Abstract
Background
Lipomatous tumours of the adrenals are almost always benign. The importance of recognising their characteristic radiological features, leading to their correct treatment, is fundamental, as there has been an increase in the identification of these lesions. Our goal was to review all lipomatous tumours of the adrenal glands, particularly myelolipomas, their imaging methods and surgical management, updated in 2011.
Methods
This was a retrospective review of articles published in the USA and Europe, from 1979 to date. The sites from which information was retrieved covered PubMed, Medscape, Clinical Imaging, Histopathology, Urologia Internationalis, Archives of Surgery, JACS, the American Urological Association, BMJ, Medline, and Springer Link. We report areas of controversies in addition to well established guidelines.
Results
We reviewed 45 articles, that confirmed, with a high level of evidence-based medicine, that the diagnosis of a lipomatous adrenal tumour is made by various imaging procedures, particularly computed tomography (CT). We emphasise the importance to their management of the initial size of the adrenal mass, its increase in size over time, in addition to the presence of symptoms.
Conclusion
Lipomatous tumours of the adrenals are most frequently benign. The diagnosis is usually made by various techniques, in particular CT. The fundamental characteristics indicating the necessity of surgical intervention are the symptoms presented, volume of the tumoral mass (>5 cm), and the increase in size of the tumour as shown in two consecutive imaging studies.
Abbreviations: US, ultrasonography; CAH, congenital adrenal hyperplasia; HU, Hounsfield unit
Keywords: Adrenal glands, Myelolipomas, Conservative management, Laparoscopic adrenalectomy
Introduction
Lipomatous adrenal tumours are hormonally inactive lesions, often benign, such as myelolipomas, lipomas, angiomyolipomas, or mature teratomas, and are rarely malignant, such as liposarcomas. The importance of recognising their characteristic radiological features, leading to their correct treatment, is primary, as there has been an increase in the identification of this lesion, often detected incidentally (incidentaloma) [1,2]), as recently emphasised by Song et al. in 2011 [3].
Primary adrenal tumours encountered in clinical practice are often functioning tumours, such as adrenal cortical adenomas or phaeochromocytomas [4]. However, the importance of asymptomatic adrenal masses discovered incidentally during the investigation of unrelated problems was studied by Geelhoed and Druy in 1982 [5]. With the increased availability of high resolution ultrasonography (US), CT and MRI, incidental adrenal lesions are increasingly reported. This leads to issues in the evaluation and management of these lesions [6]. Adrenal tumours of uncommon pathology were more frequently reported. By detecting more adrenal tumours with the newest radiological techniques, previous reports seem to have limitations and shortcomings for the selection criteria related to the surgical management of these tumours, with areas of controversy leading to different management when several factors play their role, like the size, the aggressiveness, and the natural evolution of these lipomatous tumours.
Therefore, the objective of the present review was to detail all types of lipomatous adrenal tumours, emphasising myelolipomas, reviewing their incidence, aetiology, pathology, and best diagnostic methods, to finally clarify their best therapies, as updated to 2011.
The various imaging procedures, although not easily providing a histological diagnosis, permit an assessment of the heterogeneity of the tumoral mass and its development. These factors are of significant importance in planning, for interpretation, and the correct surgical procedure. For treatment, the surgical removal of any lesion of >5 cm is mandatory [7–9].
Methods
Between November 1979 and January 2011, the information assessed included a thorough review of previous reports, including 45 articles published in the USA, Europe and Asia, on adrenal tumours, and especially lipomatous ones. We provide the most up-to-date information, areas of controversy, in addition to well established guidelines.
The information covered was retrieved from the Annals of Internal Medicine, PubMed, Medscape, Clinical Imaging, Histopathology, Urologia Internationalis, Archives of Surgery, JACS, AUA and Journal of Urology Website, the BMJ, Scandinavian Journal of Urology and Nephrology, Medline, and Springer Link.
In an initial approach, we reviewed all types of lipomatous adrenal tumours, with the emphasis on myelolipomas, their incidence, aetiology, pathology, diagnostic methods and finally the best therapies.
Results
Myelolipomas
Incidence
Lipomatous tumours in adrenal glands are uncommon, with an incidence of 4–5% [7] in primary tumours involving the adrenal. Apart from myelolipoma, rare tumours such as lipoma, teratoma, angiomyolipoma, and liposarcoma are also found.
Myelolipoma is the most common fatty tumour of the adrenal gland [10]. Gierke first described this disease in 1905, and the term myelolipoma was mentioned by Oberling in 1929. In a 1973 autopsy series, it was estimated that the prevalence at autopsy was 0.08–0.4%.
Aetiology and prognosis
In the very large series of Lam and Lo [7], in 2001, it was noted that myelolipomas were often diagnosed as either incidental radiological or postmortem findings [11]. The tumours were usually noted in late adult life (age range, 41–84 years; mean, 62). They were equally distributed on each side. Adrenal myelolipoma has been reported in the fifth to seventh decade of life, with no gender predilection, and the right adrenal is more commonly involved than the left, as reported by Nerli et al. and other series [12]. A recent study by Ramchandani and Lin [13] in 2009 confirmed that most tumours are unilateral but showed no predilection to one particular side, and that seems to be an area of controversy, including an update in May 2011. There is no sex difference reported previously, although a male predominance was noted in some series.
The aetiology of adrenal myelolipomas remains unclear; hypotheses about the origin include metaplasia of adrenal cortical cells precipitated by chronic stress or degeneration, remains of adrenal cortex or extra-medullary haematopoiesis in pathological situations [14,15]. Fewer than 300 cases were reported up to 2000. Nevertheless, their prevalence seems to be increasing, up to 10%, due to novel and enhanced imaging techniques.
Chronic adrenal stimulation and stress [16,17], as shown by the high incidence of the disease in the elderly [18], could generate the development of benign or malignant tumours. Cushing’s disease, hypertension, diabetes and obesity are often related to adrenal myelolipomas and could be characterised as major adrenal stimuli. We also speculate that the contemporary stressful lifestyle and unbalanced diet could be implicated to the pathogenesis of this tumour.
The prognosis is related to the few complications, such as rupture and retroperitoneal haemorrhage, that can occur in large myelolipomas [16].
Pathology
Gross appearance
Myelolipomas are usually <4 cm in diameter (Fig. 1), but they can attain very large sizes (>10 cm), and they can involve both adrenals [9,16,19]. To date, the largest adrenal myelolipomas reported weighed 5900 g [20], and 6 kg [21] Extra-adrenal sites for myelolipomas include the retroperitoneum, thorax and pelvis [22].
Figure 1.
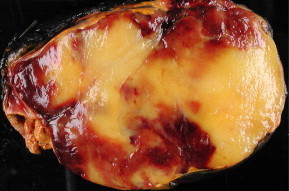
Gross appearance of an adrenal myelolipoma.
Microscopic examination
Adrenal myelolipoma is composed of mature adipose tissue and haematopoietic elements, and is surrounded by a pseudocapsule [12]. The tumour is thought to arise from metaplasia of undifferentiated stromal cells [23]. Reports on large series of patients with adrenal myelolipomas are lacking. In English language reports, Han et al. [17] and Kenney et al. [12] reviewed 20 and 46 patients with myelolipomas, respectively, based on radiological features, with no complete pathological examination in most patients.
On microscopic examination of 11 myelolipomas, the incidence of a predominance of either adipose tissue or the myeloid component was similar. The incidence of calcification (27%) suggested that it is a common feature in adrenal myelolipomas and that it might be a feature of benign adrenal lipomatous tumours on imaging. The presence of mature adipose tissue intermixed with haematopoietic elements (Fig. 2), including megakaryocytes on cytology or histology, is diagnostic of myelolipoma. Therefore, HMB 45 staining should always be used, as recommended in other studies.
Figure 2.
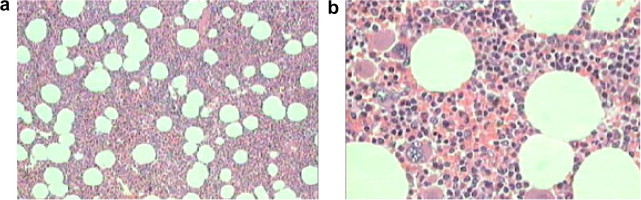
Histological criteria of myelolipoma. (a) Low-power micrograph showing a mixture of adipose tissue and bone marrow elements (×50). (b) High-power micrograph showing fat cells and all three lineages of haematopoietic marrow (×400).
Clinical presentation
Adrenal myelolipomas present as nonspecific back pain or incidental findings by imaging and necropsy. They are hormonally inactive although they might sometimes coexist with primary aldosteronism, congenital adrenal hyperplasia (CAH), phaeochromocytoma, adenoma and Cushing’s syndrome [21,24]. Most of these patients are asymptomatic, and occasionally can present with abdominal pain due to either the tumour being large, or from spontaneous haemorrhage, more likely when predominantly composed of myeloid tissue.
Awareness of this rare entity is essential to avoid extensive surgery. There is a frequent association with obesity and type 2 diabetes mellitus and hypertension, possibly coincidental [11].
Adrenal myelolipoma has been reported to coexist in association with CAH due to 21-hydroxylase or 17-hydroxylase deficiency. It is believed that excess adrenocorticotropic hormone secretion over a long period can stimulate myelolipomatous alterations in adrenal glands [24].
Diagnostic methods
US (Fig. 3) is a satisfactory method for detecting adrenal lesions, in particular if located in the right side where the adrenal gland is better visualised. The findings are of a mixed structure; intermediary because of the tissue components and typically hyperechogenic due to fat [25]. The fat appears definitely hyperechogenic in angiomyolipomas, but it is frequently minimally echogenic for the adjacent adipose cellular tissue in the other histological types.
Figure 3.
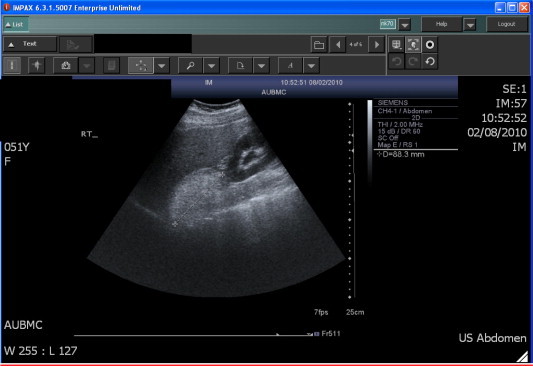
US image showing a right 8.8 cm adrenal myelolipoma in a 51-year-old woman.
CT (Figs. 4 and 5) is the procedure of choice [19,26,27], showing the negative density of the adipose tissue in the adrenal mass, and often allowing the precise location of the adrenal by multiplanar reconstructions. However, the enhancement of the soft-tissue elements is not specific, raising the problem of a precise characterisation of the lesion.
Figure 4.
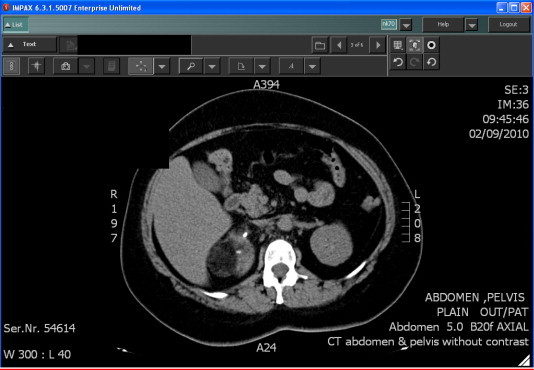
Unenhanced CT image showing the typical low density and negative attenuation value (–19 HU) of the adipose tissue in a right adrenal mass.
Figure 5.
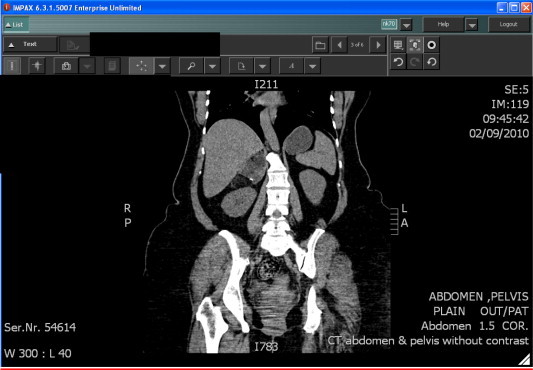
Coronal reformatted image of unenhanced CT scan showing the typical low density and negative attenuation (–19 HU) of a right adrenal mass with hyperdense foci of calcification.
Usually, adrenal myelolipomas are detected by CT, and it is the preferred primary method for evaluating the abdomen if there is a suspicious mass or lesion, because it is fast, readily available, and provides spatial resolution as well as differentiates tissues by different Hounsfield unit (HU) values. Most adrenal myelolipoma is composed of fat tissue, which has a negative HU value. The CT values are usually higher than those of retroperitoneal fat (typically measuring <−20 HU) because of an associated haematopoietic tissue component in adrenal myelolipomas. The interspersed haematopoietic element can also be enhanced by contrast medium, leading to a heterogeneous appearance. Sometimes there are high attenuation regions, probably resulting from haemorrhage or calcifications.
MRI is also very precise in detecting fat in a lesion, thanks to the T1-weighted sequences without fat suppression. In-phase and out-of-phase are also very contributory, but MRI is not more helpful than CT for characterising precisely the tissue component (haematopoietic elements, malignant tissue).
On MRI, adrenal myelolipoma shows a high signal intensity on T1-weighted images because of plentiful adipose tissue. An intermediate signal intensity on T2-weighted images can appear due to mixed fat and marrow tissues. This appearance is nonspecific and can be confused with adrenal metastases or primary adrenal cancers. By using fat-suppression MRI techniques the diagnosis can be confirmed by showing a loss of signal intensity within the fatty component. Fat-containing malignancies of the adrenal gland are exceedingly rare; thus, adrenal malignancies would not be expected to lose signal on fat-suppressed MRI. MRI shows signals indicating a lipomatous mass and soft-tissue components.
Positron-emission tomography is in theory very specific, differentiating between a benign and a malignant lesion, the latter being hypermetabolic. However, in the case of a myelolipoma, the haematopoietic elements can produce an 18fluorodeoxyglucose uptake when the lesion is benign [28]. For this reason this test should be considered of secondary importance.
The various imaging procedures permit the correct localisation of the adrenal lesion except in the case of a very large mass. Generally, they determine the adipose components of the tumour but do not precisely define its histological type [7], and often even differentiate between a benign and a malignant process [2,28]. If a definite diagnosis is needed, a fine-needle biopsy is indicated, either under US [29] or CT guidance [8,26,30], although one should consider the possibility of a ruptured mass or the development of a haemorrhage.
Lipomas
Lipoma of the adrenal is rare; it is composed only of mature adipose tissue, at times focal calcifications can develop (Fig. 6), and usually it is small. In recent years large adrenal lipomas have been reported. Sharma et al. [31] reported a symptomatic adrenal lipoma of 12 cm, weighing 225 g.
Figure 6.
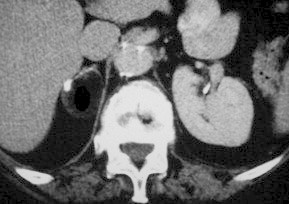
Adrenal lipoma: unenhanced CT scan showing a homogeneous, negatively dense mass with calcific deposits in the periphery.
The pathogenesis of adrenal lipoma remains unknown, although adrenal myelolipoma and lipoma were believed to be the same tumour entity [31]. Adrenal lipomas also seem to have a right-sided predominance (75%). The right side predominance of adrenal lipomas is unlikely to be the result of chance alone, and might be a clue to the origin of this tumour. More cases need to be studied.
Mature teratomas
Mature teratoma is composed of mature germinal tissue, of fat tissue, and at times calcification/bone can develop. Its size, when discovered, is sometimes significant.
Angiomyolipomas
Angiomyolipoma is a benign mesenchymal tumour originating from epithelioid perivascular cells, containing adipose tissue. It more frequently involves the kidney. The involvement of the adrenal is sporadic and most of the time involves both adrenals, and is associated with other locations, such as in the kidneys, liver, spleen and lungs, in the setting of tuberous sclerosis [32]. When the volume of the tumoral mass is significant, there is the risk of a spontaneous rupture due to abnormal elastin [7]. Some of these tumours are potentially aggressive, such as the case of the atypical or epithelioid angiomyolipoma [33], composed mainly of smooth muscular cells.
Liposarcomas
Liposarcoma involves mainly young adults aged 30–50 years. This tumour is suspected when there is a large tumoral mass composed of adipose tissue. These adipose tumours are very often asymptomatic and discovered incidentally during imaging for different pathologies. The clinical findings that might be present are a palpable mass and a flank or dorsal pain caused by the size of the tumour, by tumoral necrosis, or by spontaneous retroperitoneal haemorrhage [8]. They are hormonally inactive, but they can be associated with hormonally active lesions, such as adrenal endocrine abnormalities [19,34]. In these cases the tumour is small and frequently calcified [12].
Discussion
Imaging methods for lipomatous adrenal tumours
Detecting hypodensity within an adrenal mass is virtually diagnostic of myelolipoma on CT. Desai et al. [15] felt that biochemically a nonfunctioning radiolucent solid adrenal mass on CT, with no neovascularity on angiography, is most likely a myelolipoma. MRI is required to show the origin of the tumour, to define tissue planes when the tumour is large and heterogeneous, and to distinguish benign from malignant lesions by comparing signal-intensity ratios of adrenal to liver [24]. We found that there was no advantage in a confirmatory MRI at our institution, as US and CT were very specific most of the time.
As myelolipomas contain different proportions of fat and myeloid tissue, a definitive diagnosis using CT or MRI can be difficult, although rarely so, if only a small amount of fat is present.
To distinguish the mass from a well-differentiated liposarcoma, percutaneous fine-needle aspiration by US or CT guidance can confirm the diagnosis. Percutaneous fine-needle aspiration was not needed in our patients to diagnose an adrenal myelolipoma.
Yip et al. [35], in a study of 196 adrenalectomies in 192 patients from 2000 to 2008, found that CT or MRI characteristics predicted the presence of benign lesions with 100% specificity. Histopathology confirmed that all 66 adrenal masses with imaging characteristics suggesting benign adenoma were indeed benign lesions, and these included 61 benign adrenal adenomas and five benign non-adenomatous lesions (three myelolipomas, one composite myelolipoma/adenoma, and one ganglioliponeuroma). The authors stated that to exclude malignancy, adrenal masses with non-benign imaging characteristics should be resected. Lamas et al. [36] reported on two cases of large mixed adrenal tumours that had a heterogeneous appearance and areas of fat density on imaging, and that resulted in autonomous cortisol production leading to Cushing syndrome. The patients underwent adrenalectomy, and histology identified adrenocortical adenomas with widespread myelolipomatous metaplasia. The authors noted that although adrenal myelolipomas are usually asymptomatic, non-functioning adrenal incidentalomas, there have been a few reports of myelolipomatous masses that are associated with adrenocortical hypersecretion. Therefore, we would recommend always assessing the cortisol level before surgery of an adrenal myelolipoma, based on these findings, and on recent reports [34]. Since the reported recent increase in the incidence of adrenal myelolipomas, should it be attributed to the increase in incidentalomas and new intensive imaging techniques? Or is it due to the increased daily stress? Further prospective studies should be addressed in patients with Addison’s disease to postulate if their risk of myelolipoma is lower.
Concerning the reported male predisposition, should we recommend genetic mapping to elucidate any gender predisposition? Would there be any reason for a right-sided predominance? Further studies are needed to elucidate these issues.
Montone et al. [37] presented the pathological and radiographic features of three patients with adrenocortical neoplasms. Two patients had imaging findings that were compatible with adrenal myelolipoma. Pathologically, two of the lesions were classified as adrenocortical neoplasms of uncertain malignant potential, and one lesion was classified as an adrenocortical adenoma. All three lesions contained myelolipomatous foci throughout the neoplasm, and two of the tumours contained several pure lipomatous foci. The authors noted that imaging studies can result in a false diagnosis of a benign adrenal myelolipoma and, as a result, under-treatment.
There are sometimes limitations to these techniques, when myelolipomas enlarge enough to make the organ of origin difficult to discern on CT, resulting in a differential diagnosis that includes renal angiomyolipoma and retroperitoneal liposarcoma or lipoma. In these patients, the multiplanar capability of MRI can help to define the tissue planes and confirm that the mass is adrenal in origin. Our recommendation is to proceed with MRI when there is a large adrenal mass (>10 cm).
Management of lipomatous adrenal tumours
The management of all lipomatous tumours of the adrenals, including myelolipomas, can be either conservative or surgical. For symptomatic tumours there is no doubt; the treatment is always surgical. However, for asymptomatic ones there are divergent opinions.
Numerous authors recommend surgery for tumoral masses of >4–5 cm [2,27,29,38–40]. The National Institutes of Health state-of-the science conference [1] recommends surgical excision for lesions >6 cm, a regular follow-up for lesions of <4 cm, and a decision based on a case-by-case analysis for the tumoral masses of 4–6 cm.
Despite that, Meyer and Behrend [26] state that there is no indication for surgery of lesions of <10 cm, because up to 10 cm there is no significant correlation between the size of the tumour and its symptoms. Then, for this category of patients who had no surgery, they should have a regular follow-up by CT and/or MRI. If a change in the size of the lesion is detected, then surgery is indicated. According to the National Institutes of Health [1], the surveillance could be stopped if there is no evidence of an increase in the size of the tumour on two consecutive images at 6-month intervals, and there is no evidence of abnormal hormonal secretion.
For patients who have surgical excision of the tumour, regular surveillance should be maintained because of the risk of a contralateral lesion [26].
Reviewing previous reports, most myelolipomas are solitary when the size at presentation is usually <5 cm and surgery is not needed. According to the PubMed database, there are only seven case reports worldwide of bilateral giant adrenal myelolipomas [19], showing the rarity of this entity.
For surgical procedures, laparoscopy is the standard [4,5]; it minimises the possibility of blood loss and of postoperative complications, also resulting in a shorter hospital stay and convalescence time [4,38].
Open adrenalectomy should be performed in the case of voluminous tumours (>10 cm) or malignant tumours requiring a wide resection with satisfactory margins, to eliminate future carcinogenic development.
Conclusion
Lipomatous tumours of the adrenals are most frequently benign; uncommon are the malignant types and those that are potentially aggressive, making the question of their treatment a fundamental issue. The diagnosis of a lipomatous adrenal tumour is usually made by various imaging procedures, in particular CT. Nonetheless, the preoperative characteristic of the lesion is not easily distinguished by imaging. The three fundamental characteristics indicating the need for surgical intervention are the symptoms at presentation, the volume of the tumoral mass (>5 cm), and the increase in the volume of the tumour shown on two consecutive imaging studies.
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1.Grumbach M.M., Biller B.M., Braunstein G.D., Campbell K.K., Carney J.A., Godley P.A. Management of clinically inapparent adrenal mass (‘incidentaloma’) Ann Intern Med. 2003;138:424–429. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 2.Bellantone R., Ferrante A., Raffaelli M., Boscherini M., Rubino F., Crucciti F. Incidental discovery of adrenal neoplasm: our experience. Ann Ital Chir. 1995;66:439–448. [PubMed] [Google Scholar]
- 3.Song J.H., Mayo-Smith W.W. Incidentally discovered adrenal mass. Radiol Clin N Am. 2011;49:361–368. doi: 10.1016/j.rcl.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Lam K.Y. Adrenal tumours in Chinese. Virchows Arch a Pathol Anat Histopathol. 1992;421:13–16. doi: 10.1007/BF01607133. [DOI] [PubMed] [Google Scholar]
- 5.Geelhoed G.W., Druy E.M. Management of the adrenal ‘incidentaloma’. Surgery. 1982;92:866–874. [PubMed] [Google Scholar]
- 6.Gajraj H., Young A.E. Adrenal incidentaloma. Br J Surg. 1993;80:422–426. doi: 10.1002/bjs.1800800405. [DOI] [PubMed] [Google Scholar]
- 7.Lam K.Y., Lo C.Y. Adrenal lipomatous tumours: a 30 year clinicopathological experience at a single institution. J Clin Pathol. 2001;54:707–712. doi: 10.1136/jcp.54.9.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel V.G., Babalola O.A., Fortson J.K., Weaver W.L. Adrenal myelolipoma: report of a case and review of the literature. Am Surg. 2006;72:649–654. [PubMed] [Google Scholar]
- 9.Cristofaro M.G., Lazzaro F., Fava M.G., Aversa C., Musella M. Giant adrenal myelolipoma: a case report and review of the literature. Ann Ital Chir. 2004;75:677–681. [PubMed] [Google Scholar]
- 10.Kasperlik Zeluska A.A., Roslonowska E., Slowinska Srzednicka J., Migdalska B., Jeske W., Makowska A. Incidentally discovered adrenal mass (incidentaloma). Investigation and management of 208 patients. Clin Endocrinol. 1997;46:27–29. doi: 10.1046/j.1365-2265.1997.d01-1751.x. [DOI] [PubMed] [Google Scholar]
- 11.Bayram F., Atasoy K.C., Yalcin B., Salih M., Hekimoglu K., Uysal A.R. Adrenal myelolipoma: case report with a review of the literature. Australas Radiol. 1996;40:68–71. doi: 10.1111/j.1440-1673.1996.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 12.Kenney P.J., Wagner B.J., Rao P., Heffes C.S. Myelolipoma. CT and pathologic features. Radiology. 1998;208:87–95. doi: 10.1148/radiology.208.1.9646797. [DOI] [PubMed] [Google Scholar]
- 13.Ramchandani P, Lin E. Emedicine at Medscape <http://www.emedicine.medscape.com/article/376700-overview#showall>.
- 14.Rao P., Kenney P.J., Wagner B.J., Davidson A.J. Imaging and pathologic features of myelolipoma. Radiographics. 1997;17:1373–1385. doi: 10.1148/radiographics.17.6.9397452. [DOI] [PubMed] [Google Scholar]
- 15.Desai S.B., Dourmashkin L., Kabakow B.R., Leiter E. Myelolipoma of the adrenal gland: case report, literature review and analysis of diagnostic features. Mt Sinai J Med. 1979;46:155–159. [PubMed] [Google Scholar]
- 16.Vierna J., Laforga J.B. Giant adrenal myelolipoma. Report of a case and review of the literature. Scand J Urol Nephrol. 1994;28:301–304. [PubMed] [Google Scholar]
- 17.Han M., Burnett A.L., Fishman E.K., Marshall F.F. The natural history and treatment of adrenal myelolipoma. J Urol. 1997;157:1213–1216. [PubMed] [Google Scholar]
- 18.Yildiz L., Akpolat I., Erzurumlu K., Aydin O., Kandemir B. Giant adrenal myelolipoma: case report and review of the literature. Pathol Int. 2000;50:502–504. doi: 10.1046/j.1440-1827.2000.01071.x. [DOI] [PubMed] [Google Scholar]
- 19.Wrightson W.R., Hahm T.X., Hutchinson J.R., Cheadle W. Bilateral giant adrenal myelolipomas: a case report. Am Surg. 2002;68:588–589. [PubMed] [Google Scholar]
- 20.Boudreaux D., Waisman J., Skinner D.G., Low R. Giant adrenal myelolipoma and testicular interstitial cell tumor in a man with congenital 21-hydroxylase deficiency. Am J Surg Pathol. 1979;3:109–123. doi: 10.1097/00000478-197904000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Bhansali A., Dash R. Adrenal myelolipoma. Profile of six patients with a brief review of literature. Int J Endo Metab. 2003;1 [Google Scholar]
- 22.Chakraborty J., Paul P.C., Gumta M.K., Ghosh G., Goswami B. Adrenal myelolipoma - report of a case. J Indian Med Assoc. 2006;104:148–149. [PubMed] [Google Scholar]
- 23.Casey L.R., Cohen A.J., Wile A.G., Dietrich R.B. Giant adrenal myelolipomas. CT and MRI findings. Abdom Imaging. 1994;19:165–167. doi: 10.1007/BF00203496. [DOI] [PubMed] [Google Scholar]
- 24.Olsson C.A., Crane R.J., Klugo F.C., Selikowitz S.M. Adrenal myelolipoma. Surgery. 1973;73:665–670. [PubMed] [Google Scholar]
- 25.Scheible W., Ellenbogen P.H., Leopold G.R., Siao N.T. Lipomatous tumors of the kidney and adrenal: apparent echographic specificity. Radiology. 1978;129:163–166. doi: 10.1148/129.1.153. [DOI] [PubMed] [Google Scholar]
- 26.Meyer A., Behrend M. Presentation and therapy of myelolipoma. Int J Urol. 2005;12:239–243. doi: 10.1111/j.1442-2042.2005.01034.x. [DOI] [PubMed] [Google Scholar]
- 27.Haab F., Ponsot Y., Rolloy P., Fouret P., Gattegno B., Thibault P. Adrenal myelolipoma. Review of the literature and case report. Prog Urol. 1991;1:313–318. [PubMed] [Google Scholar]
- 28.Ludwig V., Rice M.H., Martin W.H., Kelley M.C., Delbeke D. 2-Deoxy-2 [18F] fluoro-d-glucose positron emission tomography uptake in a giant adrenal myelolipoma. Mol Imaging Biol. 2002;4:355–358. doi: 10.1016/s1536-1632(02)00018-5. [DOI] [PubMed] [Google Scholar]
- 29.Porcaro A.B., Novella G., Ficarra V., Cavalleri S., Antonrolli S.Z., Curti P. Incidentally discovered adrenal myelolipoma Report on 3 operated patients and update of the literature. Arch Ital Urol Androl. 2002;74:146–151. [PubMed] [Google Scholar]
- 30.Robbani I., Shah I., Shah O.J. Diagnosis of adrenal myelolipoma by imaging and guided biopsy. Ceylon Med J. 2003;48:24–25. doi: 10.4038/cmj.v48i1.3391. [DOI] [PubMed] [Google Scholar]
- 31.Sharma M.C., Gill S.S., Kashyap S., Nabi G., Mishra M.C. Adrenal lipoma: a case report. Urol Int. 1998;60:245–247. doi: 10.1159/000030265. [DOI] [PubMed] [Google Scholar]
- 32.Elsayes K.M., Narra V.R., Lewis J.S., Brown J.J. Magnetic resonance imaging of adrenal angiomyolipoma. J Comput Assist Tomogr. 2005;29:80–82. doi: 10.1097/01.rct.0000152863.97865.47. [DOI] [PubMed] [Google Scholar]
- 33.Nonomura A., Minato H., Kurumaya H. Angiomyolipoma predominantly composed of smooth muscle cells: problems in histological diagnosis. Histopathology. 1998;33:20–27. doi: 10.1046/j.1365-2559.1998.00426.x. [DOI] [PubMed] [Google Scholar]
- 34.Htsamatsu H., Sakai H., Tsuda S., Shigematsu I.C., Kanetake H. Combined adrenal adenoma and myelolipoma in a patient with Cushing’s syndrome: case report and review of the literature. Int J Urol. 2004;11:416–418. doi: 10.1111/j.1442-2042.2004.00815.x. [DOI] [PubMed] [Google Scholar]
- 35.Yip L., Tublin M.E., Falcone J.A., Nordman C.R., Stang M.T., Ogilvie J.B. The adrenal mass. Correlation of histopathology with imaging. Ann Surg Oncol. 2010;17:846–852. doi: 10.1245/s10434-009-0829-2. [DOI] [PubMed] [Google Scholar]
- 36.Lamas C., López L.M., Lozano E., Atienzar M., Ruiz-Mondéjar R. Myelolipomatous adrenal masses causing Cushing’s syndrome. Exp Clin Endocrinol Diabetes. 2009;117:440–445. doi: 10.1055/s-0029-1202274. [DOI] [PubMed] [Google Scholar]
- 37.Montone K.T., Rosen M., Siegelman E.S., Fogt F., Livolsi V.A. Adrenocortical neoplasms with myelolipomatous and lipomatous metaplasia: report of 3 cases. Endocr Ract. 2009;15:128. doi: 10.4158/EP.15.2.128. [DOI] [PubMed] [Google Scholar]
- 38.Ramacciato G., Paolo M., Pietromaria A., Paolo B., Francesco D., Sergio P. Ten years of laparoscopic adrenalectomy: lesson learned from 104 procedures. Am Surg. 2005;71:321–325. [PubMed] [Google Scholar]
- 39.Ares Valdes Y. Adrenal mye’lolipome Case report and bibliographic review. Arch Esp Urol. 2006;59:71–73. doi: 10.4321/s0004-06142006000100010. [DOI] [PubMed] [Google Scholar]
- 40.Sa Y.L., Xu Y.M., Qiao Y., Jin G.R., Si J.M. Adrenal myelolipoma. Clinical diagnosis and management of 26 cases. Zhonghua Wai Ke Za Zhi. 2004;42:1444–1446. [PubMed] [Google Scholar]


