Abstract
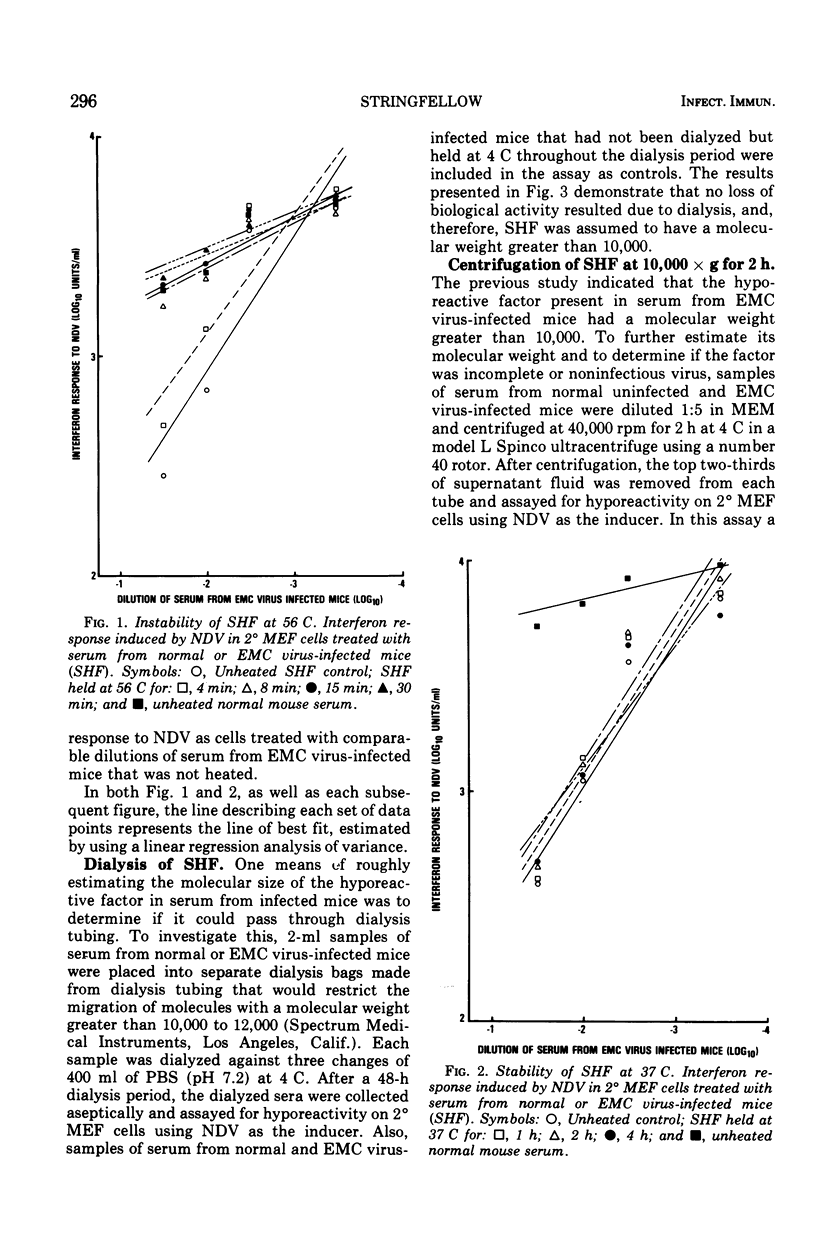
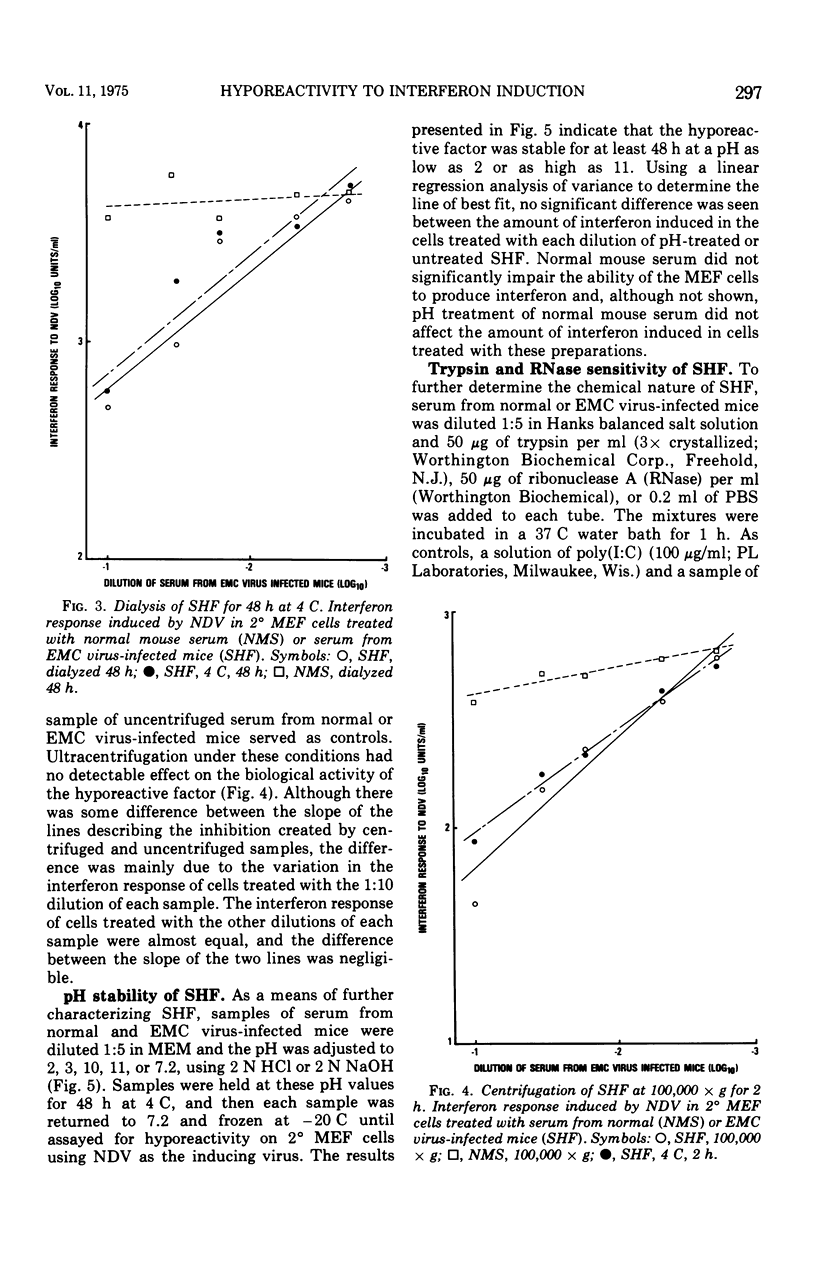
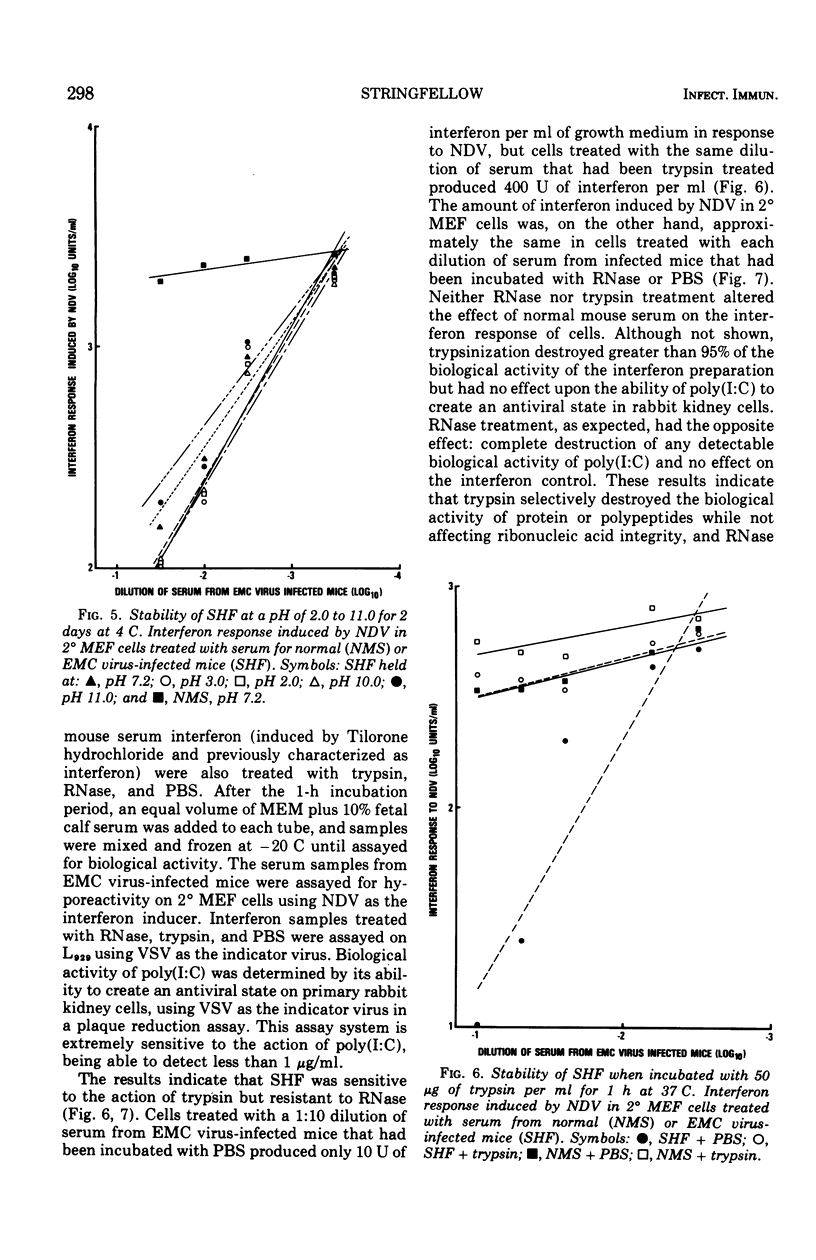
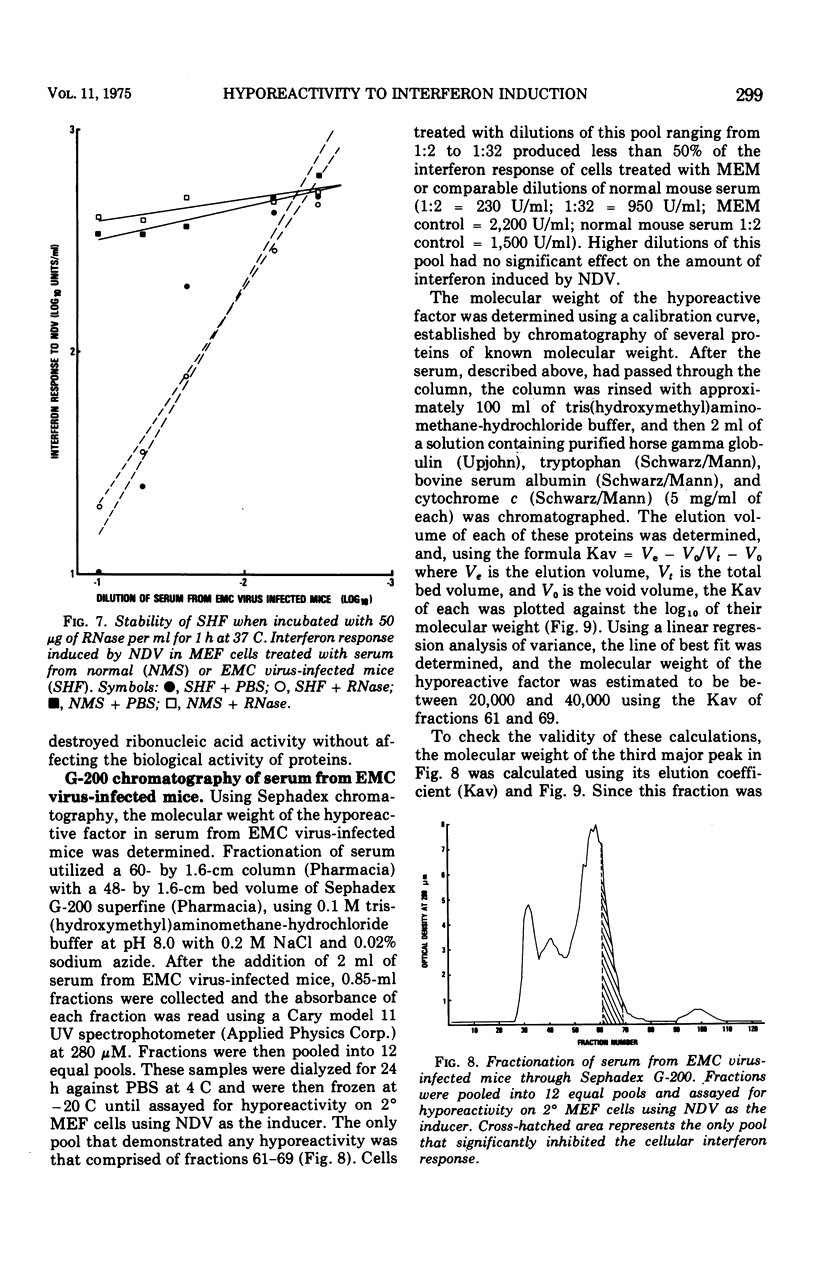
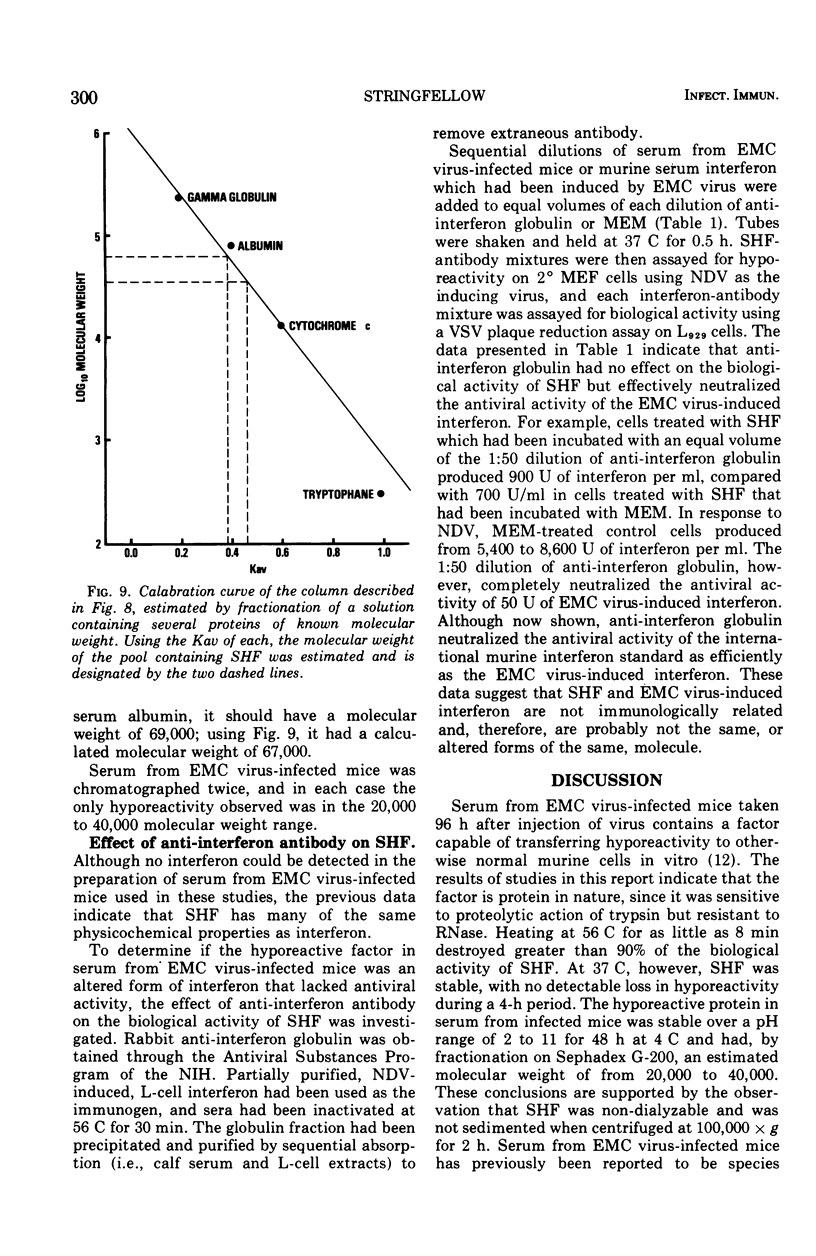
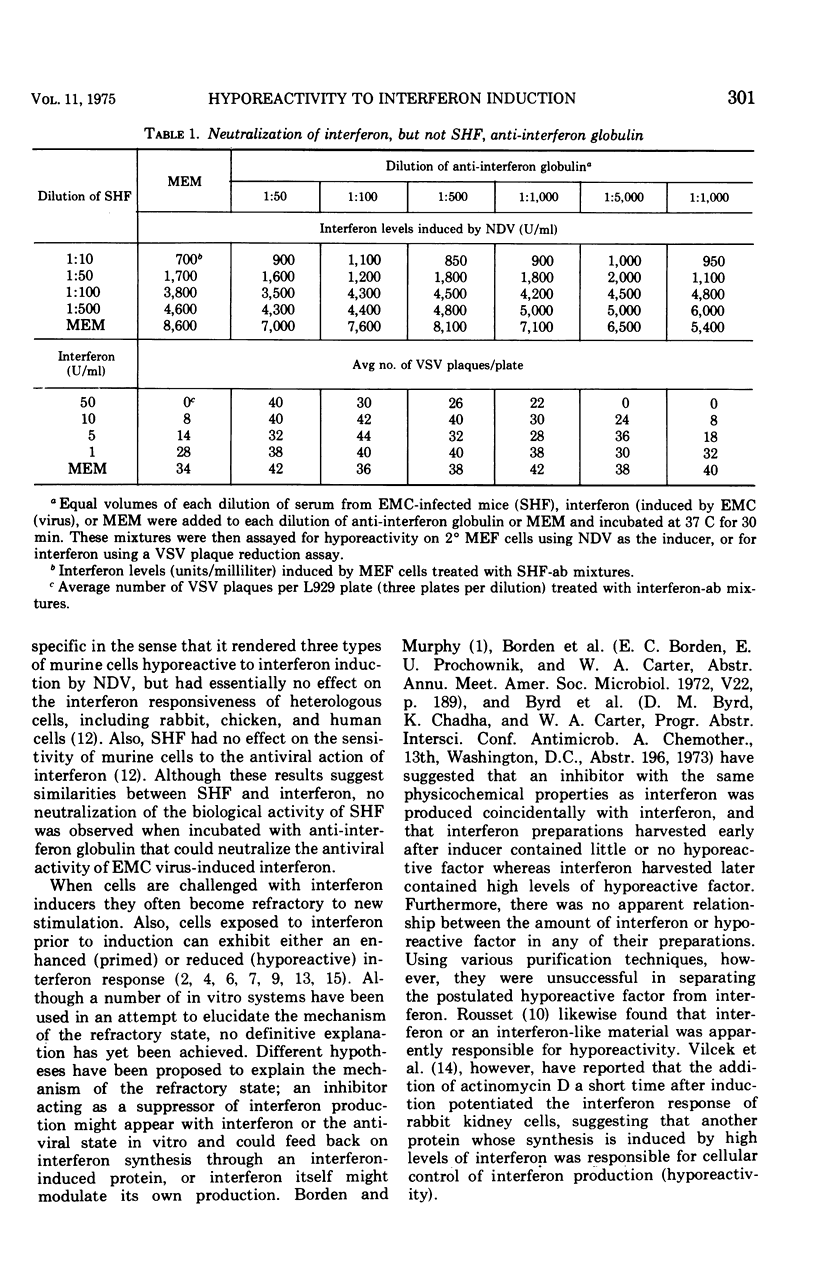
Mice infected with encephalomyocarditis virus develop a severe state of hyporeactivity to interferon induction. One mechanisms possibly responsibile for development of hyporesponsiveness in these animals is a circulating factor which can be detected in their serum 96 h after encephalomyocarditis virus infection (at the time of peak hyporeactivity in vivo). This report describes some of the physiocochemical characteristics of this serum hyporeactive factor (SHF). SHF is a protein with a molecular weight between 20,000 and 40,000 that was extremely labile at 56 C, losing greater than 90% of its biological activity in 8 min, but stable at 37 c for at least 4 h. Hyporeactive factor was also stable over a pH range of 2 to 11 for 48 h at 4 C. These results suggest that SHF is physicochemically similar to interferon. However, no interferon could be detected in the SHF preparation, and no loss in biological activity was observed when the serum factor was incubated with anti-interferon antibody, suggesting that they are separate substances.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURKE D. C., BUCHAN A. INTERFERON PRODUCTION IN CHICK EMBRYO CELLS. I. PRODUCTION BY ULTRAVIOLET-INACTIVATED VIRUS. Virology. 1965 May;26:28–35. doi: 10.1016/0042-6822(65)90022-x. [DOI] [PubMed] [Google Scholar]
- Borden E. C., Murphy F. A. The interferon refractory state: in vivo and in vitro studies of its mechanism. J Immunol. 1971 Jan;106(1):134–142. [PubMed] [Google Scholar]
- De Maeyer-Guignard J. Mouse leukemia: depression of serum interferon production. Science. 1972 Sep 1;177(4051):797–799. doi: 10.1126/science.177.4051.797. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Effect of interferon treatment on interferon production. J Immunol. 1966 May;96(5):872–877. [PubMed] [Google Scholar]
- Holtermann O. A., Havell E. A. Reduced interferon response in mice congenitally infected with lymphocytic choriomeningitis virus. J Gen Virol. 1970 Oct;9(1):101–103. doi: 10.1099/0022-1317-9-1-101. [DOI] [PubMed] [Google Scholar]
- LOCKART R. Z., Jr PRODUCTION OF AN INTERFERON BY L CELLS INFECTED WITH WESTERN EQUINE ENCEPHALOMYELITIS VIRUS. J Bacteriol. 1963 Mar;85:556–566. doi: 10.1128/jb.85.3.556-566.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H. B., Buckler C. E., Baron S. Effect of interferon on early interferon production. Science. 1966 May 27;152(3726):1274–1276. doi: 10.1126/science.152.3726.1274. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Medearis D. N., Jr Suppression of interferon and antibody and multiplication of Newcastle disease virus in cytomegalovirus infected mice. Proc Soc Exp Biol Med. 1967 Feb;124(2):347–353. doi: 10.3181/00379727-124-31740. [DOI] [PubMed] [Google Scholar]
- Paucker K., Boxaca M. Cellular resistance to induction of interferon. Bacteriol Rev. 1967 Jun;31(2):145–156. doi: 10.1128/br.31.2.145-156.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset S. Refractory state of cells to interferon induction. J Gen Virol. 1974 Jan;22(1):9–20. doi: 10.1099/0022-1317-22-1-9. [DOI] [PubMed] [Google Scholar]
- Stringfellow D. A., Glasgow L. A. Hyporeactivity due to infection: recognition of a transferable hyporeactive factor in the serum of encephalomyocarditis virus-infected mice. Infect Immun. 1974 Dec;10(6):1337–1342. doi: 10.1128/iai.10.6.1337-1342.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringfellow D. A., Glasgow L. A. Hyporeactivity of infection: potential limitation to therapeutic use of interferon-inducing agents. Infect Immun. 1972 Nov;6(5):743–747. doi: 10.1128/iai.6.5.743-747.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILCEK J., RADA B. Studies on an interferon from tickborne encephalitis virus-infected cells (IF). III. Antiviral action of IF. Acta Virol. 1962 Jan;6:9–16. [PubMed] [Google Scholar]
- VILCEK J. Studies on an interferon from tick-borne encephalitis virus-infected cells (IF). IV. Comparison of IF with interferon from influenza virus-infected cells. Acta Virol. 1962 Mar;6:144–150. [PubMed] [Google Scholar]
- Vilcek J., Rossman T. G., Varacalli F. Differential effects of actinomycin D and puromycin on the release of interferon induced by double stranded RNA. Nature. 1969 May 17;222(5194):682–683. doi: 10.1038/222682a0. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Hallum J. V. Inhibition of induction of interferon synthesis in L-cells pretreated with interferon. Virology. 1969 Mar;37(3):473–475. doi: 10.1016/0042-6822(69)90231-1. [DOI] [PubMed] [Google Scholar]



