Abstract
A 13-year-old boy presented with recurrent episodes of sudden brief posturing of the right upper and lower limbs accompanied by transient inability to speak and a tendency to smile which would sometimes break into laughter. Awareness was retained during the attack, and there was no associated emotional abnormality. The events were precipitated by walking and occurred several times in a day. The laughter was pathological in nature, and the abnormal posturing was akin to ‘paroxysmal kinesigenic dyskinesia’ (PKD). ‘Pathological laughter or crying’ is defined as an involuntary, inappropriate, unmotivated laughter, crying or both, without any associated mood change. It can occur as a result of cerebral lesions like tumors, trauma, vascular insults, multiple sclerosis and/or degenerative disorders. It can also be a component of gelastic epilepsy which is characterized by stereotyped recurrences, presence of interictal and ictal epileptiform discharges and absence of external precipitants. In our patient, however, there was no ictal or interictal EEG correlate. Paroxysmal kinesigenic dyskinesia is characterized by intermittent, involuntary movements triggered by kinesigenic stimuli and is usually familial but can also be secondary to metabolic and structural brain disorders. Magnetic Resonance Imaging (MRI), in our case, revealed multiple T2 and FLAIR hyperintense, non-enhancing lesions in the periaqueductal gray matter, pontine and midbrain tegmentum, bilateral thalami and left lentiform nucleus suggesting a diagnosis of ‘acute disseminated encephalomyelitis’, in which this unique combination of pathological laughter and PKD has not been described so far. Magnetic Resonance Spectroscopy (MRS) confirmed a demyelinating pathology, and the patient responded well to steroids.
Keywords: Pathological laughter, Gelastic epilepsy, Paroxysmal kinesigenic dyskinesia, Acute disseminated encephalomyelitis
1. Introduction
Pathological laughter and crying (PLC) is defined as involuntary and uncontrollable attacks of emotional expression as a result of a cerebral lesion [1]. It has been shown to occur due to the damage of pathways that arise in the motor areas of the cerebral cortex and descend to the brainstem putative centers for laughter and crying. The lesions ‘disinhibit’ or ‘release’ the laughter and crying centers [1,2]. Various diseases that have been associated with PLC are vascular disorders, head injuries, tumors, multiple sclerosis, amyotrophic lateral sclerosis, degenerative dementias and extrapyramidal disorders [2].
Paroxysmal kinesigenic dyskinesia (PKD) is a rare disorder characterized by sudden brief, abnormal involuntary movements precipitated by kinesigenic triggers [3,4]. It can manifest as dystonia, choreoathetosis, ballismus or any combination of these, involving muscle groups of the arms, legs, trunk, face, and/or neck [1,2]. Paroxysmal kinesigenic dyskinesia is usually familial with autosomal dominant inheritance, but it can be encountered in patients with metabolic and structural brain disorders as well which include ischemic stroke, birth injury, head injury, vascular malformations, brain tumors, multiple sclerosis, encephalitis, AIDS, drug abuse, hyperglycemia, cystinuria, hyperthyroidism, hypoparathyroidism or disorders that are psychogenic [5–7].
Here, we report a 13-year-old male with pathological laughter associated with paroxysmal kinesigenic dyskinesias secondary to acute disseminated encephalomyelitis.
2. Case report
A 13-year-old boy presented with one month history of recurrent episodes of sudden brief posturing of the right upper and lower limbs along with transient inability to speak. He could understand what others were saying, wanted to reply but could not speak. His relatives noticed a tendency to smile which would sometimes break into laughter. However, there was no feeling of elation or happiness, or any associated emotional abnormality following the event. There was no history of any aura or prodrome, nor any head or neck deviation, twitching of face, clonic jerking, frothing, salivation or any fall. There was no associated pain. These events were precipitated by walking for a few minutes but not by any other sudden stimuli. These occurred multiple times in a day and were not present during sleep. There was no history of slowness in activities of daily living, stiffness of any part, or tremulousness during rest, nor any change in posture or persistent change in gait or speech. There was no history of jaundice in the past. The family history was not contributory.
General physical, cardiovascular, chest and abdominal examinations were unremarkable. Detailed central nervous system examination revealed normal higher mental functions, and there was no deficit in the distribution of any cranial nerves. Kayser–Fleischer ring on slit-lamp examination was absent. Motor system examination showed normal bulk, tone and power in all four limbs. Deep tendon reflexes were well elicitable, and planters were bilateral flexor. There were no rigidity, bradykinesia, tremors or cerebellar signs. Sensory system examination was normal.
Multiple episodes were observed on making the patient walk for a few minutes. These were characterized by sudden abduction and external rotation of the right lower limb along with abduction and internal rotation of the right upper limb and an associated smile which, at times, would break into laughter along with speech arrest. The patient was aware of his surroundings and could follow commands; his speech took a while to clear (Fig. 1, Video 1).
Fig. 1.

By walking for a few minutes, the patient exhibited a smile and sudden abduction and external rotation of the right lower limb along with abduction and internal rotation of the right upper limb. Speech arrest occurred during the event, the patient could follow commands, and the speech took a while to clear.
Multiple episodes were observed on making the patient walk for a few minutes. These were characterized by sudden abduction and external rotation of the right lower limb along with abduction and internal rotation of the right upper limb and an associated smile which, at times, would break into laughter along with speech arrest. The patient was aware of his surroundings and could follow commands; his speech took a while to clear (Fig. 1, Video 1).
Routine investigations revealed normal hemoglobin, blood counts, blood sugar, serum electrolytes, and liver and kidney function tests. Serum calcium, phosphorous, alkaline phosphatase and thyroid function tests were normal. X-ray chest was unremarkable. Human immunodeficiency virus types 1 and 2 by ELISA were nonreactive. MRI brain revealed T1 hypointense and T2/FLAIR hyperintense lesions in pontine tegmentum, midbrain tegmentum (left > right), periaqueductal gray matter, bilateral thalamus and left lentiform nucleus, without any contrast enhancement (Figs. 2–5). MR spectroscopy through left pontine lesion showed normal NAA peak, mild elevation of choline, and no lactate peak, suggestive of a benign pathology (Fig. 6). Scalp EEG did not reveal any abnormality. On prolonged video-EEG monitoring the habitual events were recorded without any ictal correlate or any interictal discharges (Video 2).
Fig. 2.

Magnetic Resonance Imaging (MRI) brain showing T1 hypointense and T2/FLAIR hyperintense lesions in the pontine tegmentum, without any contrast enhancement.
Fig. 3.

MRI brain showing T1 hypointense and T2/FLAIR hyperintense lesions in the midbrain tegmentum (left > right), without any contrast enhancement.
Fig. 4.

MRI brain showing T1 hypointense and T2/FLAIR hyperintense lesions in the periaqueductal gray matter, without any contrast enhancement.
Fig. 5.
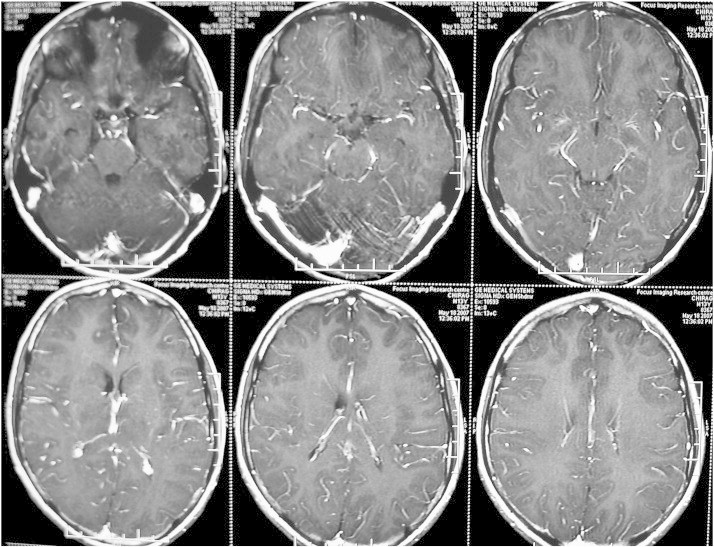
MRI brain showing T1 hypointense and T2/FLAIR hyperintense lesions in the bilateral thalamus and left lentiform nucleus, without any contrast enhancement.
Fig. 6.

Magnetic Resonance (MR) spectroscopy through left pontine lesion showing normal NAA peak, mild elevation of choline, and no lactate peak.
Routine investigations revealed normal hemoglobin, blood counts, blood sugar, serum electrolytes, and liver and kidney function tests. Serum calcium, phosphorous, alkaline phosphatase and thyroid function tests were normal. X-ray chest was unremarkable. Human immunodeficiency virus types 1 and 2 by ELISA were nonreactive. Magnetic Resonance Imaging (MRI) brain revealed T1 hypointense and T2/FLAIR hyperintense lesions in pontine tegmentum, midbrain tegmentum (left > right), periaqueductal gray matter, bilateral thalamus and left lentiform nucleus, without any contrast enhancement (Figs. 2–5). Magnetic Resonance (MR) spectroscopy through left pontine lesion showed normal NAA peak, mild elevation of choline, and no lactate peak, suggestive of a benign pathology (Fig. 6). Scalp EEG did not reveal any abnormality. On prolonged video-EEG monitoring the habitual events were recorded without any ictal correlate or any interictal discharges (Video 2).
A possibility of acute disseminated encephalomyelitis (ADEM) manifesting as pathological laughter as well as paroxysmal kinesigenic dyskinesia (PKD) was entertained, and treatment with IV methyl prednisolone 1000 mg/day for five days followed by oral steroid for two weeks in tapering doses was instituted. Before presenting to our hospital, the patient had been diagnosed as having seizures and was taking phenytoin 300 mg/day. It was gradually tapered off. There was gradual decrease in the frequency of the events, and he became completely asymptomatic in two weeks and continued to remain so at his last follow-up at eight months.
3. Discussion
Laughter is a coordinated function of facial and respiratory muscles triggered by specific stimuli and is associated with mood elevation. The expression of laughter depends on two neuronal pathways: an ‘involuntary’ or ‘emotionally driven’ system involving the amygdala, thalamic, hypothalamic and subthalamic areas and the dorsal/tegmental brainstem; and a ‘voluntary’ system originating in the premotor or frontal opercular areas leading through the motor cortex and pyramidal tract to the ventral brainstem [1,2]. The pontine nuclei act as the final common pathway for expression of emotion, phonation, rhythmic clonic expiration and facial expression. The mesencephalic central gray matter integrates input from diverse regions, acts as a processing and relay station between descending limbic diencephalic tracts and bulbar effector organs and, via the annulo-olivary tract to the cerebellum, exercises a modulating effect on all these expressions [1,2,8,9]. The cerebellum with the cerebro-ponto-cerebellar pathways coordinates smile or laughter in a given social context, and the associated mirth involves elaboration of sensory processing and its integration with past memories and the affect of the individual [9].
Pathological laughter or crying (PLC) is defined as an involuntary, inappropriate, unmotivated laughter, crying or both, without any associated mood change [1]. Release of the laughter and crying centers in the brainstem from the inhibitory effect of the motor cortex and an abnormal or an incomplete processing of the laughter mechanisms are the various hypotheses postulated for the genesis of pathological laughter [1,2]. These are based on various experimental studies in animals and on studies in patients suffering from disseminated sclerosis and tumors in the internal capsule, subthalamic region, the tegmentum and the upper pons [1]. Lesions of the midbrain and ventral pons have been shown to give rise to pathological laughter, and those of the dorsal pons to abolition of laughter [1]. The loss of sensory integration leads to mirthless laughter. In a clinico-pathological series of 53 cases (33 autopsied) with pathologic laughter and crying, the lesions were cortical in 6, corticodiencephalic area in 25, diencephalic area in 15 and, mesencephalometencephalic in 7 cases. Twenty-six patients had vascular disease of CNS, tumors (6 cases) were also seen, MS (6 cases), ALS (3 cases), and various other neurological disorders (10 cases) [10].
Pathological laughter is also a component of gelastic epilepsy. Ictal laughter is the cardinal clinical sign of gelastic seizures, where it is short-lasting, usually less than 30 s, and may occur as an isolated event [11]. Sudden emotions as a manifestation of an epileptic seizure have been recognized since the 19th century. Laughing seizures were first described by Trousseau in 1877 and then by Gowers in 1881 [12,13]. The term gelastic epilepsy was coined by Daly and Mulder and came from the Greek word ‘gelos’ meaning laughter [14]. Frontal or temporal lobe lesions (focal cortical dysplasias, tumors, tuberous sclerosis, post-infectious foci) and hypothalamic hamartoma are known to be associated with gelastic epilepsy [15]. The dysfunction of the limbic system is the most likely mechanism involved in the generation of gelastic seizures. Differentiating this condition from PLC occurring due to other causes is difficult. The diagnostic criteria laid down by Gascon and Lombroso for gelastic epilepsy, however, are explicit and state that gelastic epilepsy is characterized by stereotyped recurrences, absence of external precipitants, concomitance of other manifestations such as tonic-clonic movements, disturbances of consciousness and/or automatisms that are generally considered as epileptic, presence of interictal and ictal epileptiform discharges and absence of conditions in which pathological laughter might occur [16].
Brief stereotyped limb posturing and associated laughter can be suggestive of gelastic seizures but was probably not a manifestation of seizures in our case, as these events were precipitated by an external stimulus and the ictal and interictal EEG were normal. Moreover, there were no cortical or hypothalamic lesions in the MRI. The pathological laughter in our patient most likely resulted from lesions in the mesencephalic periaqueductal gray matter and pons. The occurrence of sudden brief dystonic posturing of the right upper and lower limbs and the inability to speak in our patient in all probability are paroxysmal kinesigenic dyskinesia, which is supported by stereotyped occurrence, precipitation by movement, retaining of consciousness, absence during sleep, and a normal EEG as observed in our case.
Paroxysmal kinesigenic dyskinesia (PKD) was first described in 1941 by Smith and Heersema [17]. Paroxysmal kinesigenic dyskinesia patients experience recurrent, sudden brief stereotypic episodes of dystonic or choreoathetotic movements induced by sudden voluntary activity. The frequency of these may be as high as 100 attacks per day. These can be severe enough to cause a patient to fall. Speech can sometimes be affected, there may be an expression of smile, but alteration of consciousness never occurs, as was seen in our case. Attacks of PKD were earlier considered as epileptic and, in fact, have been referred to as movement-induced seizures, extrapyramidal epilepsy, striatal epilepsy and subcortical epilepsy [18–21], but they are now regarded as a form of channelopathy, with good response to low doses of antiepileptic drugs like phenytoin, carbamazepine, oxcarbazepine, ethosuximide, and lamotrigine [22,23]. At the time of presentation, our patient was already on 300 mg of phenytoin/day but without any response. He, however, showed a good response to steroids and did not require any subsequent antiepileptic medication.
In children, basal ganglia involvement on neuroimaging has been reported in up to 20–39% cases of ADEM, but extrapyramidal signs like chorea and dystonia are infrequently described [24–27]. The MRI findings in our case and the therapeutic response to IV methyl prednisolone suggested a demyelinating etiology, most likely ADEM. To the best of our knowledge, our case is a rare presentation of ADEM manifesting as unique combination of ‘pathological laughter’ and ‘paroxysmal kinesigenic dyskinesia’.
The following are the supplementary data related to this article.
By walking for a few minutes, the patient exhibited a smile and sudden abduction and external rotation of the right lower limb along with abduction and internal rotation of the right upper limb. Speech arrest occurred during the event, the patient could follow commands, and the speech took a while to clear.
Video-EEG showing typical event without any ictal correlate or any interictal discharges.
Interictal and ictal EEG.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebcr.2012.11.001.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Davison C., Kelman H. Pathological laughter and crying. Arch Neurol Psychiatry. 1939;42(4):595–643. [Google Scholar]
- 2.Parvizi J., Coburn K.L., Shillcutt S.D., Coffey C.E., Lauterbach E.C., Mendez M.F. Neuroanatomy of pathological laughing and crying: a report of the American Neuropsychiatric Association Committee on Research. J Neuropsychiatry Clin Neurosci. 2009;21:75–87. doi: 10.1176/jnp.2009.21.1.75. [DOI] [PubMed] [Google Scholar]
- 3.Bruno M.K., Hallett M., Gwinn-Hardy K. Clinical evaluation of idiopathic paroxysmal kinesigenic dyskinesia: new diagnostic criteria. Neurology. 2004;63:2280–2287. doi: 10.1212/01.wnl.0000147298.05983.50. [DOI] [PubMed] [Google Scholar]
- 4.Houser M.K., Soland V.L., Bhatia K.P., Quinn N.P., Marsden C.D. Paroxysmal kinesigenic choreoathetosis: a report of 26 patients. J Neurol. 1999;246:120–126. doi: 10.1007/s004150050318. [DOI] [PubMed] [Google Scholar]
- 5.Weber Y.G., Lerche H. Genetics of paroxysmal dyskinesias. Curr Neurol Neurosci Rep. 2009;9:206–211. doi: 10.1007/s11910-009-0031-8. [DOI] [PubMed] [Google Scholar]
- 6.Depienne C., Brice A. Unlocking the genetics of paroxysmal kinesigenic dyskinesia. Oxf J Med Brain. 2011;134(Pt. 12):3431–3434. doi: 10.1093/brain/awr319. [DOI] [PubMed] [Google Scholar]
- 7.Blakeley J., Jankovic J. Secondary causes of paroxysmal dyskinesia. Adv Neurol. 2002;89:401–420. [PubMed] [Google Scholar]
- 8.Wild B., Rodden F.A., Grodd W., Ruch W. Neural correlates of laughter and humour. Brain. 2003;126:2121–2138. doi: 10.1093/brain/awg226. [DOI] [PubMed] [Google Scholar]
- 9.Parvizi J., Anderson S.W., Martin C.O., Damasio H., Damasio A.R. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124:1708–1719. doi: 10.1093/brain/124.9.1708. [DOI] [PubMed] [Google Scholar]
- 10.Hamby W.B. Thomas Books; Springfield: 1960. The case reports and autopsy records of Ambroise Paré; pp. 6–7. [Google Scholar]
- 11.Arroyo S., Lesser R.P., Gordon B. Mirth, laughter and gelastic seizures. Brain. 1993;116(Pt. 4):757–780. doi: 10.1093/brain/116.4.757. [DOI] [PubMed] [Google Scholar]
- 12.Trousseau A. 1877. pp. 89–155. (Clinique Medicale de L'Hotel-Dieu de Paris. De L'Epilepsie). [Google Scholar]
- 13.Gowers W.R. Epilepsy and other chronic convulsive diseases. William Wood and Company; New York: 1881. p. 255. [Google Scholar]
- 14.Daly D.D., Mulder D.W. Gelastic epilepsy. Neurology. 1957;7:189–192. doi: 10.1212/wnl.7.3.189. [DOI] [PubMed] [Google Scholar]
- 15.Téllez-Zenteno J.F., Serrano-Almeida C., Moien-Afshari F. Gelastic seizures associated with hypothalamic hamartomas. An update in the clinical presentation, diagnosis and treatment. Neuropsychiatr Dis Treat. 2008;4(6):1021–1031. doi: 10.2147/ndt.s2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gascon G.G., Lombroso C.T. Epileptic (gelastic) laughter. Epilepsia. 1971;12:63–76. doi: 10.1111/j.1528-1157.1971.tb03916.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith L.A., Heersema P.H. Periodic dystonia. Mayo Clin Proc. 1941;16:842–846. [Google Scholar]
- 18.Falconer M., Driver M., Serafetinides E. Seizures induced by movement: report of a case relieved by operation. J Neurol Neurosurg Psychiatry. 1963;26:300–307. doi: 10.1136/jnnp.26.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterling W. Le type spasmodique tetanoide et tetaniforme de léncephalite epidemique remarques sur lépilepsie “extra-pyramidale”. Rev Neurol (Paris) 1924;2:484–492. [Google Scholar]
- 20.Spiller W.G. Subcortical epilepsy. Brain. 1927;50:171–187. [Google Scholar]
- 21.Wimmer A. Etudes sur les syndromes extra-piramidaux: spasm de torsion infantile debutant par crises d'hemispasmes toniques (epilepsie striee) Rev Neurol (Paris) 1925;32:281-25. [Google Scholar]
- 22.Siniscalchi A., Gallelli L., Sarro G.D. Use of antiepileptic drugs for hyperkinetic movement disorders. Curr Neuropharmacol. 2010;8:359–366. doi: 10.2174/157015910793358187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unterberger I., Trinka E. Diagnosis and treatment of paroxysmal dyskinesias revisited. Ther Adv Neurol Disord. 2008;1(2):67–74. doi: 10.1177/1756285608095119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dale R.C., de Sousa C., Chong W.K., Cox T.C., Harding B., Neville B.G. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123(12):2407–2422. doi: 10.1093/brain/123.12.2407. [DOI] [PubMed] [Google Scholar]
- 25.Hynson J.L., Kornberg A.J., Coleman L.T., Shield L., Harvey A.S., Kean M.J. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56:1308–1312. doi: 10.1212/wnl.56.10.1308. [DOI] [PubMed] [Google Scholar]
- 26.Murthy S.N., Faden H.S., Cohen M.E., Bakshi R. Acute disseminated encephalomyelitis in children. Pediatrics. 2002;110(2 Pt. 1):e21. doi: 10.1542/peds.110.2.e21. [DOI] [PubMed] [Google Scholar]
- 27.Donovan M.K., Lenn N.J. Postinfectious encephalomyelitis with localized basal ganglia involvement. Pediatr Neurol. 1989;5:311–313. doi: 10.1016/0887-8994(89)90024-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
By walking for a few minutes, the patient exhibited a smile and sudden abduction and external rotation of the right lower limb along with abduction and internal rotation of the right upper limb. Speech arrest occurred during the event, the patient could follow commands, and the speech took a while to clear.
Video-EEG showing typical event without any ictal correlate or any interictal discharges.
Interictal and ictal EEG.


