Abstract
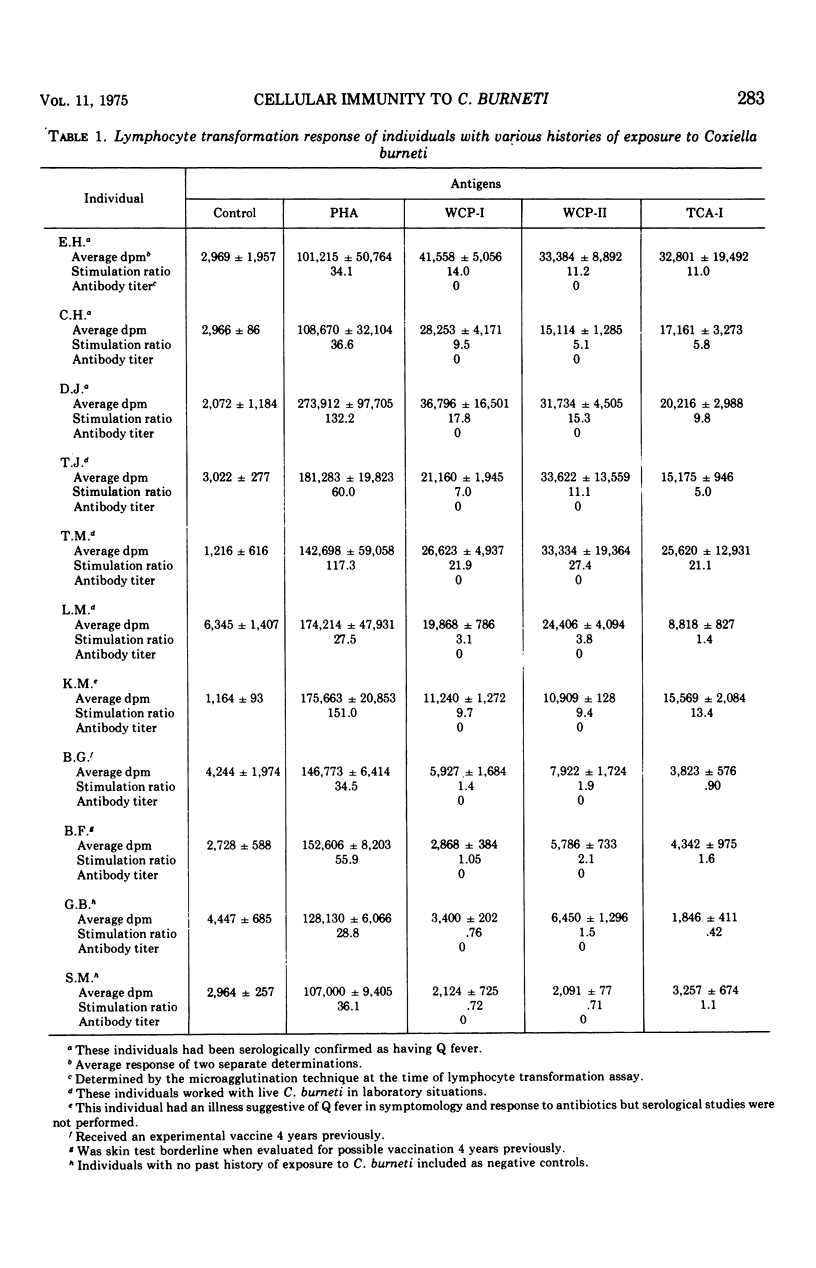
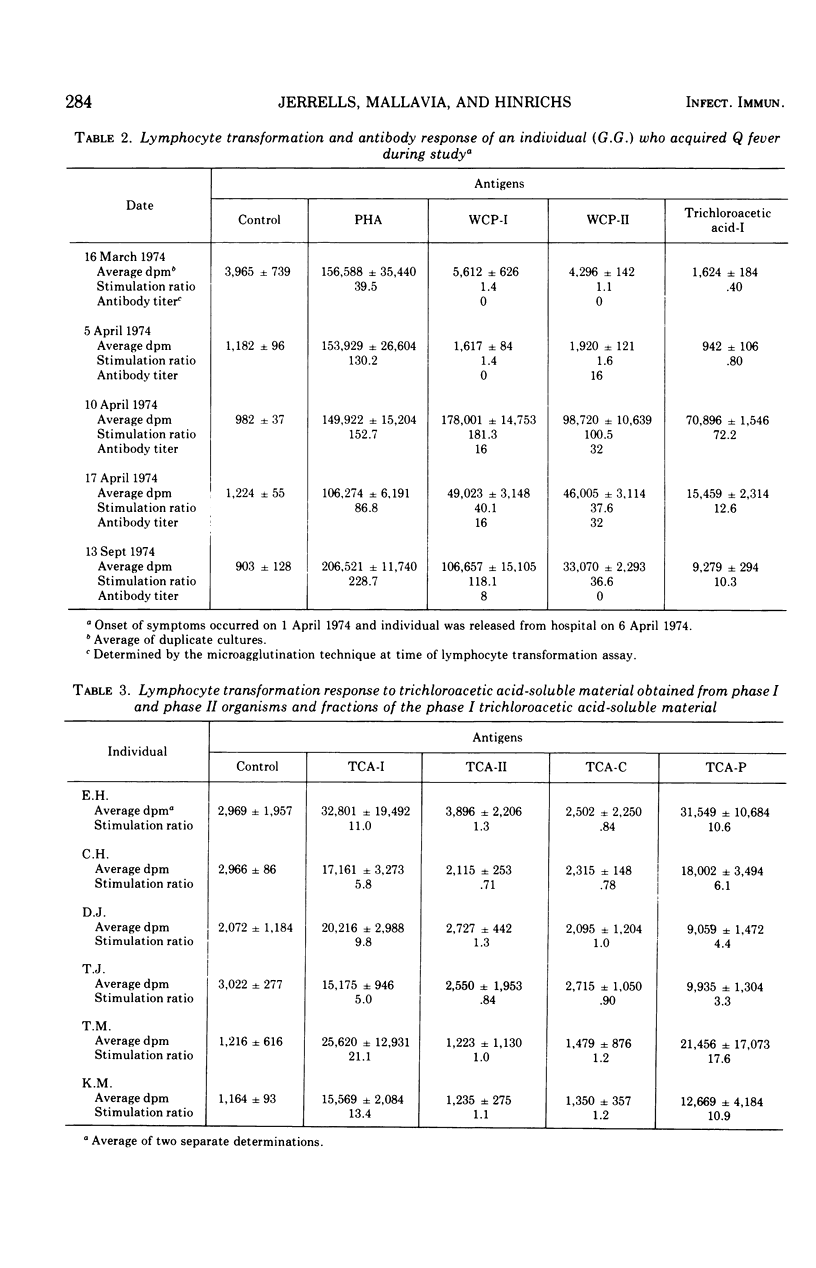
Delayed hypersensitivity to the antigens of Coxiella burneti, Nine Mile strain, was demonstrated in human subjects with various past histories of exposure to the organism by using lymphocyte transformation assays. Individuals with histories indicating exposure to C. burneti up to 8 years before the study demonstrated marked lymphocyte transformation in vitro to whole-cell antigens consisting of formalin-killed C. burneti phase I and phase II. These individuals also demonstrated a marked lymphocyte response to the trichloracetic acid-soluable phase I antigen. One individual who acquired Q fever during the study and one individual who received an experimental Q fever vaccine 4 years earlier were also evaluated by the lymphocyte transformation assay. It was also found that phase I trichloroacetic acid-soluble material was capable of acting as an antigen in the assay, whereas the phase II trichloroacetic acid-soluble material did not contain any antigenic material capable of causing lymphocyte transformation. The complete phase I trichloroacetic acid-soluble antigen, which was found to consist of protein and carbohydrate, was chemically fractionated into monospecific fractions. The fraction treated to eliminate carbohydrate was the only fraction found to elicit an in vitro response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANACKER R. L., HASKINS W. T., LACKMAN D. B., RIBI E., PICKENS E. G. CONVERSION OF THE PHASE I ANTIGEN OF COXIELLA BURNETII TO HAPTEN BY PHENOL TREATMENT. J Bacteriol. 1963 May;85:1165–1170. doi: 10.1128/jb.85.5.1165-1170.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Shepard C. C. Lymphocyte transformation in rickettsioses. J Immunol. 1971 Jan;106(1):209–216. [PubMed] [Google Scholar]
- Crăcea E., Voiculescu R., Zarnea G., Ionescu M., Botez D. Electron microscopic study of phase I and II C. burneti in the chick yolk sac by use of ferritin conjugated antibody. Z Immunitatsforsch Allerg Klin Immunol. 1970 Oct;140(4):358–365. [PubMed] [Google Scholar]
- FISET P. Phase variation of Rickettsia (Coxiella) burneti; study of the antibody response in guinea pigs and rabbits. Can J Microbiol. 1957 Apr;3(3):435–445. doi: 10.1139/m57-046. [DOI] [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- HOYER B. H., ORMSBEE R. A., FISET P., LACKMAN D. B. Differentiation of Phase I and Phase II Coxiella burnetti by equilibrium density gradient sedimentation. Nature. 1963 Feb 9;197:573–574. doi: 10.1038/197573a0. [DOI] [PubMed] [Google Scholar]
- Han T., Pauly J. Simplified whole blood method for evaluating in vitro lymphocyte reactivity of laboratory animals. Clin Exp Immunol. 1972 May;11(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Heggers J. P., Mallavia L. P., Hinrichs D. J. The cellular immune response to antigens of Coxiella burneti. Can J Microbiol. 1974 May;20(5):657–662. doi: 10.1139/m74-101. [DOI] [PubMed] [Google Scholar]
- LACKMAN D. B., BELL E. J., BELL J. F., PICKENS E. G. Intradermal sensitivity testing in man with a purified vaccine for Q fever. Am J Public Health Nations Health. 1962 Jan;52:87–93. doi: 10.2105/ajph.52.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNETTE E. H., CLARK W. H., JENSEN F. W., TOOMB C. J. Q fever studies. XV. Development and persistence in man of complement-fixing and agglutinating antibodies to Coxiella burnetii. J Immunol. 1952 May;68(5):591–598. [PubMed] [Google Scholar]
- LUOTO L., BELL J. F., CASEY M., LACKMAN D. B. Q FEVER VACCINATION OF HUMAN VOLUNTEERS. I. THE SEROLOGIC AND SKIN-TEST RESPONSE FOLLOWING SUBCUTANEOUS INJECTIONS. Am J Hyg. 1963 Jul;78:1–15. [PubMed] [Google Scholar]
- ORMSBEE R. A. A method of purifying Coxiella burnetii and other pathogenic Rickettsiae. J Immunol. 1962 Jan;88:100–108. [PubMed] [Google Scholar]
- ORMSBEE R. A., BELL E. J., LACKMAN D. B. Antigens of Coxiella burnetii. I. Extraction of antigens with non-aqueous organic solvents. J Immunol. 1962 Jun;88:741–749. [PubMed] [Google Scholar]
- Ormsbee R. A., Peacock M., Tallent G., Munoz J. J. An analysis of the immune response to rickettsial antigens in the guinea pig. Acta Virol. 1968 Jan;12(1):78–82. [PubMed] [Google Scholar]
- STOKER M. G., FISET P. Phase variation of the Nine Mile and other strains of Rickettsia burneti. Can J Microbiol. 1956 May;2(3):310–321. doi: 10.1139/m56-036. [DOI] [PubMed] [Google Scholar]



