Abstract
Objective
Despite many efforts to prevent ureteric stenosis in a transplanted kidney, this complication occurs in 3–5% of renal transplant recipients. Balloon dilatation (BD) is a possible minimally invasive approach for treatment, but reports to date refer only to the antegrade approach; we analysed our experience with retrograde BD (RBD) and reviewed previous reports.
Patients and methods
From October 2008 to February 2011, eight patients after renal transplantation (RTX) underwent RBD for transplant ureteric stenosis at our hospital. We retrospectively analysed the outcome and reviewed previous reports.
Results
The eight recipients (five men and three women; median age 55 years, range 38–69) were treated with one or two RBDs for transplant ureteric stenosis. There were no complications. The median (range) time after RTX was 4.5 (2.5–11) months. Long-term success was only achieved in one recipient, while five patients were re-operated on (three with a new implant, two by replacement of transplanted ureter with ileum) after a median (range) of 2.8 (0.7–7.0) months after unsuccessful RBD(s). For two recipients the success remained unclear (one graft loss due to other reasons, one result pending). When the first RBD was unsuccessful there was no improvement with a second.
Conclusion
RBD is technically feasible, but our findings and the review of previous reports on antegrade ureteric dilatation suggest that the success rate is low when the ureter is dilated at ⩾10 weeks after RTX. From our results we cannot recommend RBD for transplant ureteric stenosis at ⩾10 weeks after RTX, while previous reports show favourable results of antegrade BD in the initial 3 months after RTX.
Abbreviations: RTX, renal transplantation; (R)BD, (retrograde) balloon dilatation; PNS, percutaneous nephrostomy
Keywords: Renal transplantation, Ureteric stricture, Balloon dilatation
Introduction
The transplanted ureter is highly susceptible to a compromised blood supply, as after explantation the blood supply to the ureter is exclusively from the renal pelvic arteries, which run in the peri-ureteric adventitia and might be injured during explantation. Therefore, ureteric problems outnumber all other surgical complications that occur after renal transplantation (RTX), e.g. lymphocele formation, bleeding, or transplant vessel thrombosis. Stenosis and leakage of the ureter occur in about 5% of cases, while ureteric necrosis is a rare event (0.5%) [1–3].
Ureteric complications mostly appear in the initial 3 months after RTX. If detected in time and treated adequately, they do not impair graft or patient survival [3–5]. There are many options for treating transplant ureteric stenosis. Minimally invasive methods include insertion of a ureteric stent (JJ or J) and percutaneous nephrostomy (PNS), which are usually used to overcome acute renal insufficiency rather than providing long-term relief. Also, minimally invasive antegrade balloon dilatation (BD) has been described as a promising alternative to open re-operation of a stenosed renal transplant ureter [6–15].
The RTX programme at the University Hospital Duesseldorf started in 1968, and is one of the largest programmes in Germany, having transplanted 2347 kidneys (2011 deceased donors/336 living donors) until the end of 2010, with >100 annual RTX over the past 5 years. During the study period, 262 RTX were performed (200 from deceased donors, 62 from living donors). Because of the intensified cooperation between surgeons and urologists in RTX at our institution since September 2008, retrograde BD (RBD), performed by staff in the Department of Urology, became part of the increased options for treating of transplant ureteric stenosis. All patients presenting with a ureteric stenosis after RTX received a first therapeutic approach by RBD. In this retrospective study we aimed to evaluate the outcome of RBD in renal transplant recipients who developed ureteric stenoses. Furthermore, we compared our findings of RBD with a review of previous reports, where only the percutaneous, antegrade approach was described, for the optimal treatment of transplant ureteric stenosis.
Patients and methods
We retrospectively analysed all renal transplant recipients who underwent RBD of a stenosed transplant ureter in our department. Due to the new implementation of this technique, interventions were between September 2008 and March 2011. The technique of RTX used at our institution is an extraperitoneal approach with end-to-side anastomosis of the renal vessels with the recipient’s external iliac vein and artery, and an extravesical ureteric anastomosis (full-thickness anastomosis of the spatulated ureter with the recipients bladder, nonantirefluxive). As a study on the effect of ureteric stent insertion vs no stenting in RTX ended in December 2009, and found a favourable effect of transplant ureteric stenting, a ureteric stent was routinely placed during RTX from 2010. Patients were followed regularly, including transplant ultrasonography done by hospital or external nephrologists. When postrenal obstruction was suspected, patients were treated in cooperation with the Department of Urology.
RBD of ureteric stenosis was done under general anaesthesia and antibiotic prophylaxis. Briefly, the transplant ureteric orifice was identified by transurethral cystoscopy. After retrograde transplant ureterography and exact localisation of the stricture site, a guidewire (ZipWire™ or Sensor™, Boston Scientific, Pierreux, France) was advanced to the transplant renal pelvis. The balloon dilation system (Cook Urological Inc., Spencer, Indiana, USA) was introduced into the ureter over a guidewire and the balloon extended with a 1:1 mixture of contrast medium/sodium chloride to the maximum diameter (6 mm/18 F) and maximum pressure (2.0 MPa) at the stricture site. Thus, the stricture was initially seen as a hourglass-like impression of the dilated balloon, which disappeared on maximum balloon expansion. Then the dilator was kept in place for 3–5 min. As we wanted to apply as little contrast medium as necessary in this retrograde approach, and as in low-volume retrograde ureterography a stenosis might be not detected after dilatation of the stricture, we slid the inflated balloon of the dilator through the rest of the transplant ureter to assure its patency over the whole length. At the end of the RBD procedure, a JJ stent (Visiostar Standard™, 7 F/22 cm, Urovision, Germany) was inserted, the cystoscope was withdrawn, and a 16 F Foley catheter was placed for 1–3 days (Fig. 1). The stent was removed with a flexible cystoscope under local anaesthesia at 14–21 days after the intervention. After this, the follow-up consisted of regular ultrasonographic assessment and creatinine assay in the Department of Nephrology. When there was an increase in creatinine level and/or dilatation of the transplant renal pelvis, the patient was re-evaluated for further intervention (stent insertion, repeated RBD, open operation).
Figure 1.
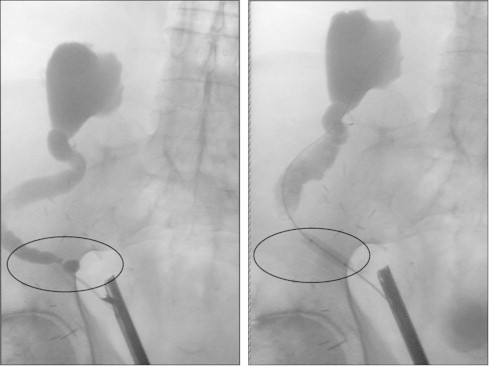
Retrograde balloon dilation. Stenosis of the distal transplant ureter of a renal transplant with 2 ureters (second ureter not opacified). The right image shows successful dilation with full expansion of the balloon.
We also searched PubMed and Medline for articles on the treatment of transplant ureteric stenosis and evaluated our findings in the light of this overview.
Results
Between September 2008 and March 2011, eight recipients (five men and three women) of renal transplants (seven first transplants, one third transplant) from one living and seven deceased donors, were treated with single or repeated RBD of the stenosed transplant ureter. The median (range) age at RTX was 55 (38–69) years. Demographic data, underlying diseases and transplant-related data are summarised in Table 1. There were no complications from the RBD.
Table 1.
Summary of demographic data, underlying diseases, transplant-related data, and complication management. RTX: renal transplantation; ADPKD: autosomal polycystic kidney disease; GN: glomerulonephritis; d = post transplant day
| Patient | Sex | Age at RTX (years) | Underlying disease | Number of RTX | Living (LD)/deceased donor (DD) | Stent during RTX | Stricture site | Number of dilations | Success | Problem (P)/definitive management (DM) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | GT | m | 43 | ADPKD | 1 | LD | No | Distal ureteral/neo-ostium | 1 | Yes |
P: transplant ureter necrosis ureteroureterostomy d4 DM: dilation |
| 2 | ZW | m | 38 | Fam. cystic kidney disease | 1 | DD | No | Distal ureteral/neo-ostium | 2a | No |
P: 2 transplant ureters implanted separately DM: neoimplantation of both ureters |
| 3 | AS | m | 75 | Nephrosclerosis | 1 | DD | Yes | Distal ureteral/neo-ostium | 2 | ? |
P: poor graft function, recurrent CMV-pneumonia/sepsis DM: transplant nephrectomy |
| 4 | HW | f | 66 | ? | 3 | DD | Yes | Prox. ureteral | 1 | No | DM: ileal interposition |
| 5 | PH | m | 44 | Mesangioprolif. GN | 1 | DD | Yes | Middle 1/3 | 1 | No | DM: neoimplantation of transplant ureter |
| 6 | GA | f | 44 | ? | 1 | DD | Yes | Distal ureteral/neo-ostium | 1 | No | DM: replacement of ureter with ileum |
| 7 | CW | f | 68 | Nephrocalcinosis | 1 | DD | Yes | Distal ureteral/neo-ostium | 2 | No | DM: neoimplantation of Transplant ureter |
| 8 | KH | m | 69 | ? | 1 | DD | Yes | Distal ureteral/neo-ostium | 2 | Pending |
P: incomplete duplex ureter leakage DM: neoimplantation of 1 of the 2 ureters d 42, sec. ureter could be left as implanted |
Only one of the two ureters could be dilated in the first attempt, the second ureter was dilated 10 days later. DM, definitive management; ADPKD, autosomal polycystic kidney disease; CKD, cyctic kidney disease; GN, glomerulonephritis; LD, living donor; DD, deceased donor; DU, distal ureteric; neo-o, neo-ostium; mesangiop GN, mesangioproliferative glomerulonephritis; CMV, cytomegalovirus.
Half of the recipients were treated with one RBD and the other half with two, for six distal, one middle and one proximal ureteric stenosis. The median (range) time from RTX to first RBD was 4.5 (2.5–11.4) months, and the median time from unsuccessful first RBD to final operative management was 2.8 (0.7–7.0) months, with a median follow-up after definitive management of 4.1 (1.9–6.6) months. The median (range) follow-up after RTX was 11.4 (7.3–25) months (Table 2).
Table 2.
Time until first dilation, definitive management, and follow-up after RTX. ∗ = case with success of first dilation and pending case excluded.
| Patient initials | Interval between RTX and 1st dilation (months) | Interval between RTX and definitive management (months) | Interval between 1st dilatation and definitive management (months) | Follow-up after RTX (months) | Follow-up after 1st dilation (months) | Interval between definitive management and last follow-up | |
|---|---|---|---|---|---|---|---|
| 1 | GT | 5.1 | Success | Success | 21.3 | 16.2 | Success |
| 2 | ZW | 11.4 | 18.4 | 7.0 | 25.0 | 13.6 | 6.6 |
| 3 | AS | 5.2 | 8.2 | 3.0 | 9.6 | 4.4 | 1.4 |
| 4 | HW | 3.0 | 3.7 | 0.7 | 11.4 | 8.4 | 7.7 |
| 5 | PH | 4.0 | 6.5 | 2.5 | 12.5 | 8.5 | 6.0 |
| 6 | GA | 4.5 | 7.9 | 3.4 | 9.8 | 5.3 | 1.9 |
| 7 | CW | 2.5 | 5.2 | 2.7 | 7.3 | 4.8 | 2.1 |
| 8 | KH | 8.2 | Pending | Pending | 9.4 | 1.2 | Pending |
| Median time (months) | 4.5∗ | 7.2∗ | 2.8∗ | 11.4∗ | 8.4∗ | 4.1∗ | |
Long-term success was achieved in one recipient only. In this patient a uretero-ureterostomy between the native and the transplant ureter had been formed 4 days after the RTX, because the initial vesico-ureteric anastomosis was stenosed. This uretero-ureterostomy also strictured and therefore was treated with RBD after 5.1 months. Five transplant ureters re-stenosed and had to be re-operated on with transplant ureter neo-implantation into the bladder (three) or replacement of the transplant ureter with ileum (two). For two recipients the success remains unclear. In one patient with reduced transplant function not related to ureteric stenosis, immunosuppression had to be stopped and the graft explanted, as the recipient suffered from repeated severe infections including cytomegalovirus pneumonia. Another recipient was treated recently and the stent was still in place at the time of this report.
Discussion
Stenosis of the transplant ureter mostly occurs at the site of ureteric anastomosis and has been described in 0.5–5% of adult [1–4] and 3–10% of paediatric recipients [16–18]. The type of ureteric anastomosis with the recipient bladder (Lich-Gregoir, Politano-Leadbetter) does not affect the occurrence of ureteric stenosis [19]. Anastomosis of the transplant ureter with the recipient’s native ureter was not found to increase the risk of ureteric stenosis by some authors [20], while others detected a greater risk compared with anastomosis with the recipient’s bladder [21]. In paediatric recipients, PUV were found to be associated with ureteric stenosis after RTX, possibly because remodelling of collagen I/III also affects the ureteric anastomosis site of the transplant ureter in these children [18]. A very rare cause of ureteric stenosis in paediatric recipients might be a BK virus infection; to date, in one case report this was found to be the underlying cause [22]. In the recipients at our centre, regular controls for BK virus in the peri- and postoperative phase of RTX are part of the routine assessments. In one recipient 60,000 copies of BK virus were detected once, but as this was several months after the formation of a ureteric stenosis, and 5 weeks after RBD of the strictured ureter without BK virus detection immediately before and afterwards, BK virus infection as the underlying cause for the ureteric stricture appears unlikely.
Possible risk factors for adult recipients are donor age >65 years, delayed graft function, the existence of more than two transplant arteries or a double ureter, and the duration of organ transport (cold ischaemic time) [3,4,23–25]. In the present patients, two donor kidneys which developed ureteric strictures had a double ureter, and one was found to have multiple arteries. As the anatomy of a donor organ cannot be changed, the implanting surgeon/urologist should be aware of these risk factors for ureteric stenosis during the operation. Moreover, this should lead to a closer follow-up in the early phase after RTX to ensure timely detection of possible ureteric complications. Insertion of a ureteric stent during RTX significantly prevents ureteric stenosis and (as it also prevents complications due to ureteric leakage) should be used routinely in all RTX [5]. Therefore, we have started to insert ureteric stents in all RTX procedures.
Many treatment options have been described for transplant ureteric stenosis, ranging from minimally invasive procedures to open re-operation. Generally, PNS or stenting can be done but usually provide no long-term relief. Nevertheless, in selected cases ureteric stenting might provide a definitive solution [26], as can the insertion of a subcutaneous artificial ureter (Detour™, Mentor-Porges, Le Plessis Robinson, France), which has been described for about 20 renal transplant recipients so far [27,28]. Another minimally invasive method is endoscopic transplant ureterotomy, which has been reported for only ≈20 cases. The success rate was >50% [29,30] but there are too few cases to recommend this as a general approach.
BD of the ureter is a minimally invasive approach for treating transplant ureteric stenosis, and an alternative to open re-operation with re-implantation of the transplant ureter, a new anastomosis of the native ureter with the renal transplant pelvis, performing a Boari flap/psoas hitch or replacement of the transplant ureter with ileum [31–34]. Over the past 23 years, experience with ≈200 cases of BD of a stenosed transplant ureter has been reported (Table 3). Interestingly, all studies used the technique of antegrade BD via an initially placed PNS. Complications were not severe and mostly consisted of limited haematuria and/or UTI. A half to two-third of these interventions were reported to have been successful after single or repeated BD, with a tendency to a higher success rate the earlier the BD was performed after RTX [6–15].
Table 3.
Publications on dilation of transplant ureter stenosis after renal transplantation.
| Reference | Year of publication | Number of transplants | Number of ureteral strictures treated by balloon dilation | Interval between transplantation & balloon dilation | Site of stricture | Success rate pts (%) | Complications |
|---|---|---|---|---|---|---|---|
| Voegeli [6] | 1988 | ? | 14a | nn | nn | 11/14 (79%) | None |
| Lojanapiwat [7] | 1994 | 692 | 21a,b | 14 × <3 months 7 × >3 months |
17 UVJ 4 UPJ |
12/21 (57%) <3 months: 10/14 (71%) >3 months: 2/ 7 (29%) |
“no perforation of ureter/pelvic area” |
| Fontaine [8] | 1997 | ? | 44a | 13 × <3 months 31 × >3 months |
nn | 13/44 (30%) <3 months: 8/13 (62%) >3 months: 5/31 (16%) |
38% UTI 14% limited hematuria |
| Peregrin [9] | 1997 | 1074 | 23a | 13 × <3 months 10 × >3 months (2 × >years) |
nn | 10/23 (43%) <3 months: 6/13 (46%) >3 months: 4/10 (40%) |
Limited hematuria = “most common complication” |
| Collado [10] | 1998 | 472 | 18a | 8 months (1–30) | 16 UVJ 1 UPJ 1 UPJ & UVJ |
7/18 (39%) <3 months: 4/5 (80%) >3 months: 3/13 (23%) |
None |
| Yong [11] | 1999 | ? | 9a | 6 × <3 months (1–2.5) 3 × >3 months (4–5) |
8 UVJ 1 UPJ |
8/9 (89%) <3 months: 6/6 (100%) >3 months: 2/3 (66%) |
None |
| Kristo [12] | 2003 | 622 | 9a | median 7 months (3–122) | 9 UVJ | 9/9 (100%) | None |
| Bachar [13] | 2004 | 422 | 21a,c | 1.5–30 months early group 1.5 months late group 10.5 months |
19 × UVJ 2 × UPJ 1 × mid ureter |
13/21 (62%) 7/12 (58%) 6/9 (66%) |
38% UTI hematuria – most common |
| Juaneda [14] | 2005 | 1000 | 45a | 6.8 months (0.01–64) | nn | 20/45 (45%) | 2 × sepsis with subsequent graft loss 15 × steroid sensitive AR |
| Bromwich [15] | 2006 | 207 | 9a | 17 months (1–276) | 3 × UVJ 5 × UPJ 1 UPJ & UVJ |
4/9 (45%) | None |
| This study (retrograde) | 2011 | 262d | 8 | 4.5 months (3–11) | 6 × UVJ 1 × middle 1 × UPJ |
1/8 (12%) | none |
UVJ = uretero-vesical junction; UPJ = uretero-pelvic junction; AR = acute rejection; UTI = urinary tract infection; nn = not named/data missing.
Antegrade (percutaneous) dilation via transplant nephrostomy
Dilation not with balloon but with ureteral stents.
11 of these patients were referred from other centres.
Number of renal transplantations at the Heinrich Heine University Hospital during the study period from 10/2008 to 02/2011.
The most important conclusions to be drawn from previously published data are: (i) BD within 3 months after RTX is more successful than later treatment; (ii) if initial BD fails, the success rate of further dilatation is <25%; (iii) BD does not compromise subsequent ureteric operations that might become necessary if BD fails to achieve long-term relief.
In our small study, the first on RBD, the time after RTX was >10 weeks. This might be why the success rate was very low, even though the one patient successfully treated with BD had been treated 5.1 months after RTX. Also, our study is the first to evaluate the retrograde approach to transplant ureter BD. The present limited success rate compared to antegrade BD might be due to as yet unidentified factors of the chosen approach. Overall, our experience confirms the findings published in other series; if a first BD fails, further BD does not improve the outcome. Therefore, we have changed our algorithm for ureteric stenosis after RTX. An initial attempt with RBD is only offered to recipients with early transplant ureteric stenoses (<10 weeks); if this attempt fails, open re-operation is our therapy of choice. Nevertheless, depending on the individual setting, BD might be tried in recipients developing stenosis later after RTX if risk factors for ureteric transplant stenosis are favourable (low donor age, single donor ureter, single transplant artery, short cold ischaemic time). We are well aware that the limitations of this study are the retrospective approach and the small sample size.
In conclusion, our findings of this first study on RBD of transplant ureteric stenoses confirm the results published by others: late treatment of ureteric stenosis is often unsuccessful, irrespective of the approach (retrograde and antegrade). Recipients developing transplant ureteric stenosis should be followed closely. Early-stage treatment with single BD and subsequent open operation in case of unsuccessful BD should be offered within 10–12 weeks after RTX. In case of late occurrence (>12 weeks after RTX), re-operation and neo-implantation of the transplant ureter should be considered as the first choice.
References
- 1.Davari H.R., Yarmohammadi H., Malekhosseini S.A., Salahi H., Bahador A., Salehipour M. Urological complications in 980 consecutive patients with renal transplantation. Int J Urol. 2006;13:1271–1275. doi: 10.1111/j.1442-2042.2006.01539.x. [DOI] [PubMed] [Google Scholar]
- 2.Emiroglu R., Karakayall H., Sevmis S., Akkoç H., Bilgin N., Haberal M. Urologic complications in 1275 consecutive renal transplantations. Transplant Proc. 2001;33:2016–2017. doi: 10.1016/s0041-1345(00)02772-x. [DOI] [PubMed] [Google Scholar]
- 3.Karam G., Hétet J.F., Maillet F., Rigaud J., Hourmant M., Soulillou J.P. Late ureteral stenosis following renal transplantation. Risk factors and impact on patient and graft survival. Am J Transplant. 2006;6:352–356. doi: 10.1111/j.1600-6143.2005.01181.x. [DOI] [PubMed] [Google Scholar]
- 4.Streeter E.H., Little D.M., Cranston D.W., Morris P.J. The urological complications of renal transplantation: a series of 1535 patients. BJU Int. 2002;90:627–634. doi: 10.1046/j.1464-410x.2002.03004.x. [DOI] [PubMed] [Google Scholar]
- 5.Wilson CH, Bhatti AA, Rix DA, Manas DM. Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst Rev 2005;19:CD004925. [DOI] [PubMed]
- 6.Voegeli D.R., Crummy A.B., McDermott J.C., Jensen S.R. Percutaneous dilation of ureteral strictures in renal transplant patients. Radiology. 1988;169:185–188. doi: 10.1148/radiology.169.1.3047784. [DOI] [PubMed] [Google Scholar]
- 7.Lojanapiwat B., Mital D., Fallon L., Koolpe H., Raja R., Badosa F. Management of ureteral stenosis after renal transplantation. J Am Coll Surg. 1994;179:21–24. [PubMed] [Google Scholar]
- 8.Fontaine A.B., Nijjar A., Rangaraj R. Update on the use of percutaneous nephrostomy/balloon dilation for the treatment of renal transplant leak/obstruction. J Vasc Interv Radiol. 1997;8:649–653. doi: 10.1016/s1051-0443(97)70625-0. [DOI] [PubMed] [Google Scholar]
- 9.Peregrin J., Filipová H., Matl I., Vítko S., Làcha J. Percutaneous treatment of early and late ureteral stenosis after renal transplantation. Transplant Proc. 1997;29:140–141. doi: 10.1016/s0041-1345(96)00038-3. [DOI] [PubMed] [Google Scholar]
- 10.Collado A., Caparrós J., Guirado L., Rosales A., Martí J., Solà R. Balloon dilatation in the treatment of ureteral stenosis in kidney transplant recipients. Eur Urol. 1998;34:399–403. doi: 10.1159/000019773. [DOI] [PubMed] [Google Scholar]
- 11.Yong A.A., Ball S.T., Pelling M.X., Gedroyc W.M., Morgan R.A. Management of ureteral strictures in renal transplants by antegrade balloon dilatation and temporary internal stenting. Cardiovasc Intervent Radiol. 1999;22:385–388. doi: 10.1007/s002709900412. [DOI] [PubMed] [Google Scholar]
- 12.Kristo B., Phelan M.W., Gritsch H.A., Schulam P.J. Treatment of renal transplant ureterovesical anastomotic strictures using antegrade balloon dilation with or without holmium:YAG laser endoureterotomy. Urology. 2003;62:831–834. doi: 10.1016/s0090-4295(03)00655-1. [DOI] [PubMed] [Google Scholar]
- 13.Bachar G.N., Mor E., Bartal G., Atar E., Goldberg N., Belenky A. Percutaneous balloon dilatation for the treatment of early and late ureteral strictures after renal transplantation: long-term follow-up. Cardiovasc Intervent Radiol. 2004;27:335–338. doi: 10.1007/s00270-004-0163-9. [DOI] [PubMed] [Google Scholar]
- 14.Juaneda B., Alcaraz A., Bujons A., Guirado L., Díaz J.M., Martí J. Endourological management is better in early-onset ureteral stenosis in kidney transplantation. Transplant Proc. 2005;37:3825–3827. doi: 10.1016/j.transproceed.2005.09.199. [DOI] [PubMed] [Google Scholar]
- 15.Bromwich E., Coles S., Atchley J., Fairley I., Brown J.L., Keoghane S.R. A 4-year review of balloon dilation of ureteral strictures in renal allografts. J Endourol. 2006;20:1060–1061. doi: 10.1089/end.2006.20.1060. [DOI] [PubMed] [Google Scholar]
- 16.Englesbe M.J., Lynch R.J., Heidt D.G., Thomas S.E., Brooks M., Dubay D.A. Early urologic complications after pediatric renal transplant: a single-center experience. Transplantation. 2008;86:1560–1564. doi: 10.1097/TP.0b013e31818b63da. [DOI] [PubMed] [Google Scholar]
- 17.Nuininga J.E., Feitz W.F., van Dael K.C., de Gier R.P., Cornelissen E.A. Urological complications in pediatric renal transplantation. Eur Urol. 2001;39:598–602. doi: 10.1159/000052510. [DOI] [PubMed] [Google Scholar]
- 18.Smith K.M., Windsperger A., Alanee S., Humar A., Kashtan C., Shukla A.R. Risk factors and treatment success for ureteral obstruction after pediatric renal transplantation. J Urol. 2010;183:317–322. doi: 10.1016/j.juro.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Masahiko H., Kazunari T., Tokumoto T., Ishikawa N., Yagisawa T., Toma H. Comparative study of urosurgical complications in renal transplantation: intravesical versus extravesical ureterocystoneostomy. Transplant Proc. 2000;32:1844–1846. doi: 10.1016/s0041-1345(00)01458-5. [DOI] [PubMed] [Google Scholar]
- 20.Faenza A., Nardo B., Fuga G., Liviano-D’Arcangelo G., Grammatico F., Montalti R. Urological complications in kidney transplantation: ureterocystostomy versus uretero-ureterostomy. Transplant Proc. 2005;37:2518–2520. doi: 10.1016/j.transproceed.2005.06.079. [DOI] [PubMed] [Google Scholar]
- 21.Nie Z.L., Zhang K.Q., Li Q.S., Jin F.S., Zhu F.Q., Huo W.Q. Urological complications in 1, 223 kidney transplantations. Urol Int. 2009;83:337–341. doi: 10.1159/000241679. [DOI] [PubMed] [Google Scholar]
- 22.Rajpoot D.K., Gomez A., Tsang W., Shanberg A. Ureteric and urethral stenosis: a complication of BK virus infection in a pediatric renal transplant patient. Pediatr Transplant. 2007;11:433–435. doi: 10.1111/j.1399-3046.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 23.Fuller T.F., Deger S., Büchler A., Roigas J., Schönberger B., Schnorr D. Ureteral complications in the renal transplant recipient after laparoscopic living donor nephrectomy. Eur Urol. 2006;50:535–540. doi: 10.1016/j.eururo.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 24.Figueiredo A.J., Parada B.A., Cunha M.F. Ureteral complications: analysis of risk factors in 1000 renal transplants. Transplant Proc. 2003;35:1087–1088. doi: 10.1016/s0041-1345(03)00319-1. [DOI] [PubMed] [Google Scholar]
- 25.Shokeir A.A., Osman Y., Ali-El-Dein B., El-Husseini A., El-Mekresh M., Shehab-El-Din A.B. Surgical complications in live-donor pediatric and adolescent renal transplantation: study of risk factors. Pediatr Transplant. 2005;9:33–38. doi: 10.1111/j.1399-3046.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 26.Burgos F.J., Bueno G., Gonzalez R., Vazquez J.J., Diez-Nicolás V., Marcen R. Endourologic implants to treat complex ureteral stenosis after kidney transplantation. Transplant Proc. 2009;41:2427–2429. doi: 10.1016/j.transproceed.2009.06.068. [DOI] [PubMed] [Google Scholar]
- 27.Giessing M., Schnorr D., Loening S.A. Artificial ureteral replacement following kidney transplantation. Clin Transplant. 2006:578–579. [PubMed] [Google Scholar]
- 28.Azhar R.A., Hassanain M., Aljiffry M., Aldousari S., Cabrera T., Andonian S. Successful salvage of kidney allografts threatened by ureteral stricture using pyelovesical bypass. Am J Transplant. 2010;10:1414–1419. doi: 10.1111/j.1600-6143.2010.03137.x. [DOI] [PubMed] [Google Scholar]
- 29.Bhayani S.B., Landman J., Slotoroff C., Figenshau R.S. Transplant ureter stricture. Acucise endoureterotomy and balloon dilation are effective. J Endourol. 2003;17:19–22. doi: 10.1089/089277903321196733. [DOI] [PubMed] [Google Scholar]
- 30.Gdor Y., Gabr A.H., Faerber G.J., Wolf J.S., Jr. Holmium:yttrium-aluminum-garnet laser endoureterotomy for the treatment of transplant kidney ureteral strictures. Transplantation. 2008;85:1318–1321. doi: 10.1097/TP.0b013e31816c7f19. [DOI] [PubMed] [Google Scholar]
- 31.Lapointe S.P., Charbit M., Jan D., Lortat-Jacob S., Michel J.L., Beurton D. Urological complications after renal transplantation using ureteroureteral anastomosis in children. J Urol. 2001;166:1046–1048. [PubMed] [Google Scholar]
- 32.Salomon L., Saporta F., Amsellem D., Hozneck A., Colombel M., Patard J.J. Results of pyeloureterostomy after ureterovesical anastomosis complications in renal transplantation. Urology. 1999;53:908–912. doi: 10.1016/s0090-4295(98)00624-4. [DOI] [PubMed] [Google Scholar]
- 33.Schult M., Küster J., Kliem V., Brunkhorst R., Nashan B., Oldhafer K.J. Native pyeloureterostomy after kidney transplantation: experience in 48 cases. Transplant Int. 2000;13:340–343. doi: 10.1007/s001470050711. [DOI] [PubMed] [Google Scholar]
- 34.Krings F., Stippel D., Vorreuther R. Reconstruction of a totally necrotic renal collecting system and ureter after living donor transplantation using the Boari flap technique. Eur J Surg. 1997;163:73–76. [PubMed] [Google Scholar]


