Abstract
Objective
We aimed to analyse donor and recipient predictors of graft survival in children who received live-donor renal grafts.
Patients and methods
The study comprised 273 children who received live-donor renal transplants at our center between March 1976 and October 2010. The follow-up ranged from 6 months to 25 years. Donor variables included donor age, gender, donor/recipient body weight ratio (DR BWR), ABO blood groups, human leukocyte antigen, and DR mismatching. Donor-specific problems, e.g., ischemia time during surgery and number of renal arteries, were included. Recipient variables included recipient age, sex, original kidney disease, ischemia time, acute tubular necrosis (ATN) after transplantation, immunosuppression, number of acute rejection episodes, re-transplantation, and development of hypertension.
Results
Independent variables with a sustained effect on the 5- and 10-year graft survival on multivariate analysis were: ATN after transplant, number of acute rejections, hypertension, and DR BWR. At the last follow-up, 185 patients (67.8%) had a functioning graft, while 82 (30.0%) had graft failure. Only six patients (0.02%) were lost to follow-up.
Conclusion
Donor and recipient variables that affect short- and long-term graft survival in children with a live-donor renal allograft are DR BWR, number of acute rejections, ATN and hypertension after transplant. Considering these variables provides a better outcome.
Abbreviations: ESKD, end-stage kidney disease; NAPRTCS, North American Pediatric Renal Transplant Cooperative Study; UNOS, United Network for Organ Sharing; HLA, human leukocyte antigen; DR, donor/recipient; BWR, body weight ratio; ATN, acute tubular necrosis
Keywords: Kidney transplantation, Children, Live donor, Outcome
Introduction
Despite recent improvements in chronic dialysis therapies for children, the quality of a child’s life with a successful renal transplant is far better than life on chronic dialysis. Dialysis is now viewed only as a bridge to subsequent renal transplantation. A successful kidney transplant from a living donor is now considered the most effective renal-replacement therapy to end-stage kidney disease (ESKD) in children. Growth and development are maximized, and the long-term results from several centers are excellent. Transplant results of several large pediatric studies reported from the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) registry [1] and the United Network for Organ Sharing (UNOS) Scientific Registry [2] were quite promising, with outcomes comparable to those of adult recipients. Many of the major factors influencing renal transplant survival (donor and recipient variables) have been identified through analysis of the NAPRTCS and UNOS registries [3,4].
The effect of donor variables on renal allograft outcome in children were also evaluated recently in the Collaborative Transplant Study [5], using deceased donors. The authors recommended that kidneys from deceased donors up to the age of 49 years be allocated to children, and that an acceptable human leukocyte antigen (HLA)-A + B + donor/recipient (DR) match be attempted in patients with a relatively common HLA phenotype, as they found a hierarchical relationship for the effect of increasing numbers of mismatches on graft survival. Finally, they advised avoiding transplants with two HLA-DR mismatches, to reduce the risk of non-Hodgkin lymphoma after transplant among pediatric recipients.
In this study, the donor and recipient predictors of graft survival in children who received living-related donor grafts are of special interest. The study offers patients of the same race, donor source and receiving the same management, thus overcoming the influence of organ preservation, ischemia and the allelic differences between the recipients and their donors.
Patients and methods
This study comprised 273 children receiving live-donor renal transplants at our center between March 1976 and October 2010. The follow-up period ranged from 6 months to 25 years.
The kidney donors underwent an extensive medical, physical, and radiological examination. The algorithm for donor evaluation and assessment in our center was published previously [6]. Contraindications to donation had included age >60 years or <21 years, Mycobacterium tuberculosis infection, diabetes mellitus, proteinuria, microscopic hematuria, impaired renal function, hypertension, coronary heart disease and positive serology for HIV, and hepatitis B and C.
As kidney weight and body weight (BW) correlate directly, so the ratio between the weight of the kidney graft and that of the recipient can be expressed approximately by the ratio between the BWs of the donor and recipient. This DR BW ratio (BWR) is considered when matching the graft to recipients [7]. Patients were classified into two groups; a low DR BWR of <1.2 (group 1), and a high DR BWR of >1.2 (group 2).
For recipients, the exclusion criteria for kidney transplantation were sensitization with positive lymphocytotoxic cross match, type I diabetes mellitus, active infection, malignancies, and significant cardiac, pulmonary and hepatic disease. A biopsy-confirmed diagnosis of renal pathology was used to identify the causes of chronic kidney disease.
Triple immunosuppression (prednisolone + cyclosporin A + azathioprine) was mainly used for the transplant recipients. Most patients who received a transplant before 1988 were treated with daily oral administration of 7.5–15 mg prednisolone (the mean daily dose at 6 months after transplantation was 0.3 mg/kg, and was reduced thereafter), and 2.5 mg/kg azathioprine as combined therapy. From 1988 to 1998, a triple-therapy regimen comprising prednisolone (5–10 mg), with the mean daily dose of 0.25 mg/kg at 6 months after transplant, azathioprine 2 mg/kg and cyclosporin A was used. Cyclosporin whole-blood minimum levels were kept at 100–150 ng/mL. Tacrolimus and mycophenolate mofetil were introduced as a primary therapy in 1998, and sirolimus was used in addition to prednisolone in 2002. Tacrolimus blood levels were maintained at 10–15 ng/μL in the first month and 5–10 ng/μL thereafter.
Acute rejection episodes were diagnosed according to Banff classification of renal allograft pathology [8]. Renal pathology was assessed as confirmation before anti-rejection treatment was initiated. Acute rejection episodes were treated with intravenous bolus doses of methyl prednisolone (250–500 mg/day for 3–5 days). Antibody-mediated rejection and steroid-resistant cases were treated with anti-thymocyte globulin, plasmaphoresis on alternate days for 10 days, or rituximab. Graft function was monitored by periodic estimation of serum creatinine levels and creatinine clearance.
Possible donor and recipient variables that might affect graft survival were assessed using univariate and multivariate analysis. Donor variables included donor age, gender, DR consanguinity, ABO blood groups, HLA, and DR mismatching. Donor-specific problems, e.g., infections, ischemia time during surgery and number of renal arteries were included. Recipient variables included recipient age, sex, original kidney disease, ischemia time, acute tubular necrosis (ATN) after transplant, immunosuppression, number of acute rejections, re-transplantation, and the development of hypertension after transplant.
The probability of graft survival was calculated using the Kaplan–Meier method. Differences were determined using the log-rank test, with P < 0.05 considered to indicate statistical significance. Significant factors were further examined in a multivariate analysis to determine those that acted independently (P < 0.05). The risk factors were evaluated by the corresponding hazard ratio using a Cox model, with P < 0.05 considered to indicate significance.
Results
Graft and patient survival to the last follow-up are shown in Fig. 1; patient survival was 89.5% and 78.3% at 5 and 10 years, respectively, while graft survival was 82.8% and 60%, respectively. The effect of 11 donor variables on the 5- and 10-year survival rates were examined by univariate analysis (Table 1); only three variables gave significant results, i.e., the number of HLA-A and -B mismatching, the side of kidney donation and the DR BWR.
Figure 1.
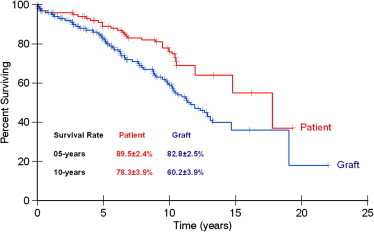
Actuarial patient and graft survival.
Table 1.
Univariate analysis of the effect of donor variables on pediatric renal allograft survival.
| Variable | N (%) of cases | Survival rate |
Log rank P | |
|---|---|---|---|---|
| 5 years | 10 years | |||
| Age, years | ||||
| <30 | 51 (18.7) | 0.826 | 0.481 | 0.151 |
| 31–40 | 132 (48.4) | 0.833 | 0.653 | |
| 41–50 | 76 (27.8) | 0.849 | 0.613 | |
| >50 | 14 (5.1) | 0.581 | 0.596 | |
| Sex | ||||
| Male | 83 (30.4) | 0.839 | 0.628 | 0.858 |
| Female | 190 (69.6) | 0.822 | 0.587 | |
| Recipient sex match | ||||
| Male–male | 55 (20.1) | 0.863 | 0.571 | 0.335 |
| Male–female | 125 (45.8) | 0.857 | 0.578 | |
| Female–male | 28 (10.3) | 0.866 | 0.689 | |
| Female–female | 65 (23.8) | 0.762 | 0.625 | |
| Blood groups | ||||
| Same | 211 (77.3) | 0.833 | 0.578 | 0.478 |
| Different (but compatible) | 62 (22.7) | 0.844 | 0.664 | |
| Consanguinity | ||||
| Parents | 217 (79.5) | 0.859 | 0.631 | 0.221 |
| Siblings | 21 (7.7) | 0.835 | 0.506 | |
| Other relatives | 11 (4.0) | 0.707 | 0.530 | |
| Unrelated | 24 (8.8) | 0.689 | 0.470 | |
| Number of renal arteries | ||||
| 1 | 235 (86.1) | 0.821 | 0.682 | 0.468 |
| 2 | 36 (13.2) | 0.862 | – | |
| >2 | 1 (0.4) | 0.590 | – | |
| Number of HLA-A and -B mismatching | ||||
| 0 | 7 (2.97) | 0.800 | 0.800 | 0.012 |
| 1 | 33 (14.0) | 0.866 | 0.653 | |
| 2 | 205 (75.1) | 0.853 | 0.613 | |
| 3 | 22 (9.4) | 0.468 | 0.301 | |
| 4 | 6 (2.6) | 1.0 | 1.0 | |
| Number of HLA-DR mismatching | ||||
| 1 | 250 (91.6) | 0.800 | 0.800 | 0.319 |
| 2 | 23 (8.4) | 0.829 | 0.585 | |
| Donated kidney | ||||
| Right | 192 (70.3) | 0.798 | 0.561 | |
| Left | 81 (29.7) | 0.916 | 0.691 | 0.02 |
| Infection bilharziasis | ||||
| No | 270 (98.9) | 0.834 | 0.594 | 0.15 |
| Yes | 3 (1.1) | 1.0 | 1.0 | |
| CMV IgG testing | ||||
| No | 241 (88.3) | 0.832 | 0.594 | 0.41 |
| Yes | 32 (11.7) | 0.885 | 0.885 | |
| DR BWR | ||||
| <1.2 | 80 | 0.825 | 0.538 | 0.003 |
| >1.2 | 151 | 0.917 | 0.846 | |
CMV, cytomegalovirus.
All children received their graft from living donors, predominantly from related donors (91.2%), mostly parents (79.5%), whereas sibling and other related donors comprised 11.7%. The 5- and 10-year survival rate by univariate analysis were higher in related than in unrelated transplantation, although the difference was not statistically significant (P = 0.221). Unrelated donations were due to congenital or hereditary disorders, e.g., Alport syndrome, polycystic kidney disease, contraindicating related transplantation, or possibly lack of a suitable family member due to renal, medical or surgical contraindications to donation, despite being highly motivated. Middle-aged donors were generally chosen, the donor mean (SD) age being 37.6 (7.8) years. Most donors were aged 21–50 years, and only 5.1% of donors were aged >50 years. Although there was no observed difference in survival at 5 years, the 10-year graft survival rate was less from relatively young (21–30 years) and relatively old donors (>50 years), enforcing our policy of choosing middle-aged donors, although this difference in survival was not statistically significant. Females constituted most donors (69.6%), mainly mothers of the children, and siblings to a lesser extent. There was no statistically significant effect on graft survival at 5 and 10 years (P = 0.858). DR sex matching showed no apparent difference in either male or female donation to either male or female recipients (P = 0.335).
The side of donor nephrectomy showed a statistically significant difference. Donation of the left kidney gave a higher survival rate at 5 and 10 years than a right kidney (P = 0.024) on univariate analysis, although the mean split radioisotope clearance (GFR) of the donated kidneys were comparable (60.5 and 58.4 mL/min for the right and left kidney, respectively).
Table 1 shows the significant effect of HLA-A and -B mismatching on the 5- and 10-year survival rates (P = 0.012). There was a decline in survival rate as more mismatched loci were present. HLA-DR mismatching had no significant effect on graft survival (P = 0.319).
Interactive recipient variables with the previous donor variables were also assessed. The effect of nine recipient variables on the 5- and 10-year graft survival were examined by univariate analysis and shown in Table 2. Five variables were statistically significant, i.e., age of the recipients, number of acute rejection crises, ATN after transplant, immunosuppression, and hypertension. There was no significant difference in the effect of the original kidney disease on graft survival, probably because in more than half the patients with ESKD, no specific pathology was recognized due to shrunken or fibrotic kidneys with no demonstrable pathology.
Table 2.
Univariate analysis of the effect of recipient variables on pediatric renal allograft survival.
| Variable | N (%) of cases | Survival rate |
Log rank P | |
|---|---|---|---|---|
| 5 years | 10 years | |||
| Age, years | ||||
| 5–10 | 60 (22.0) | 0.745 | 0.529 | 0.04 |
| 10–17 | 213 (78.0) | 0.842 | 0.621 | |
| Sex | ||||
| Male | 180 (65.9) | 0.834 | 0.509 | 0.858 |
| Female | 93 (34.1) | 0.727 | 0.569 | |
| Original kidney disease | ||||
| Chronic glomerulonephritis | 56 (20.5) | 0.799 | 0.510 | 0.801 |
| Chronic pyelonephritis | 31 (11.4) | 0.834 | 1.00 | |
| Amyloidosis | 2 (0.7) | 1.800 | 0.675 | |
| Hereditary nephritis | 10 (3.7) | 0.750 | 0.500 | |
| Obstructive uropathy | 18 (6.6) | 0.830 | 0.634 | |
| ESKD | 156 (57.1) | 0.814 | 0.617 | |
| Number of acute rejections | ||||
| None | 115 (42.1) | 0.931 | 0.824 | 0.001 |
| 1 | 69 (25.3) | 0.830 | 0.587 | |
| > 1 | 89 (32.6) | 0.759 | 0.494 | |
| Ischemia intervals, min | ||||
| < 45 | 111 | 0.826 | 0.576 | 0.629 |
| > 45 | 162 | 0.832 | 0.652 | |
| ATN after transplant | ||||
| Yes | 18 (6.6) | 0.677 | 0.271 | 0.002 |
| No | 255 (93.4) | 0.848 | 0.621 | |
| Immunosuppression | ||||
| Azathioprine-based | 13 (4.8) | 0.462 | 0.308 | 0.001 |
| Cyclosporin-based | 26 (9.5) | 0.833 | 0.441 | |
| Triple therapy | 141 (51.6) | 0.833 | 0.628 | |
| Tacrolimus-based | 92 (33.7) | 0.932 | 0.828 | |
| Rapamycin-based | 1 (0.4) | 0.00 | 0.00 | |
| Hypertension | ||||
| No | 132 (48.4) | 0.834 | 0.812 | 0.05 |
| Yes | 141 (51.7) | 0.831 | 0.538 | |
| Transplant received | ||||
| 1st | 270 (98.9) | 0.839 | 0.607 | 0.129 |
| Re-transplant | 3 (1.1) | 0.667 | 0.333 | |
ATN had a statistically significant effect and correlated with the 5- and 10-year graft survival on univariate analysis. P = 0.002, although there was no significant effect of ischemia times on early or long-term graft survival (P = 0.629). The availability of cyclosporin and newer agents like tacrolimus and mycophenolate mofetil has led to a significant improvement in graft survival rates compared to the traditional protocols using azathioprine (P < 0.001).
Donor and recipient variables which were statistically significant were entered in a multivariate analysis using a Cox proportional-hazards regression model, to detect independent variables with a sustained effect on the 5- and 10-year graft survival rates. Variables that maintained their significance were ATN, number of acute rejection episodes, hypertension and DR BWR (Table 3).
Table 3.
Predictors of long-term pediatric renal graft survival by multivariate analysis (Cox proportional hazards regression model).
| Variables | Regression estimate (B) | SE | Relative risk (95% CI), ExpB | P |
|---|---|---|---|---|
| ATN | ||||
| No | Ref. | – | 1.00 | – |
| Yes | 1.43 | 0.55 | 4.18 (1.43,12.24) | 0.009 |
| Number of acute rejections | ||||
| No rejection | Ref. | – | 1.000 | – |
| One rejection | 0.98 | 0.50 | 2.66 (1.0–7.1) | 0.050 |
| >one rejection | 1.66 | 0.44 | 4.83 (2.0–11.52) | <0.001 |
| Hypertension | ||||
| No | Ref. | – | 1.000 | – |
| Yes | 0.66 | 0.33 | 1.94 (1.0–3.67) | 0.042 |
| DR BWR | ||||
| <1.2 | Ref. | – | 1.000 | – |
| >1.2 | 0.71 | 0.29 | 2.0 (1.15–3.61) | 0.015 |
At the last follow-up, 185 patients (67.8%) had a functioning graft, while 82 (30.0%) had graft failure (Table 4). Only six patients (0.02%) were lost to follow-up.
Table 4.
Condition of the graft at the last follow-up.
| Status | N (%) of cases |
|---|---|
| Living with functioning graft | 175 (64.1) |
| Living on dialysis | 58 (21.2) |
| Died with functioning graft | 10 (3.7) |
| Died with failed graft | 24 (8.8) |
| Lost follow-up | 6 (2.2) |
| Total | 273 (100) |
Discussion
The graft survival rates of pediatric kidney transplant recipients have shown a steady improvement in the last two decades among North American children, for both living and deceased donor transplants [9]. Data from NAPRTCS have shown better 5- and 7-year graft survival rates for children who received a kidney transplant from living donors (85.2% vs. 76.9% at 5 years, and 78% vs. 65% at 7 years for living and deceased donors, respectively) [10]. Analysis of the UNOS database showed that living donation gives better outcomes for pediatric recipients, does not require time on the waiting list and should be recommended whenever possible [9].
In this study, there were lower allograft survival rates for recipients aged <10 years of age than those aged 10–17 years, on univariate analysis of the rates at 5 and 10 years (P = 0.04), but the multivariate analysis did not confirm this finding. Gulati et al. [9] reported that older children (10–17 years) had poorer graft survival; the authors explained their results as possibly due to not adhering to medications, or that rejection episodes might be also more refractory to therapy, and require customized approaches to reverse the specific rejection mechanisms and salvage the graft.
There was no significant effect of a specific donor age group in the present series at 5 and 10 years of follow-up. In our series, living donors aged 21–60 years were included in the programme. However, on the contrary, the Organ Procurement and Transplantation Network/UNOS database [11] showed a graft survival advantage by using live-donor kidneys from donors aged <55 years for the pediatric renal transplant population, while kidneys from ⩾55-year-old live donors had poorer allograft survival than for younger live donors. However, survival was comparable to deceased-donor recipients.
The clinical importance of nephron mass as well as renal functional adaptation has been studied in the context of comparing pediatric and adult kidney transplantation [12]. In this study the effect of DR BWR mismatch was assessed; the donation of adult-sized kidneys had no adverse effect on graft function. On the contrary, there was a significantly better long-term survival at 5 and 10 years, suggesting better survival with a relatively large nephron mass. This was confirmed by univariate and multivariate analysis. This was also confirmed by Lezaic et al. [13], who compared the function of living adult kidney grafts transplanted into adult and child recipients, and concluded that the function of adult kidney grafts in adult and child recipients had suffered no adverse events that would be expected to affect subsequent graft function, and adult kidney grafts would have a lower GFR even if this does not reflect the graft’s full compensatory capacity. Dubourg et al. [14] also supported this view.
In adult transplantation, the effect of DR BWR mismatch on patient and graft outcome was recently reported by our group [7]. The DR BWR was a significant predictor of graft survival; a low DR BWR contributed to inferior long-term graft survival.
In this study there was no significant difference between graft survival of kidneys obtained from either related or unrelated donors; both groups were comparable at 5 and 10 years after surgery, on univariate analysis. Emotionally related (but genetically unrelated) living donors are generally accepted by many transplant centers, as this can shorten the waiting time for transplantation, especially in children. Several studies showed that the outcome of renal transplantation from unrelated living donors is excellent and better than cadaver transplants [15]. Similar to our results, Humar et al. [16] showed a comparable graft survival rate between unrelated and related kidney transplants despite more frequent complications, including chronic rejection in the group of unrelated transplants.
ABO-identical living-related kidney transplants in this study had a similar graft outcome to those with different (but compatible) transplants. These results were contradicted by Park et al. [17], who reported better graft survival in ABO-identical transplants.
In this study, HLA-A and -B matching had an effect on graft survival at 5 and 10 years after transplantation, while HLA-DR mismatch was not significantly correlated with graft survival. However, the multivariate analysis did not confirm these results. Takemoto et al. [18] also found that recipients of HLA-matched kidney have better outcomes, as defined by lower rates of rejection and higher rates of graft and patient survival, than recipients of HLA-mismatched kidneys. Gritsch et al. [19] reported that the 5-year survival rates for cadaver grafts were identical in grafts with no HLA-DR mismatches and in those with two HLA-DR mismatches (71%), and that the odds of developing a panel-reactive antibody titer of >30% by the time of second transplantation did not increase significantly in the presence of HLA mismatches. They finally recommended accepting HLA-DR mismatched kidneys from deceased donors aged ⩽35 years for transplantation into children with ESKD. However, Oplez and Dohler [20] clearly showed that the number of mismatches correlated significantly with the rates of graft survival and rejection. In this study, all the kidneys offered to our children were retrieved from living-related donors and we did not accept any HLA-DR mismatched grafts. In addition, mismatches in HLA-A or -B were generally abandoned in our series.
Ghoneim and Refaie [21] supported the conclusion of Gritsch et al. [19], who conducted a study with the aim of shortening the waiting time or dialysis of pediatric renal transplant candidates, and hence minimizing the morbidity of these individuals. However, they recommended that the suggestion to use HLA-DR mismatched grafts should be analyzed cautiously.
In this study the multiplicity of donor renal arteries had no significant effect on graft survival rates. Similar results were reported by Benedetti et al. [22]. A contradictory view was provided by Roza et al. [23], who found that the use of grafts with multiple arteries was associated with a greater risk to allograft survival and a higher incidence of vascular complications.
In this study there was an independent effect of ATN on graft survival at 5 and 10 years; this result was previously confirmed in the report of NAPRTCS and US renal data system [24]. They found that ATN after kidney transplantation results in a 20–35% worse graft survival rate than in recipients without ATN, at all times after transplantation.
In our study, the mean split renographic clearance (GFR) of the donated kidneys was comparable (60.5 and 58.4 mL/min for the right and left kidney, respectively). However, on univariate analysis donation of left kidney offered a better survival advantage over the right kidney, but multivariate analysis did not support this observation.
In this work, more clinical acute rejection crises had an independent negative effect on the overall graft survival rate. Patients with no rejection crises had the best graft survival at 5 and 10 years, while frequent rejection episodes had the lowest survival rates. The same conclusion was reached by Benfield et al. [25], who reported better short-term graft survival rates in rejection-free recipients. A similar conclusion was reported by Tejani and Sullivan [26]. Furthermore, they indicated that acute rejections ultimately result in chronic rejection and graft loss. In the NAPRTCS report [9], although there was an increase in the 1-year graft survival rate, half of all graft failures were due to rejection, and chronic rejection was the most common cause, accounting for 35% of the failures.
In this study, on univariate analysis tacrolimus-based immunosuppression offered the highest overall survival rates (93.2% and 82.8% at 5 and 10 years, respectively) followed by the cyclosporin-based therapy, and the lowest survival rates were when azathioprine was used (46% and 30.8% at 5 and 10 years, respectively). These results were not confirmed by the multivariate regression analysis. NAPRTCS reported a decline in the use of cyclosporin A, anti-thymocyte globulin, OKT3 and anti-interleukin-2 receptor blockers, with increased use of tacrolimus, mycophenolate mofetil in addition to steroid-sparing regimens [27].
In this study, hypertension after kidney transplantation had an independent effect, by multivariate analysis, on graft survival after 10 years of follow-up. This was also shown by Mitsnefes et al. [28], who concluded that the development of hypertension after transplantation is a significant and independent predictor of poor long-term transplant function, regardless of the number of rejection episodes or transplant function at 1 year. Furthermore, Sorof et al. [29] noted that hypertension is a significant risk for the development of graft dysfunction, and an independent predictor of graft survival.
In conclusion, donor and recipient variables that affect short- and long-term graft survival of pediatric live-donor renal allografts are DR BWR, number of acute rejections, ATN and hypertension. Considering these variables provides a better outcome.
References
- 1.Tejani A., Stablein D.M., Donaldson L., Harmon W.E., Alexander S.R., Kohaut E. Steady improvement in short-term graft survival of pediatric renal transplants: the NAPRTCS experience. Clin Transplant. 1999:95–110. [PubMed] [Google Scholar]
- 2.Cecka J.M., Gjertson D.W., Terasaki P.I. Pediatric renal transplantation. A review of the UNOS data. Pediatr Transplant. 1997;1:55–64. [PubMed] [Google Scholar]
- 3.Tejani A., Stablein D.M., Sullivan E.K., Alexander S.R., Fine R.N., Harmon W.E. The impact of donor source, recipient age, pre-operative immunotherapy and induction therapy on early and late acute rejections in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Pediatr Transplant. 1998;2:318–324. [PubMed] [Google Scholar]
- 4.Gjertson D.W., Cecka J.M. Determinants of long-term survival of pediatric kidney grafts. Reports to the United Network for Organ Sharing Kidney Transplant Registry. Pediatr Transplant. 2001;5:5–15. doi: 10.1034/j.1399-3046.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- 5.Oplez G., Döhler B. Pediatric kidney transplantation: analysis of donor age, HLA match and post-transplant non-Hodgkin lymphoma: a Collaborative Transplant Study Report. Transplantation. 2010;90:292–297. doi: 10.1097/TP.0b013e3181e46a22. [DOI] [PubMed] [Google Scholar]
- 6.Wafa E.W., Donia A.F., Ali El-Dein B., El Agroudy A.E., Rifaie A., Moustafa A. Evaluation and selection of potential kidney donors. J Urol. 2004;171:1424–1427. doi: 10.1097/01.ju.0000116431.65651.58. [DOI] [PubMed] [Google Scholar]
- 7.El Agroudy A., Hassan N.A., Bakr M.A., Foda M.A., Shokeir A.A., Shehab El-Dein. Effect of donor/recipient body weight mismatch on patient and graft outcome in living donor kidney transplantation. Am J Nephrol. 2003;23:294–299. doi: 10.1159/000072819. [DOI] [PubMed] [Google Scholar]
- 8.Solez K., Colvin R.B., Racusen L.C., Haas M., Sis B., Mengel M. Banff 07 classification of renal allograft pathology. Updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 9.Gulati A., Sarwal M.M. Pediatric renal transplantation. An overview and update. Current Opin Pediatrics. 2010;22:189–196. doi: 10.1097/MOP.0b013e32833683fd. [DOI] [PubMed] [Google Scholar]
- 10.North American Pediatric Renal Trials and Collaborative Studies. 2008 Annual Report. An overview of the current state of pediatric kidney transplantation across the United States. The data provide future directions for changing practices and guiding research to improve outcomes in pediatric kidney transplantation. Available at http://www.naprtcs.org.
- 11.Dale-Shall A.W., Smith J.M., McBride M.A., Hingorani S.R., McDonald R.A. The relationship of donor source and age on short-and long-term allograft survival in pediatric renal transplantation. Pediatr Transplant. 2009;13:711–718. doi: 10.1111/j.1399-3046.2008.01054.x. [DOI] [PubMed] [Google Scholar]
- 12.Parada B., Figueiredo A., Nunes P., Bastos C., Macario F., Roseiro A. Pediatric renal transplantation: comparative study with renal transplantation in adult population. Transplant Proc. 2005;37:2771–2774. doi: 10.1016/j.transproceed.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 13.Lezaic V., Naumovic R., Stanic M., Marin Kovic J., Kostic M., Paco-Antic A. Factors affecting graft function in pediatric and adult recipients of adult live-donor kidney transplants. Pediatr Transplant. 2007;11:906–913. doi: 10.1111/j.1399-3046.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- 14.Dubourg L., Cochat P., Hadj-Aissa A., Tyden G., Berg U.B. Better long-term functional adaptation to the child’s size with pediatric compared to adult kidney donors. Kidney Int. 2002;62:1454–1460. doi: 10.1111/j.1523-1755.2002.kid576.x. [DOI] [PubMed] [Google Scholar]
- 15.Cecka J.M. Results of more than 1000 recent living-unrelated donor transplant in the US. Transplant Proc. 1999;31:234. doi: 10.1016/s0041-1345(98)01515-2. [DOI] [PubMed] [Google Scholar]
- 16.Humar A., Durand B., Gillingham K., Payne W.D., Sutherland W.E., Matas A.J. Living unrelated donors in kidney transplants: better long-term results than with non-HLA-identical living related donors? Transplantation. 2000;69:1942–1945. doi: 10.1097/00007890-200005150-00033. [DOI] [PubMed] [Google Scholar]
- 17.Park K., Kim Y.S., Kim M.S., Kim S.I., Oh C.K., Han D.S. A 16-year experience with 1275 primary living donor kidney transplants: univariate and multivariate analysis of risk factors affecting graft survival. Transplant Proc. 1996;28:1578–1579. [PubMed] [Google Scholar]
- 18.Takemoto S., Port F.K., Claas F.H., Duquesnoy R.J. HLA matching for kidney Transplantation. Hum Immunol. 2004;65:1489–1505. doi: 10.1016/j.humimm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Gritsch H.A., Veale J.L., Leichtman A.B., Guidinger M.K., Magee J.C., MaDonald R.A. Should pediatric patients wait for HLA-DR-matched renal transplants? Am J Transplant. 2008;8:2056–2061. doi: 10.1111/j.1600-6143.2008.02320.x. [DOI] [PubMed] [Google Scholar]
- 20.Oplez G., Dohler B. Pediatric kidney transplantation. Analysis of donor age, HLA match, and post transplant non-Hodgkin lymphoma: a Collaborative Transplant Report. Transplantation. 2010;90:292–297. doi: 10.1097/TP.0b013e3181e46a22. [DOI] [PubMed] [Google Scholar]
- 21.Ghoneim M.A., Refaie A.F. Is matching for human leucocyte antigen-DR beneficial in pediatric kidney transplantation. Nature Clin Prac Nephrol. 2009;5:70–71. doi: 10.1038/ncpneph1017. [DOI] [PubMed] [Google Scholar]
- 22.Benedetti E., Troppmann C., Gillingham K., Sutherland D.E., Payne W.D., Dunn D.L. Short-and long-term outcomes of kidney transplants with multiple renal arteries. Ann Surg. 1995;221:406–414. doi: 10.1097/00000658-199504000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roza A.M., Perloff L.J., Naji A., Grossman R.A., Barker C.F. Living-related donors with bilateral multiple renal arteries. A twenty-year experience. Transplantation. 1989;47:397–399. [PubMed] [Google Scholar]
- 24.Sarwal M.M., Cecka J.M., Millan M.T., Salvatierra O. Adult-size kidneys without acute tubular necrosis provide exceedingly superior long-term graft outcomes for infants and small children. Transplantation. 2000;70:1728–1736. doi: 10.1097/00007890-200012270-00012. [DOI] [PubMed] [Google Scholar]
- 25.Benfiled M.R., McDonald L., Sullivan E.K., Stablein D.M., Tejani A. The 1997 annual renal transplantation in children. Report of the American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Pediatr Transplant. 1999;3:152–167. doi: 10.1034/j.1399-3046.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 26.Tejani A., Sullivan E.K. The impact of acute rejection on chronic rejection. A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) Pediatr Transplant. 1999;4:107–111. doi: 10.1034/j.1399-3046.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro R. Living donor kidney transplantation in pediatric recipients. Pediatr Transplant. 2006;10:844–850. doi: 10.1111/j.1399-3046.2006.00557.x. [DOI] [PubMed] [Google Scholar]
- 28.Mitsnefes M.M., Khoury P.R., McEnery P.T. Early post-transplantation hypertension and poor long-term renal allograft survival in pediatric patients. J Pediatr. 2003;143:98–103. doi: 10.1016/S0022-3476(03)00209-9. [DOI] [PubMed] [Google Scholar]
- 29.Sorof J., Sullivan E., Tejani A., Portman R.J. Anti-hypertensive medication and renal allograft failure: a North American Pediatric Renal Transplant Cooperative Study Report. J Am Soc Nephrol. 1999;6:1324–1330. doi: 10.1681/ASN.V1061324. [DOI] [PubMed] [Google Scholar]


