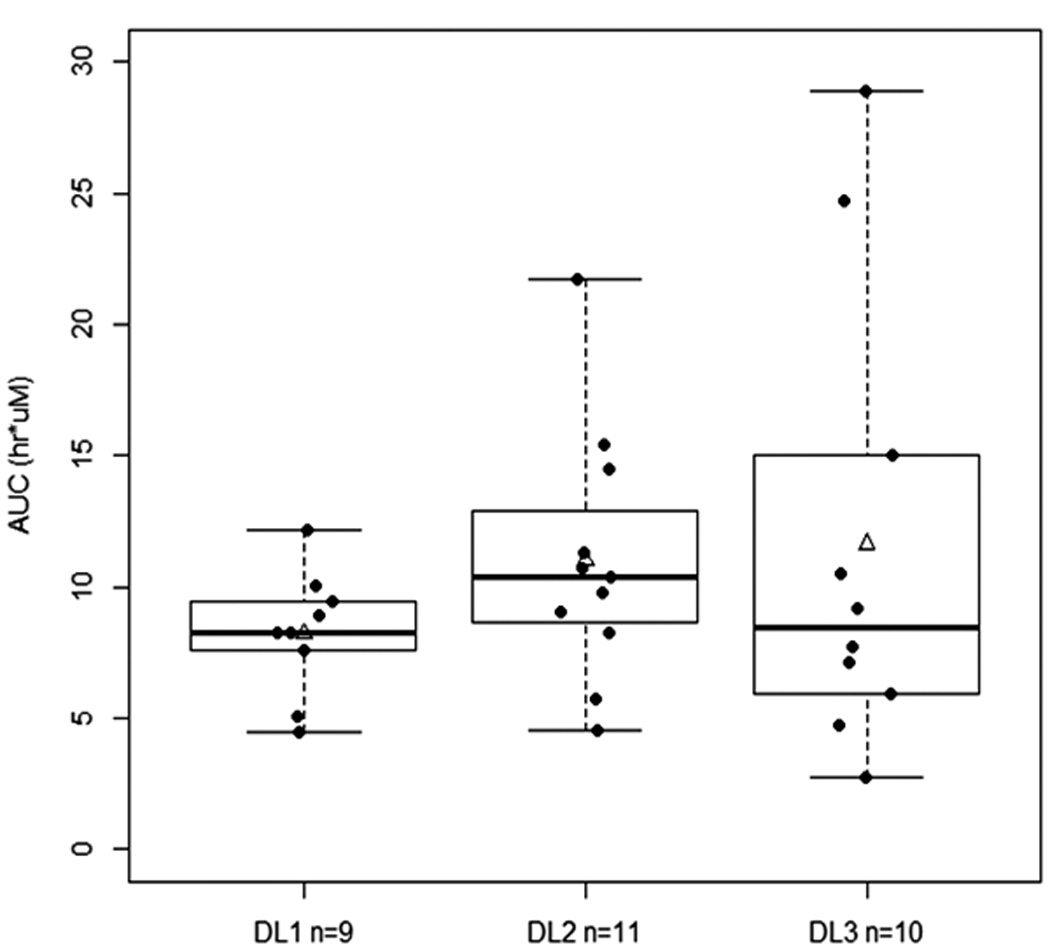
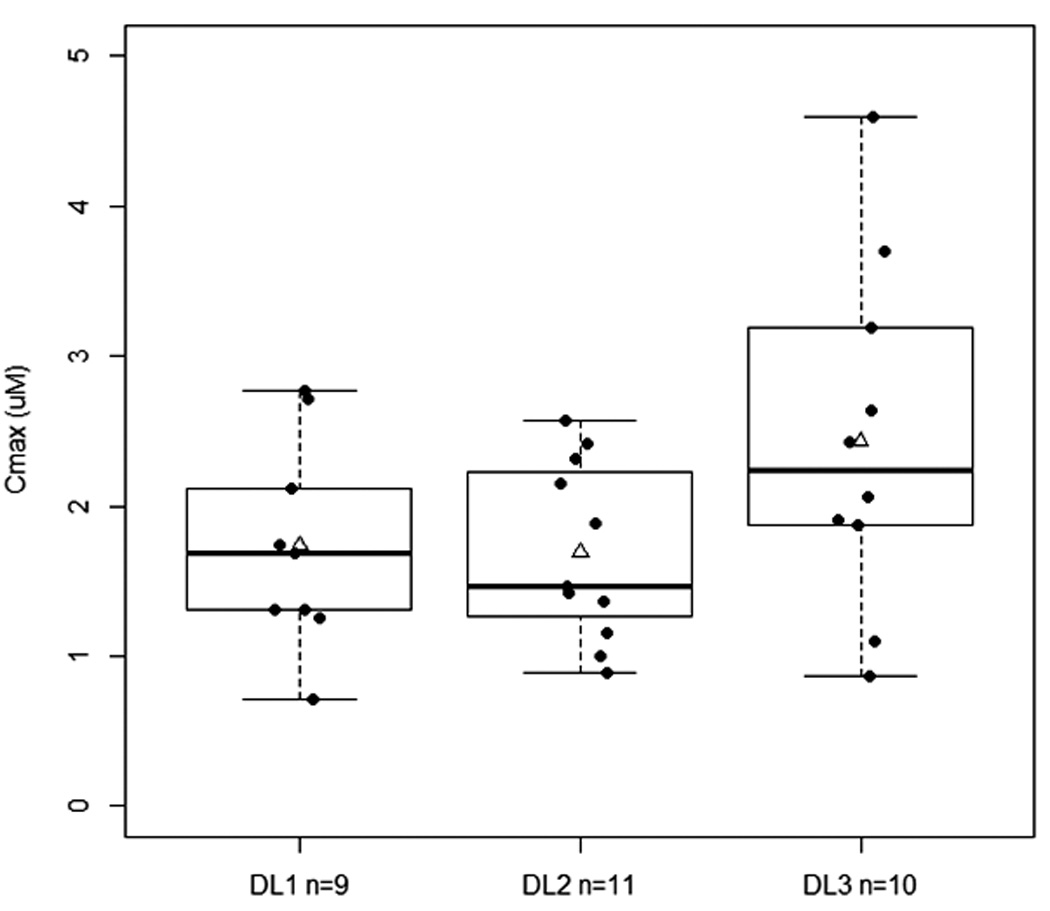
A. Relationship between C1D1 AUC0-∞ and dose levels (P=0.36, Kruskal-Wallis test). B. Relationship between C1D1 Cmax and dose levels (P=0.11, ANOVA). DL1, Dose level 1= 30/30 mg; DL2, dose level 2=30/50 mg; and DL3, dose level 3=50/50 mg. Solid line within the box represents the median, the lower and upper box borders represent the first and third quartiles, and the whiskers extend to the minimum and maximum values. The mean is marked with a triangle.
Among the 35 patients assessable for toxicity and response, 30 patients had evaluable PK data on day 1, cycle 1. Only 2 patients experienced tumor lysis syndrome, with no obvious differences in AUC0-∞ and Cmax values. Other than a higher median AUC0-∞ in those who experienced fatigue (10.48 vs. 8.25 hr*µM, P=0.06), no other noteworthy associations were observed between PK parameters and other selected toxicities (diarrhea, neutropenia, leucopenia). Higher AUC0-∞ and Cmax were observed in patients who achieved PR (Figure 2); the median AUC0-∞ in those with PR, SD, and PD was respectively, 13.79, 8.24, and 8.90 hr*µM (P=0.04), whereas the mean Cmax was 2.77 ± 1.20, 1.51 ± 0.60, and 1.82 ± 0.70 µM, respectively (P=0.16 not assuming equal variances). These results are similar to that reported in the NCI-5746 trial7, where the average AUC0-∞ was significantly higher in those with PR than those with SD/PD.
Figure 2.
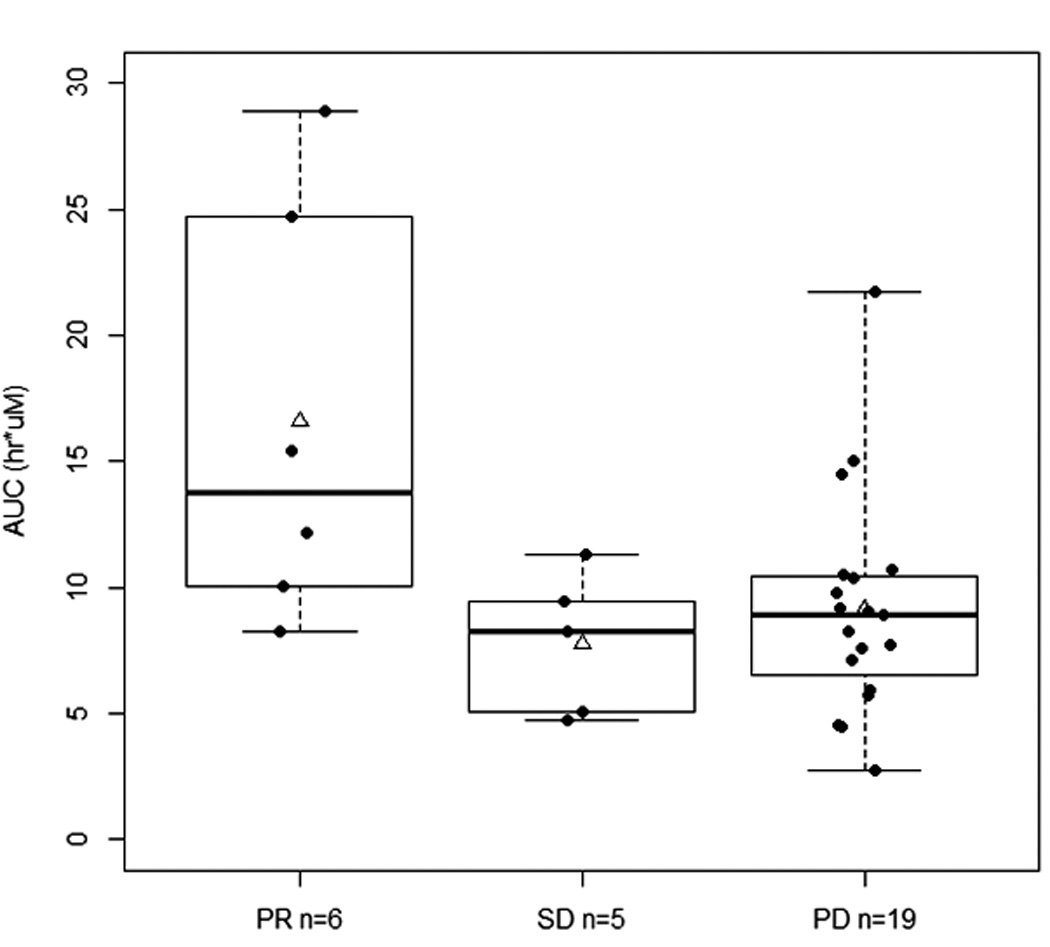
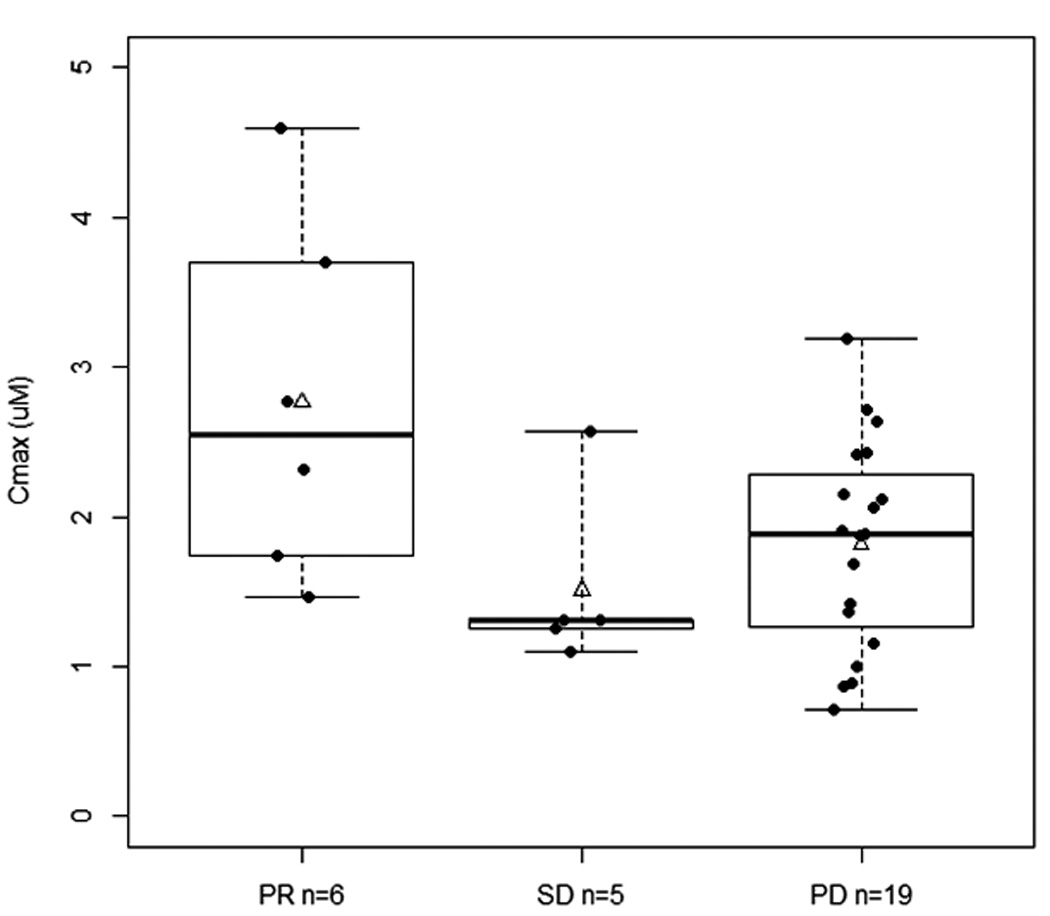
Flavopiridol exposure and response. A. Relationship between best response and C1D1 AUC0-∞. (P=0.04, Kruskal-Wallis test; *P<0.05). B. Relationship between best response and C1D1 Cmax (P=0.16, ANOVA not assuming equal variances). PR, partial response; SD, stable disease; PD, progressive disease. Solid line within the box represents the median, the lower and upper box borders represent the first and third quartiles, and the whiskers extend to the minimum and maximum values. The mean is marked with a triangle.
DISCUSSION
When given according to a pharmacokinetically derived hybrid dosing schedule, the results of this phase 1 study indicate that the cyclin-dependent kinase inhibitor flavopiridol can be safely and tolerably administered to patients with relapsed or refractory non-Hodgkin’s lymphoma. Clinical benefit was observed in 26% of patients including 14% with partial responses and a small proportion who were enabled to proceed with allogeneic or autologous transplant that resulted in sustained remission. Hematologic and non-hematologic toxicities were common but manageable, and no dose-limiting toxicities were observed. MTD was not reached in the two cohorts where accrual was completed (indolent and intermediate-grade B-cell NHL) at the planned maximum dose of 100 mg/m2 (50 mg/m2 bolus + 50 mg/m2 continuous infusion) weekly for 4 consecutive weeks of a 6 week cycle.
Broader investigation of this agent has been hampered by concerns for TLS and the relative complexity of supportive measures. Only 2 patients treated on the present study developed evidence of biochemical tumor lysis, and no patients developed electrolyte disturbances sufficiently severe to warrant hemodialysis. Another recent study of NHL patients employing similar doses of flavopiridol and an identical hybrid infusion schedule likewise reported no hyperacute TLS events.15 In contradistinction to CLL, extensive prophylaxis such as that employed here may not be required when treating NHL. Similarly, flavopiridol-induced cytokine release syndrome (CRS), likely a consequence of treatment-induced increases in IL-6 expression,16 was uncommon secondary to near universal dexamethasone pre-medication, adopted after successful prophylaxis among flavopiridol-treated CLL patients.10
While not designed to estimate efficacy, the results of our study suggest that flavoipiridol is active in NHL. In this group of heavily pre-treated patients, the response rate was 14% and stable disease was observed in an additional 12%, for an overall disease control rate of 26%. Further, flavopiridol treatment facilitated subsequent allogeneic stem cell transplant procedure in 3 patients, including one patient who is alive and in continuous remission nearly 5 years after transplant. Responses were observed in all subtypes of B-cell NHL, most notably indolent B-cell and mantle. That 3 of 6 patients with T-cell histologies demonstrated stable disease is also noteworthy, since this group of patients has limited options for effective therapy, particularly in relapse.
Prior studies of flavopiridol utilizing various other dosing schemes proved disappointing.5,6 However, those infusion schedules failed to account for significant human-plasma protein binding. The pharmacokinetics of flavopiridol in this lymphoma population are similar to data reported for other hematologic malignancies where a similar hybrid dosing schedule was employed.8,14,17 We once again observed an association between response and pharmacokinetics as previously reported.8 However, unlike the findings from studies in CLL, responses were observed at all dose levels, and there was no clear dose-response relationship for efficacy. While the planned dose-escalation was incomplete in some cohorts, the number of patients withdrawing for toxicity appeared to increase proportional to dose. Patients treated at the lowest doses in the present study achieved the longest exposure to the drug (doses, cycles). In a concurrent study of CLL patients, further modifications to the administration schedule (28- versus 42-day cycles) required primary prophylaxis with white blood cell growth factors, but mitigated treatment-related toxicities, particularly fatigue.10 Efficacy was actually improved with that shorter schedule, and the authors speculated that was likely because the patients completed a longer course of therapy.
Although definitive conclusions cannot be drawn from the small sample presented here, the potential for efficacy at lower doses suggests that flavopiridol or other cyclin-dependent kinase inhibitor might be profitably explored in combination. Preclinical data have shown synergy between flavopiridol and several other cytotoxic agents, and several recent clinical studies have demonstrated the feasibility of such combinations in clinical practice. For instance, our group has shown that flavopridol can be safely incorporated into a fludarabine-based regimen. When flavopiridol was administered according to a hybrid schedule similar to that studied here in combination with fludarabine (25 mg/m2 on days 1–5) and rituximab (375 mg/m2 day 1), the overall response rate was 82% and the median progression-free survival was 25.6 months in a group of patients with both previously untreated and relapsed MCL and indolent B-cell NHL.18 More recently, the feasibility of combining flavopiridol with bortezomib was demonstrated in a phase 1 trial of patients with recurrent or refractory B-cell neoplasms.15 In that study, 41% of patients responded when bortezomib (1.3 mg/m2/day on days 1, 4, 8, 11) was given with escalating doses of flavopiridol administered according to the same hybrid schedule. Reported toxicities were generally those expected from the single agents. As yet unreported studies of regimens incorporating cyclosphosphamide, rituximab, and lenalidomide should help to further characterize the toxicities of flavopiridol in combination. Our group has also recently described a novel mechanism of flavopiridol mediated death involving endoplasmic reticulum (ER) stress that evokes a protective autophagy response19. Disruption of autophagy, either biochemically or genetically, results in enhanced sensitization to flavopirido, justifying combination studies of flavopiridol with chloroquine.
In conclusion, we have shown that flavopiridol can be delivered to patients with relapsed and refractory lymphoid malignancies at potentially effective doses and with manageable toxicity. Further modification of the dosing schedule reported here might further limit toxicity and permit a longer duration of therapy, thereby increasing the potential for objective response and facilitating broader investigation in combination regimens. However, the promising single-agent activity of this first-generation, non-selective cyclin-dependent kinase inhibitor in NHL clearly suggests that other agents in this class merit further study in lymphoid malignancies other than CLL, both alone and in combination. Second- and third- generation compounds, such as dinaciclib (formerly SCH-727965) and the orally bioavailable agents TG02 and BAY1000394, more specifically inhibit select cyclin-dependent kinases and offer potential pharmacokinetic advantages over flavopiridol. Results from ongoing studies of these agents will better characterize the clinical potential of cyclin-dependent kinase inhibition as a strategy for treating lymphoid malignancies.
ACKNOWLEDGEMENTS
This study was funded through a Leukemia and Lymphoma Society SCOR grant, the D Warren Brown Foundation, and NIH/NCI grants P01-CA81534, N01-CM-62207, and U01-CA-076576.
References
- 1.Rapoport AP, Simons-Evelyn M, Chen T, et al. Flavopiridol induces apoptosis and caspase-3 activation of a newly characterized Burkitt’s lymphoma cell line containing mutant p53 genes. Blood Cells Mol Dis. 2001;27:610–624. doi: 10.1006/bcmd.2001.0428. [DOI] [PubMed] [Google Scholar]
- 2.Venkataraman G, Maududi T, Ozpuyan F, et al. Induction of apoptosis and down regulation of cell cycle proteins in mantle cell lymphoma by flavopiridol treatment. Leuk Res. 2006;30:1377–1384. doi: 10.1016/j.leukres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Myatt D, Johnson L, Baumli S, Siligardi G. The binding of flavopiridol to blood serum albumin. Chirality. 2010;22(Suppl 1):E40–E43. doi: 10.1002/chir.20925. [DOI] [PubMed] [Google Scholar]
- 4.Arguello F, Alexander M, Sterry JA, et al. Flavopiridol induces apoptosis of normal lymphoid cells, causes immunosuppression, and has potent antitumor activity In vivo against human leukemia and lymphoma xenografts. Blood. 1998;91:2482–2490. [PubMed] [Google Scholar]
- 5.Kouroukis CT, Belch A, Crump M, et al. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:1740–1745. doi: 10.1200/JCO.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 6.Lin TS, Howard OM, Neuberg DS, Kim HH, Shipp MA. Seventy-two hour continuous infusion flavopiridol in relapsed and refractory mantle cell lymphoma. Leuk Lymphoma. 2002;43:793–797. doi: 10.1080/10428190290016908. [DOI] [PubMed] [Google Scholar]
- 7.Byrd JC, Shinn C, Waselenko JK, et al. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood. 1998;92:3804–3816. [PubMed] [Google Scholar]
- 8.Phelps MA, Lin TS, Johnson AJ, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–2645. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin TS, Ruppert AS, Johnson AJ, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–6018. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 12.Phelps MA, Rozewski DM, Johnston JS, et al. Development and validation of a sensitive liquid chromatography/mass spectrometry method for quantitation of flavopiridol in plasma enables accurate estimation of pharmacokinetic parameters with a clinically active dosing schedule. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;868:110–115. doi: 10.1016/j.jchromb.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stephens DM, Ruppert AS, Blum K, et al. Flavopiridol treatment of patients aged 70 or older with refractory or relapsed chronic lymphocytic leukemia is a feasible and active therapeutic approach. Haematologica. 97:423–427. doi: 10.3324/haematol.2011.047324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blum W, Phelps MA, Klisovic RB, et al. Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed or refractory acute leukemias. Haematologica. 2010 doi: 10.3324/haematol.2009.017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holkova B, Perkins EB, Ramakrishnan V, et al. Phase I trial of bortezomib (PS-341; NSC 681239) and alvocidib (flavopiridol; NSC 649890) in patients with recurrent or refractory B-cell neoplasms. Clin Cancer Res. 17:3388–3397. doi: 10.1158/1078-0432.CCR-10-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messmann RA, Ullmann CD, Lahusen T, et al. Flavopiridol-related proinflammatory syndrome is associated with induction of interleukin-6. Clin Cancer Res. 2003;9:562–570. [PubMed] [Google Scholar]
- 17.Karp JE, Smith BD, Resar LS, et al. Phase 1 and pharmacokinetic study of bolus-infusion flavopiridol followed by cytosine arabinoside and mitoxantrone for acute leukemias. Blood. 117:3302–3310. doi: 10.1182/blood-2010-09-310862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol. 28:418–423. doi: 10.1200/JCO.2009.24.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahoney E, Lucas DM, Gupta SV, et al. ER stress and autophagy: new discoveries in the mechanism of action and drug resistance of the cyclin-dependent kinase inhibitor flavopiridol. Blood. 120:1262–1273. doi: 10.1182/blood-2011-12-400184. [DOI] [PMC free article] [PubMed] [Google Scholar]


