Abstract
Purpose
To analyse the long-term outcome in relation to multiple graft arteries (MGA) in live-donor renal transplantation, and assess its effect on graft and patient survival.
Patients and methods
Between March 1976 and November 2009, a total of 2100 live-donor renal transplants were carried out at our centre. Patients were stratified according to the number of graft arteries into two groups, i.e. MGA (two or more arteries; 237 patients) and single-graft artery (SGA; 1863 patients). Variables assessed included patient demographics, site of vascular anastomosis, ischaemia time, onset of diuresis, delayed graft function, acute tubular necrosis (ATN), acute rejection, vascular and urological complications. Moreover, long-term patient and graft survival were compared among both groups. Patients were followed up for a mean (SD) of 112 (63) months.
Results
Grafts with MGA were associated with a prolonged ischaemia time (P = 0.001) and ATN (P = 0.005). Vascular thrombosis (arterial and venous) had a higher incidence in MGA (2.5%) than SGA (0.6%) (P = 0.01). Both groups were not significantly different for the onset of diuresis, acute rejection and urological complications (P = 0.16, 0.23 and 0.85, respectively). Graft and patient survival were comparable in both groups. The mean (SD) 1-, 5-, 10- and 20-year graft survival rates (%) for MGA were 96.1 (1.26), 86.6 (2.39), 61.3 (4.42) and 33.8 (7.23), and 97.5 (0.36), 86.8 (0.84), 66.0 (1.35) and 37.3 (2.76) for SGA (P = 0.54).
Conclusions
Although there was a higher incidence of prolonged ischaemia time, ATN and vascular thrombosis in live-donor renal transplants with MGA, it did not adversely affect patient or graft survival. The early, intermediate- and long-term follow-up showed an outcome comparable to that in patients with SGA.
Abbreviations: MGA, multiple graft artery; SGA, single graft artery; ATN, acute tubular necrosis; US, ultrasonography
Keywords: Renal transplantation, Multiple arteries, Live donors
Introduction
It is generally accepted that renal transplantation is the optimum treatment for patients with end-stage renal disease. Organ shortage is still the major challenge in renal transplantation; the living-donor pool is an increasing source for organs, and ensuring that this pool maintains optimum outcomes is invaluable. The presence of multiple graft arteries (MGA) is the most frequently detected anatomical variation during kidney transplantation. Unilateral multiple renal arteries were detected in 23% of donors, while they were detected bilaterally in 10% [1]. Renal allografts with MGA require meticulous surgical techniques to obtain successful results.
The incidence of surgical and medical complications after renal transplantation with MGA is greatly debated. Many authors reported equal complication rates in patients with MGA to those with a single graft artery (SGA) [2,3], while others reported a greater incidence of vascular and urological complications with MGA [4–7]. Most of the present series contain few patients with no long-term follow-up. To the best of our knowledge, the present study represents the largest single-centre experience with many patients and a long-term follow-up to be reported.
We report the prevalence, demographics and surgical techniques of MGA in a large cohort of patients in a single tertiary centre over 33 years, in patients with live-donor renal transplantation. Furthermore, several pre-transplant, technical and post-transplant risk factors were compared among patients with MGA or SGA. In addition, the vascular and urological complications were analysed. Finally, the long-term outcome in relation to MGA on patient and graft survival were calculated and compared to those of SGA.
Patients and methods
Between March 1976 and November 2009, a total of 2100 renal transplants were carried out at our centre. All patients received kidneys from living donors, harvested through open flank incision. Donor renal vessels (in our early experience) were evaluated by conventional angiography, then MR angiography; currently vessels are evaluated by multidetector CT. Split renal function was evaluated by diuretic renography. For the purpose of analysis, patients were stratified according to the number of graft arteries into two groups, i.e. SGA and MGA (two or more arteries). The MGA group included 237 (11.2%) patients while the SGA group comprised of 1863 (88.8%). In the MGA group there were 214 patients with double, 21 with triple, one with four and one with five arteries. There were 176 males and 61 females, with a mean (SD, range) age of 29.5 (11.69, 5–60) years. There were 227 first, nine second and one third transplants. In all, 230 patients were treated with dialysis before transplantation, while the remaining seven had a pre-emptive renal transplant. In the SGA group there were 1385 males and 478 females, with a mean (SD, range) age of 29.6 (10.54, 5–62) years. There were 1791 first, 70 second and two third transplants. In all, 1793 patients were treated with dialysis before transplantation, while the remaining 70 had a pre-emptive renal transplant. A summary of demographic characteristics of both groups is given in Table 1.
Table 1.
Characteristics of patients in the MGA (237 patients) and SGA (1863 patients) groups.
| Variable | SGA | MGA | P |
|---|---|---|---|
| n (%): | |||
| Before transplant | |||
| Recipient age (years) | |||
| ⩽18 | 270 (14.5) | 39 (16.5) | |
| 19–30 | 770 (41.3) | 98 (41.4) | |
| 31–40 | 546 (29.3) | 58 (24.5) | |
| 41–50 | 230 (12.3) | 31 (13.1) | |
| >50 | 47 (2.5) | 11 (4.6) | 0.21 |
| Recipient sex | |||
| Male | 1385 (74.3) | 176 (74.3) | |
| Female | 478 (25.7) | 61 (25.7) | 0.93 |
| Donor age (years) | |||
| <30 | 737 (39.6) | 69 (29.1) | |
| 30–40 | 621 (33.3) | 81 (34.2) | |
| 41–50 | 334 (17.9) | 60 (25.3) | |
| >50 | 171 (9.2) | 27 (11.4) | 0.004 |
| Donor sex | |||
| Male | 889 (47.7) | 107 (45.1) | |
| Female | 974 (52.3) | 130 (54.9) | 0.45 |
| Human leukocyte antigen mismatch | |||
| Zero match | 149 (7.9) | 17 (7.2) | |
| One | 215 (11.5) | 22 (9.3) | |
| Two | 911 (48.9) | 128 (54) | |
| Three | 289 (15.6) | 32 (13.5) | |
| Four | 117 (6.3) | 18 (7.6) | 0.51 |
| Inapplicable | 182 (9.8) | 20 (8.4) | |
| Consanguinity | |||
| Related | 1539 (82.6) | 196 (82.7) | |
| Unrelated | 324 (17.4) | 41 (17.3) | 0.97 |
| Transplant received | |||
| First | 1791 (96.1) | 227 (95.8) | |
| Second | 70 (3.8) | 9 (3.8) | |
| Third | 2 (0.1) | 1 (0.4) | 0.48 |
| Technical | |||
| Main renal artery to: | |||
| Internal iliac | 1605 (86.2) | 192 (81) | |
| External iliac | 118 (6.3) | 15 (6.3) | |
| Common iliac | 110 (5.9) | 20 (8.4) | |
| Aorta | 30 (1.6) | 10 (4.2) | 0.015 |
| Renal vein to: | |||
| External iliac | 1694 (91) | 210 (88.6) | |
| Common iliac | 33 (1.8) | 6 (2.5) | |
| Inferior vena cava | 136 (7.3) | 21 (8.9) | 0.21 |
| Primary urinary re-continuity | |||
| Politano-Leadbetter | 166 (8.9) | 4 (1.7) | |
| Lich-Gregoir | 1667 (89.5) | 227 (95.8) | |
| Uretero-ureteric | 23 (1.2) | 6 (2.5) | |
| Pelvi-ureteric | 3 (0.2) | 0 | |
| Ileal conduit | 4 (0.2) | 0 | 0.001 |
| Ischaemia time (min) | |||
| ⩽30 | 220 (11.8) | 6 (2.5) | |
| 31–60 | 1475 (79.2) | 130 (54.9) | |
| >60 | 168 (9) | 101 (42.6) | 0.001 |
| Delayed graft function | |||
| Immediate | 1722 (92.4) | 213 (89.9) | |
| Delayed | 141 (7.6) | 24 (10.1) | 0.16 |
| After transplant | |||
| ATN | |||
| No | 1776 (95.3) | 216 (91.1) | |
| Yes | 87 (4.7) | 21 (8.9) | 0.005 |
| Acute rejection | |||
| No | 1081 (58) | 147 (62) | |
| Yes | 782 (42) | 90 (38) | 0.23 |
| Immunosuppression: Steroid | |||
| + Aza | 282 (15.1) | 27 (11.4) | |
| + CsA | 158 (8.5) | 15 (6.3) | |
| + CsA + Aza | 1037 (55.7) | 123 (51.9) | |
| + Tacrolimus based | 310 (16.6) | 61 (25.7) | |
| + Sirolimus based | 76 (4.1) | 11 (4.6) | 0.008 |
Aza, Azathioprine; CsA, cyclosporin A.
Surgical technique
We usually use a right para-rectal incision with an extraperitoneal approach. The iliac vessels, aorta or inferior vena cava were used for vascular anastomosis, depending on the recipient size. The aorta and common iliac artery were more frequently used in MGA (P = 0.01). Table 1 summarizes the types of arterial and venous anastomoses of both groups, while Table 2 lists the mode of arterial anastomosis in MGA. Ureteric anastomosis was established through vesico-ureteric re-implantation in the vast majority of patients in both groups (Table 1). Most patients were treated with cyclosporin-based immunosuppression, including methyl prednisolone and azathioprine, while mycophenolate mofetil, tacrolimus and sirolimus were used in a few patients in both groups (Table 1).
Table 2.
Mode of vascular anastomosis in the MGA group.
| Artery | Renal artery, n (%) |
||||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | |
| Internal iliac | 192 (81) | 102 (43) | 10 (48) | 0 | 0 |
| External iliac | 15 (6) | 61 (26) | 4 (19) | 0 | 0 |
| Common iliac | 20 (8) | 14 (6) | 1 (5) | 0 | 0 |
| Inferior epigastric | 0 | 48 (21) | 6 (29) | 1/1 | 0 |
| Aorta | 10 (24) | 5 (2) | 0 | 0 | 0 |
| Ligated | 0 | 7 (3) | 0 | 0 | 1/1 |
After surgery patients were followed daily by measurement of serum creatinine and electrolytes, a complete blood count and routine bedside Doppler and grey scale ultrasonography (US). At discharge, MR angiography and urography were used as a baseline study. All patients were then strictly followed weekly for 3 months, monthly for 6 months and every 3 months, by urine analysis, serum creatinine level, complete blood count and drug level. Doppler US was used if there was decreased urine output or rising serum creatinine level. A graft biopsy was taken for cases of clinically unexplained graft impairment. The mean (SD, range) follow-up was 112 (63, 13–352) months.
The MGA and SGA groups were assessed for patient demographics, site of vascular anastomosis, ischaemia time (the period of ischaemia from ligation of the renal artery on the donor side until restoration of vascularity of the graft on the recipient side), onset of diuresis, and delayed graft function. The occurrence of acute tubular necrosis (ATN) and incidence of acute rejection episodes were also assessed. Moreover, the incidence of vascular complications (renal artery thrombosis and renal artery stenosis), postoperative haemorrhage, lymphocele and urinary leakage or obstruction were compared between the groups. In addition, the mean serum creatinine level at 1, 3 and 5 years and the last follow-up was assessed. Finally, the long-term patient and graft survival rates were compared among patients of both groups.
Data were stored in an electronic database. The Pearson, chi-square and Student’s t-tests were used to determine the statistical significance of differences. Survival of grafts and patients was calculated using the Kaplan–Meier technique, with differences in survival assessed using the log-rank test, with P < 0.05 considered to indicate significance.
Results
Recipients with MGA had a mean (SD) serum creatinine level of 1.45 (0.86) mg/dL, compared to 1.37 (0.65) mg/dL in those with SGA (not significantly different; P = 0.09). The mean serum creatinine level at all intervals showed no significant difference between the two groups (Table 3).
Table 3.
Serum creatinine at different times during the follow-up.
| Time (years) |
n, mean (SD) serum creatinine (mg/dL) |
P | |
|---|---|---|---|
| SGA | MGA | ||
| 1 | 1712, 1.37 (0.65) | 221, 1.45 (0.09) | 0.09 |
| 3 | 1518, 1.60 (0.92) | 206, 1.70 (0.99) | 0.13 |
| 5 | 1247, 1.68 (1.01) | 155, 1.80 (1.09) | 0.15 |
| Last | 1404, 2.20 (2.10) | 186, 2.30 (2.50) | 0.38 |
Table 1 shows the comparison of the different pre-transplant, technical and post-transplant variables between the groups. There was no significant difference between the groups for recipient age (P = 0.21), recipient sex (P = 0.93), donor sex (P = 0.45), human leukocyte antigen mismatch (P = 0.51), number of renal transplants received either first, re-transplant or third transplant (P = 0.48), onset of diuresis (P = 0.16) and acute rejection episodes (P = 0.23). Patients with MGA had a significantly longer cold ischaemia time (P = 0.001); the mean (SD, range) cold ischaemia time was 62.2 (19.64, 30–132) min, vs. 44.7 (12.2, 18–120) min in the SGA group. When multiple vessels were used, the prolonged ischaemia time was >60 min in 42.6% of patients, vs. 9% in the SGA group (P = 0.001). ATN confirmed by biopsy was more common in patients with MGA (8.9% vs. 4.7%, P = 0.005).
Vascular and haemorrhagic complications occurred in 14 patients with MGA (5.9%), compared with 62 (3.3%) with SGA; the difference was statistically significant (P = 0.001; Table 4). Renal allograft arterial thrombosis was recorded in six patients (2.5%) with MGA, compared with 13 (0.6%) in the SGA group (statistically significant difference, P = 0.001; Table 4). Renal vein thrombosis occurred in four patients with SGA (0.2%), but there were no cases of venous thrombosis in the MGA group (P = 0.38; Table 4). The graft was saved in 15 (65%) and lost in eight (35%) patients with arterial and venous thrombosis. Significant haemorrhage which required active intervention was reported in seven transplants with MGA (3%) and in 37 with SGA (2%) (no significant difference, P = 0.38; Table 4). The causes of haemorrhage in both groups are also shown in Table 4. There were no deaths directly related to haemorrhagic, thrombotic or stenotic vascular complications, or as a result of their surgical exploration.
Table 4.
Surgical complications, and causes of haemorrhage in the explored patients, in the SGA and MGA groups.
| Complication, n (%) | SGA | MGA | P |
|---|---|---|---|
| Vascular and haemorrhagic | 62 (3) | 14 (6) | 0.001 |
| Renal artery thrombosis | 13 (0.6) | 6 (2.5) | <0.001 |
| Renal vein thrombosis | 4 (0.2) | 0 | 0.38 |
| Renal artery stenosis | 8 (0.4) | 1 (0.4) | 0.98 |
| Haemorrhage | 37 (2) | 7 (3) | 0.32 |
| Urological | |||
| Overall | 131 (7) | 19 (8) | 0.68 |
| Urinary fistula | 43 (2) | 5 (2) | 0.87 |
| Urinary obstruction | 30 (2) | 4 (2) | 0.95 |
| Lymphocele | 285 (15.3) | 41 (17.3) | 0.42 |
| Causes of haemorrhage in explored patientsa | |||
| Rupture graft | 11 (0.6) | 2 (0.8) | |
| Site of vascular anastomosis | 7 (0.4) | 3 (1.3) | |
| Slipped ligature over inferior epigastric artery | 1 (0.05) | 0 | |
| Tunnel of vesico-ureteric anastomosis | 1 (0.05) | 0 | |
| Graft biopsy | 4 (0.2) | 0 | |
| Nonspecific bleeding | 9 (0.5) | 2 (0.8) | |
Four patients were treated by percutaneous drainage of the haematoma.
Overall urological complications were comparable among patients with MGA (19, 8%) and those with SGA (131, 7%) (no significant difference, P = 0.68; Table 4). Ureteric leakage occurred in five patients with MGA (2.1%) compared with 2.3% with SGA (not significant P = 0.85; Table 4). In these five patients the ureteric leakage developed at mean (SD) interval of 14.4 (8.2) days after surgery. Urinary leakage via the drains, increasing serum creatinine level, appearance of newly diagnosed peri-graft collection or abdominal or graft tenderness were the presenting symptoms. Percutaneous nephrostomy and JJ ureteric stents were successful in two patients, while in the remaining three extensive leakage required a repeat ureteroneocystostomy.
Ureteric obstruction was present in four patients with MGA compared to 30 with SGA (1.6%) (no significant difference, P = 0.95; Table 4). Progressive increase in serum creatinine level with graft hydronephrosis by US provoked further investigation of the patients with MR urography for the configuration of the pelvicalyceal system, degree of hydronephrosis, and to detect the site and length of the obstructed segment. Diuretic renography provided a functional evaluation and outlined the obstruction. Percutaneous nephrostomy is the initial step in diagnosis and treatment. Antegrade pyelo-ureterography via the nephrostomy showed the configuration of the pelvicalyceal system and the ureteric dilatation above the obstructed segment. One patient was successfully treated with antegrade balloon dilatation. The remaining three required surgical intervention in the form of repeat ureteroneocystostomy in one, uretero-ureterostomy in another and pelvi-ureteric anastomosis in the third.
The early-, intermediate- and long-term graft and patient survival were comparable among patients of both groups. The 1-, 5-, 10- and 20-year graft and patient survival rates in the two groups are shown in Table 5 and Fig. 1A and B.
Table 5.
Graft and patient survival rates.
| Time (years) | Mean (SD) survival (%) |
P | |
|---|---|---|---|
| SGA | MGA | ||
| Graft | |||
| 1 | 97.5 (0.36) | 96.1 (1.26) | |
| 5 | 86.8 (0.84) | 86.6 (2.39) | |
| 10 | 66.0 (1.35) | 61.3 (4.42) | |
| 20 | 37.3 (2.76) | 33.8 (7.23) | 0.54 |
| Patients | |||
| 1 | 96.3 (0.44) | 96.6 (1.18) | |
| 5 | 89.5 (0.75) | 91.6 (1.92) | |
| 10 | 77.5 (1.21) | 81.4 (3.50) | |
| 20 | 51.9 (2.76) | 42.9 (9.72) | 0.98 |
Figure 1.
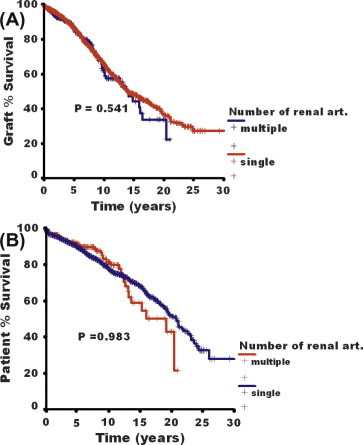
Graft (A) and patient (B) survival rates in patients with SGA or MGA (P = 0.54 and 0.98, respectively).
Discussion
There has been a continuous increase in patient and graft survival rates due to improved surgical techniques, newly developed immunosuppressive regimens, and postoperative monitoring and follow-up. The widespread application of transplantation is severely limited by the donor organ shortage. Thus, every organ must be used optimally. Theoretically, in live-donor renal transplantation, MGA carry potential risks, e.g. a prolonged warm ischaemia time, with an increased incidence of ATN, and acute rejection with concomitant prolonged hospital stay and graft dysfunction [4–6]. Contrary to the deceased donor, the use of the Carrel aortic patch allows graft harvesting with a common ostium, facilitating the arterial anastomosis with the recipient iliac vessels. However, in live-donor transplantation this technique is not applicable.
To the best of our knowledge, the present study represents the largest single-centre experience with a long-term follow-up of patients with MGA; we followed patients up to 20 years. In our study, renal transplantation with MGA had no negative effect on graft survival, in accordance with the results of others [7–11]. The mean (SD) 1-year graft survival rate in our patients with MGA was 96.1 (1.26)% and higher than the 90.9% rate reported by Kuo et al. [12]. Also, in our series the 5-year graft survival in patients with MGA was 86.6 (2.39)% similar to the 86% reported by Ghazanfar et al. [13].
A greater incidence of vascular complications in patients with MGA than in those with SGA was reported by some authors [3–5,13], but others showed no association between MGA and an increased risk of vascular complications [7–9]. In our series, the occurrence of vascular complications was significantly higher in patients with MGA (5.9% vs. 3.3%, P = 0.001). This can be explained by the fact that small-calibre arteries in MGA are vulnerable to thrombosis with any attack of hypotension, which accelerates the coagulation process. Analysis of the vascular complication components showed that a greater incidence was found only in renal artery thrombosis (P = 0.001), while there was no significant difference in renal artery stenosis (P = 0.98), renal vein thrombosis (P = 0.38) or haemorrhage (P = 0.32). Ghazanfar et al. [13] reported an increased incidence of vascular complications of 8.9% with MGA, vs. 2.8% with SGA, respectively (P < 0.05), which is a higher rate that in the present study.
Some studies showed a higher incidence of urological complications in patients with MGA [3–6]. Carter et al. [4] reported 17% urological complications in 36 patients with MGA; this was explained by the occlusion of a small lower polar artery that lead to ureteric necrosis. Similarly, a higher incidence of 60% for renal artery multiplicity in patients with ureteric complications was reported by Fuller et al. [5]. In contrast, the present patients had a comparable incidence of urological complications in the MGA and SGA groups. This can be attributed to preservation of the ‘golden triangle’ between the lower pole and the ureter during donor nephrectomy, that ensures a good ureteric blood supply. In addition, in the presence of a lower polar artery, a precise vascular anastomosis with interrupted sutures was used.
The value of administering anticoagulants after transplantation is debated, and to date there is no widely accepted consensus to support its use. Heparin was found to decrease thromboembolic complications, with no effect on lymph drainage or bleeding sequelae [14]. Two different groups of investigators showed that low molecular-weight heparin can reduce or even abolish the thrombotic sequelae without increasing postoperative surgical bleeding [15,16]; Humar et al. [17] denied the need for anticoagulation for low-risk renal transplants, and advised restricting it to a short course of heparinization only for patients at high risk. In our institute we have performed a prospective randomized study including 75 patients, randomized to one of three arms; one group received no anticoagulants, one received conventional unfractionated heparin, and the third received low molecular weight heparin in low-risk renal transplants. We concluded that postoperative heparin administration in low-risk live-donor renal transplantation is associated with a significant decrease in haemoglobin level, as well as prolonged and excessive lymph drainage, with no improvement in graft outcome, and should not be routine in low-risk live-donor renal transplantation [18]. An extensive review of previous reports failed to find one study discussing the role of heparin administration in renal transplants with MGA. Prospective randomized trials are needed to assess the value of postoperative heparinization in patients with MGA in live-donor renal transplants.
The high rejection rate in our series might be attributed to a strict follow-up of all our patients, with a meticulous registration of all patient data in regular outpatient clinical visits. Acute rejection episodes were carefully diagnosed by biopsy and the appropriate management was applied.
In conclusion, in a large cohort of patients in one centre, with a long-term follow-up, grafts with MGA were associated with a higher incidence of a prolonged ischaemia time and ATN. Despite a greater incidence of vascular thrombosis in the MGA than the SGA groups, graft and patient survival rates were comparable and there was no significant effect on urological complications.
References
- 1.Novick A.C., Magnusson M., Braun W.E. Multiple-artery renal transplantation, emphasis on extracorporal methods of arterial reconstruction. J Urol. 1979;122:731–735. doi: 10.1016/s0022-5347(17)56578-7. [DOI] [PubMed] [Google Scholar]
- 2.Ali-El-Dein B., Osman Y., Shokeir A.A., Shehab El-Dein A.B., Sheashaa H., Ghoneim M.A. Multiple arteries in live donor renal transplantation: surgical aspects and outcomes. J Urol. 2003;169:2013–2017. doi: 10.1097/01.ju.0000067637.83503.3e. [DOI] [PubMed] [Google Scholar]
- 3.Roza A.M., Perloff L.J., Naji A., Grossman R.A., Barker C.F. Living-related donors with bilateral multiple renal arteries: a twenty-year experience. Transplantation. 1989;47:397–399. [PubMed] [Google Scholar]
- 4.Carter J.T., Freise C.E., McTaggart R.A., Mahanty H.D., Kang S.M., Chan S.H. Laparoscopic procurement of kidneys with multiple renal arteries is associated with increased ureteral complications in the recipient. Am J Transplant. 2005;5:1312–1318. doi: 10.1111/j.1600-6143.2005.00859.x. [DOI] [PubMed] [Google Scholar]
- 5.Fuller T.F., Deger S., Büchler A., Roigas J., Schönberger B., Schnorr D. Ureteral complications in the renal transplant recipient after laparoscopic living donor nephrectomy. Eur Urol. 2006;50:535–540. doi: 10.1016/j.eururo.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 6.El-Mekresh M., Osman Y., Ali-El-Dein B., El-Diasty T., Ghoneim M.A. Urological complications after living-donor renal transplantation. BJU Int. 2001;87:295–306. [PubMed] [Google Scholar]
- 7.Ratner L.E., Hiller J., Sroka M., Weber R., Sikorsky I., Montgomery R.A. Laparoscopic live donor nephrectomy removes disincentives to live donation. Transplant Proc. 1997;29:3402–3403. doi: 10.1016/s0041-1345(97)00955-x. [DOI] [PubMed] [Google Scholar]
- 8.Benedetti E., Troppmann C., Gillingham K., Sutherland D.E., Payne W.D., Dunn D.L. Short- and long-term outcomes of kidney transplants with multiple renal arteries. Ann Surg. 1995;221:406–414. doi: 10.1097/00000658-199504000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin J.L. Microvascular reconstruction ‘bench’ surgery for donor kidneys before transplantation: techniques and results. J Urol. 1989;142:23–27. doi: 10.1016/s0022-5347(17)38652-4. [DOI] [PubMed] [Google Scholar]
- 10.Han D., Choi S., Kim S. Microsurgical reconstruction of multiple arteries in renal transplantation. Transplant Proc. 1998;30:3004–3005. doi: 10.1016/s0041-1345(98)00905-1. [DOI] [PubMed] [Google Scholar]
- 11.Guerra E.E., Didone E.C., Zanotelli M.L., Vitola S.P., Cantisani G.P., Goldani J.C. Renal transplants with multiple arteries. Transplant Proc. 1992;24:1868. [PubMed] [Google Scholar]
- 12.Kuo P.C., Plotkin J.S., Stevens S.A., Cribbs A., Johnson L.B. Outcome of laparoscopic donor nephrectomy in obese patients. Transplantation. 2000;69:180–182. doi: 10.1097/00007890-200001150-00031. [DOI] [PubMed] [Google Scholar]
- 13.Ghazanfar A., Tavakoli A., Zaki M.R., Pararajasingam R., Campbell T., Parrott N.R. The outcomes of living donor renal transplants with multiple renal arteries: a large cohort study with a mean follow-up period of 10 years. Transplant Proc. 2010;42:1654–1658. doi: 10.1016/j.transproceed.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 14.Ubhi C., Lam F., Mavor A., Giles G. Subcutaneous heparin therapy for cyclosporine-immunosuppressed renal allograft recipients. Transplantation. 1989;48:886–887. doi: 10.1097/00007890-198911000-00038. [DOI] [PubMed] [Google Scholar]
- 15.Alkhunaizi A., Olyaei A., Barry J.M., deMattos A.M., Conlin M.J., Lemmers M.J. Efficacy and safety of low molecular weight heparin in renal transplantation. Transplantation. 1988;66:533–534. doi: 10.1097/00007890-199808270-00020. [DOI] [PubMed] [Google Scholar]
- 16.Lundin C., Bersztel A., Wahlberg J., Wadström J. Low molecular weight heparin prophylaxis increase the incidence of lymphocele after kidney transplantation. Ups J Med Sci. 2002;107:9–15. doi: 10.3109/2000-1967-137. [DOI] [PubMed] [Google Scholar]
- 17.Humar A., Johnson E.M., Gillingham K., Sutherland D.E., Payne W.D., Dunn D.L. Venous thromboembolic complications after kidney and kidney-pancreas transplantation: a multivariate analysis. Transplantation. 1998;65:229–234. doi: 10.1097/00007890-199801270-00015. [DOI] [PubMed] [Google Scholar]
- 18.Osman Y., Kamal M., Soliman S., Sheashaa H., Shokeir A., Shehab El-Dein A.B. Necessity of routine postoperative heparinization in non-risky live-donor renal transplantation: results of a prospective randomized trial. Urology. 2007;69:647–651. doi: 10.1016/j.urology.2006.12.017. [DOI] [PubMed] [Google Scholar]


