Abstract
BACKGROUND
Growth differentiation factor-15 (GDF-15) is a stress-responsive cytokine produced in cardiovascular cells under conditions of inflammation and oxidative stress, and is emerging as an important prognostic marker in individuals with and without existing cardiovascular disease. Thus, we examined the clinical and genetic correlates of circulating GDF-15 levels, which have not been collectively investigated.
METHODS
A total of 2,991 participants of the Framingham Offspring Study free of clinically overt cardiovascular disease underwent measurement of plasma GDF-15 levels (mean age 59 years, 56% women). Clinical correlates of GDF-15 were examined in multivariable analyses. A genome-wide association study of GDF-15 levels was then conducted, including participants of the Framingham Offspring Study and the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study.
RESULTS
GDF-15 was positively associated with age, smoking, antihypertensive treatment, diabetes, worse kidney function, and non-steroidal anti-inflammatory drug use, but it was negatively associated with total and high-density lipoprotein cholesterol. Clinical correlates accounted for 38% of inter-individual variation in circulating GDF-15, whereas genetic factors account for up to 38% of residual variability (h2=0.38; P=2.5 × 10−11). We identified one genome-wide significant locus, which included the GDF15 gene, on chromosome 19p13.11 associated with GDF-15 concentrations (smallest P=2.74−32 for rs888663). Conditional analyses revealed two independent association signals at this locus (rs888663 and rs1054564), which were associated with altered cis-gene expression in blood cell lines.
CONCLUSIONS
In ambulatory individuals, both cardiometabolic risk factors and genetic factors play an important role in determining circulating GDF-15 concentrations, and contribute similarly to overall variation.
Keywords: Epidemiology, Genetics, Risk factors, Cardiovascular diseases
Growth differentiation factor-15 (GDF-15) is a member of the transforming growth factor-β cytokine superfamily (1). GDF-15 is weakly produced in most tissues under physiologic conditions (2); however, expression in cardiomyocytes, vascular smooth muscle, and endothelial cells is strongly upregulated in response to oxidative stress and inflammation (3). Its prominent anti-apoptotic, anti-hypertrophic, and anti-inflammatory actions in cardiovascular disease models suggest that GDF-15 may play a counter-regulatory role in the context of cardiovascular injury (2, 4, 5). In patients, GDF-15 has been detected in myocardium after ischemia/reperfusion injury, and in atherosclerotic plaques (4, 6).
Elevated GDF-15 concentrations have been associated with adverse prognosis in patients with acute coronary syndromes (7–11) and chronic heart failure (12, 13). More recently, GDF-15 has been associated with surrogate measures of atherosclerosis, cardiovascular events, and overall and cardiovascular mortality in community-dwelling adults (14–16). Though there are limitations of these longitudinal observational studies, GDF-15 appears to be an important biomarker of cardiovascular disease severity, conferring additional prognostic information beyond established clinical risk factors and biomarkers, including natriuretic peptides and C-reactive protein (CRP) (14, 16).
Considering the emerging role of GDF-15 as a prognostic biomarker, we sought to understand its genetic and clinical correlates, which may offer potential insights into the GDF-15 pathway and its relation to cardiovascular disease. While previous studies have highlighted the importance of certain clinical factors that determine GDF-15 concentrations in the community, the individual and collective contribution of genetic factors influencing GDF-15 have not been evaluated in this setting. We hypothesized that circulating GDF-15 levels would be associated with clinical measures of cardiometabolic risk, and that genetic factors would explain a significant portion of the inter-individual variability in GDF-15 levels. To examine genetic correlates, we estimated heritability in a family-based cohort, and conducted a genome-wide association study to explore the association of specific genetic loci and circulating GDF-15 levels.
Methods
STUDY SAMPLES
The Framingham Heart Study (FHS) is a longitudinal observational, community-based cohort initiated in 1948 to prospectively investigate cardiovascular disease and related risk factors. The children (and spouses of the children) of the original cohort, recruited in 1971, have been examined approximately every 4 years since (Framingham Offspring Cohort) (17). GDF-15 levels were measured at the sixth examination of the offspring cohort (1996–1998). Of 3,532 eligible participants, we excluded participants with missing biomarker measurements (n=82), prevalent heart failure (n=38), left ventricular (LV) systolic dysfunction by echocardiography (defined as LV fractional shortening < 0.30 or mild or greater LV systolic dysfunction by visual inspection) (n=302), or missing covariates (n=60). We additionally excluded participants with previous myocardial infarction (n=59) from the clinical correlates analyses, leaving 2,991 participants for this analysis. All participants provided written informed consent and the study protocol was approved by the Institutional Review Board at Boston University Medical Center.
For genetic analyses, we used additional data from 898 individuals enrolled in the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study, a community-based cohort of elderly individuals living in Uppsala, Sweden (Supplemental Data Methods) (15).
CLINICAL ASSESSMENT
All FHS participants underwent a routine medical history, physical examination, and laboratory testing. Blood pressure (BP) was the average of 2 seated measurements. Participants regularly smoking cigarettes during the year before the baseline examination were considered current smokers. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dL, non-fasting blood glucose ≥200 mg/dL, or the use of insulin or oral hypoglycemic medications. Total and high density lipoprotein cholesterol levels were obtained, and LV hypertrophy was defined using previously reported ECG criteria (18). Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation (19). Metabolic syndrome was defined as the presence of ≥3 of the following 5 criteria: waist circumference ≥40 inches in men or ≥35 inches in women; triglycerides ≥150 mg/dL or treatment with a fibrate or niacin; HDL cholesterol <40 mg/dL in men or <50 mg/dL in women; systolic BP ≥130 mm Hg or diastolic BP ≥85 mm Hg or drug treatment for hypertension; or, fasting glucose ≥100 mg/dL or drug treatment for elevated glucose (20).
LABORATORY TESTING
Morning blood samples were collected after an overnight fast, and centrifuged immediately for storage at −70°C. GDF-15 levels in FHS samples were measured with a pre-commercial, automated electrochemiluminescent immunoassay on a Cobas e 411 analyzer (Roche Diagnostics, Mannheim, Germany). The assay has a limit of detection below 10 ng/L, a linear measuring range up to 20,000 ng/L, and an inter-assay imprecision of 2.3% and 1.8% at GDF-15 concentrations of 1,100 ng/L and 17,200 ng/L, respectively. GDF-15 values obtained with the electrochemiluminescent assay correlate closely with the values measured with a previously described immunoradiometric assay (r = 0.980, slope 1.049, intercept −136 ng/L, n = 45 samples with GDF-15 concentrations ranging from 567 to 13,334 ng/L) (21) that was used previously to determine the concentration of GDF-15 in the PIVUS samples (15). High-sensitivity CRP and B-type natriuretic peptide (BNP) were measured as previously described (22).
GENOME-WIDE GENOTYPING AND IMPUTATION
Genotyping in FHS samples was conducted using the Affymetrix 500K mapping array and the Affymetrix 50K gene-focused MIP array. Genotypes from the Affy 500k mapping array were called using Chiamo. For imputation of genotypes to the HapMap set of 2.5 million SNPs (CEU population, release 22, build 36; http://hapmap.org), a hidden Markov model was used as implemented in MACH (version 1.0.15) (23). Genome-wide genotyping in PIVUS was performed using the Illumina HumanOmniExpress BeadChip. Genotypes were called using GenCall implemented in GenomeStudio. Imputation up to the same HapMap reference panel was performed using IMUTEv2 (24).
STATISTICAL ANALYSES
Apparently Healthy Sample (FHS)
In order to examine reference values in a healthy subset of our sample, 1,159 participants without major medical comorbid conditions, including prevalent coronary heart disease, heart failure, atrial fibrillation, diabetes mellitus, hypertension, obesity (body mass index ≥30 kg/m2), valvular heart disease (defined as systolic murmur ≥3/6 in severity or diastolic murmur of any severity), pulmonary disease (FEV1 <lower limit of normal), or serum creatinine ≥2.0 mg/dl. Simple empirical estimates for the 2.5th, 10th, 50th, 90th, and 97.5th percentiles were examined by 10-year age and sex categories.
Clinical Correlates (FHS)
GDF-15 levels were log-transformed due to right-skewed distribution. To examine the association of GDF-15 with clinical covariates, a forward selection linear regression model was used, with P<0.05 for entry. Age and sex were forced into the models; candidate covariates included systolic BP, diabetes mellitus, body mass index, cigarette smoking, total and high-density lipoprotein cholesterol, hypertension treatment, LV hypertrophy, atrial fibrillation, eGFR, and non-steroidal anti-inflammatory drug (NSAID) use. The latter was considered a potential clinical correlate, because NSAIDs are known to induce GDF15 gene expression (25).
In secondary analyses, the association of GDF-15 and the metabolic syndrome was examined in age- and sex-adjusted models. Cardiovascular risk factors were examined in aggregate using the Framingham Cardiovascular Disease (CVD) risk score (26); the association of risk score and GDF-15 levels was examined using age- and sex-adjusted analyses. Generalized estimating equations were used to account for familial correlations in secondary analyses.
Heritability (FHS)
Heritability of GDF-15 was estimated with variance-component models using Sequential Oligogenic Linkage Analysis Routines (27). Heritability estimates were age- and sex-adjusted, and multivariable-adjusted (age, sex, systolic BP, anti-hypertensive medication use, diabetes mellitus, and smoking status).
Genome-wide association study, replication, and meta-analysis (FHS and PIVUS)
The associations of genetic variants and GDF-15 concentrations in FHS were tested with an additive genetic model using linear mixed effects models to accommodate pedigree data (28). We adjusted for age, sex, systolic blood pressure, anti-hypertensive medication use, diabetes mellitus, and smoking status. Results were considered genome-wide significant at P <5 × 10−8. In secondary analyses, we tested the interaction term of NSAID*SNP for genome-wide significant hits within FHS.
A separate genome-wide association study was then performed in PIVUS, in order to replicate FHS findings in an independent cohort and meta-analyze results. Due to differences in GDF-15 distribution between FHS and PIVUS, inverse normal transformation was determined to be most appropriate for meta-analysis of genetic data. Genome-wide association analyses were performed in PIVUS using an additive model and linear regression, adjusted for the same covariates as FHS analyses. Results of both cohorts were meta-analyzed using fixed-effects with inverse variance weighting. Heterogeneity in allelic effects between FHS and PIVUS were assessed by means of Cochran’s Q-statistic. Imputed results were filtered for a minor allele frequency <0.01, and the imputed ratio or info score was examined for quality control, with an acceptable ratio of >0.4. The genomic control parameter lambda was 1.05 in FHS, 1.01 in PIVUS, and 1.03 in the meta-analysis and analyses were therefore not adjusted for population stratification. We conducted conditional analyses, conditioning on each of the most significant GDF-15 SNPs in FHS (rs749451), PIVUS (rs1054564), and the meta-analysis (rs888663). Genome-wide association analyses were performed separately in each study, accounting for each SNP alone, and in combinations of two SNPs, and results meta-analyzed to determine whether genome-wide significant SNPs represented independent signals. Secondary analyses were performed, adjusting for BMI (correlated with GDF-15 levels in PIVUS but not FHS) and also adjusting for two principal components to allow for population structure.
IN SILICO ASSOCIATION OF GENETIC VARIANTS AND CLINICAL TRAITS
The association of three independent genome-wide significant variants (rs888663, rs749451, and rs1054564) and lipid traits was examined in a previously published genome-wide association study of >100,000 individuals of European descent (29). These GDF-15 variants were searched against a collected database of expression SNPs (eSNPs) to examine association with cis-gene expression levels across different tissue types (Supplemental Data Methods).
Results
STUDY SAMPLE
Characteristics of the overall sample of 2,991 FHS participants are shown in Table 1. The mean age was 59 years, and 56% were women. The median GDF-15 level (25th to 75th percentiles) in the general FHS sample was 1,020 ng/L (803–1,362) in men and 1,017 ng/L (809–1,297) in women.
Table 1.
Characteristics of 2,991 FHS and 898 PIVUS participants
| FHS | PIVUS | |||
|---|---|---|---|---|
| Men (n=1,316) | Women (n=1,675) | Men (n=437) | Women (n=450) | |
| Age, years | 59 (10) | 59 (10) | 70 (0.2) | 70 (0.2) |
| Systolic blood pressure, mmHg | 130 (17) | 127 (20) | 146 (22) | 153 (23) |
| Diastolic blood pressure, mmHg | 78 (9) | 74 (9) | 79 (10) | 78 (10) |
| Body-mass index, kg/m2 | 28 (4) | 27 (6) | 27 (4) | 27 (5) |
| Diabetes mellitus, % | 11 | 9 | 12 | 9 |
| Anti-hypertensive treatment, % | 28 | 24 | 29 | 30 |
| Smoker, % | 15 | 16 | 11 | 13 |
| Total cholesterol, mg/dl | 200 (36) | 212 (38) | 200 (37) | 222 (37) |
| HDL cholesterol, mg/dl | 44 (12) | 58 (16) | 54 (14) | 64 (16) |
| GDF-15, ng/L | 1,180 (637) | 1,153 (615) | 1,257 (421) | 1,162 (390) |
| eGFR, ml/min/1.73m2 | 91 (42) | 89 (43) | 84 (21) | 78 (21) |
| NSAID use, % | 9 | 13 | n.a. | n.a. |
Values are mean (standard deviation) unless otherwise indicated.
FHS, Framingham Heart Study; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors study; HDL, high-density lipoprotein; GDF-15, growth differentiation factor-15; eGFR, estimated glomerular filtration rate; NSAID, non-steroidal anti-inflammatory drug; n.a., not available
GDF-15 concentrations in the subset of apparently healthy individuals free of major medical comorbidities (n=1,159), shown in Table 2, varied with age but not by sex. The median GDF-15 concentration was 901 ng/L (744–1,136). For men aged 30–39 years, the median and 90th percentile of GDF-15 were 651 and 1,197 ng/L, whereas for men aged 70–79 years, the corresponding values were 1,389 and 2,030 ng/L, respectively. Within the apparently healthy sample, 21% had a GDF-15 level above the previously established upper reference limit of >1,200 ng/L (21).
Table 2.
GDF-15 concentrations (ng/L) in 1,159 apparently healthy FHS participants without major cardiovascular, renal, or pulmonary disease
| Age | Percentile | ||||||
|---|---|---|---|---|---|---|---|
| Group (years) |
n | 2.5th | 10th | 50th | 90th | 97.5th | |
| Men | 30–39 | 19 | 473 | 483 | 651 | 1,197 | 1,498 |
| 40–49 | 121 | 535 | 571 | 726 | 1,081 | 1,250 | |
| 50–59 | 197 | 574 | 655 | 863 | 1,414 | 1,838 | |
| 60–69 | 104 | 723 | 805 | 1,115 | 1,711 | 2,197 | |
| 70–79 | 27 | 893 | 1,084 | 1,389 | 2,030 | 5,006 | |
| Women | 30–39 | 14 | 520 | 543 | 745 | 1,007 | 1,085 |
| 40–49 | 180 | 488 | 561 | 762 | 1,172 | 1,574 | |
| 50–59 | 275 | 557 | 668 | 890 | 1,269 | 1,786 | |
| 60–69 | 167 | 652 | 784 | 1,025 | 1,553 | 1,833 | |
| 70–79 | 55 | 845 | 939 | 1,277 | 1,717 | 2,562 | |
CLINICAL CORRELATES OF GDF-15 IN FHS
In multivariable analyses, GDF-15 levels were similar in men and women. GDF-15 was positively associated with age, diabetes, antihypertensive treatment, smoking, and NSAID use, but negatively associated with total cholesterol, HDL cholesterol, and eGFR (Table 3). The R2 for this model was 0.38.
Table 3.
Clinical correlates of GDF-15 in 2,991 FHS participants
| Clinical Characteristic | estimated coefficient |
s.e. | P Value |
|---|---|---|---|
| Age, per 10 years | 0.211 | 0.006 | <0.0001 |
| Sex, men vs women | 0.006 | 0.013 | 0.64 |
| Diabetes, yes vs no | 0.142 | 0.020 | <0.0001 |
| Hypertension treatment, yes vs no | 0.073 | 0.014 | <0.0001 |
| Smoking, yes vs no | 0.220 | 0.016 | <0.0001 |
| Total cholesterol, per 38 mg/dl | −0.028 | 0.006 | <0.0001 |
| HDL cholesterol, per 16 mg/dl | −0.024 | 0.007 | 0.0003 |
| eGFR, per 42 ml/min/1.73m2 | −0.032 | 0.006 | <0.0001 |
| NSAID use, yes vs no | 0.050 | 0.018 | 0.006 |
The regression coefficients indicate the increase in log-GDF-15 in the presence vs absence of the trait for dichotomous variables, and per 1 standard deviation increase as noted in continuous variables. The following variables were not significant in the forward selection model (P > 0.05): systolic blood pressure, body-mass index, left ventricular hypertrophy, and atrial fibrillation.
s.e., standard error; HDL, high-density lipoprotein; eGFR, estimated glomerular filtration rate; NSAID, non-steroidal anti-inflammatory drug;
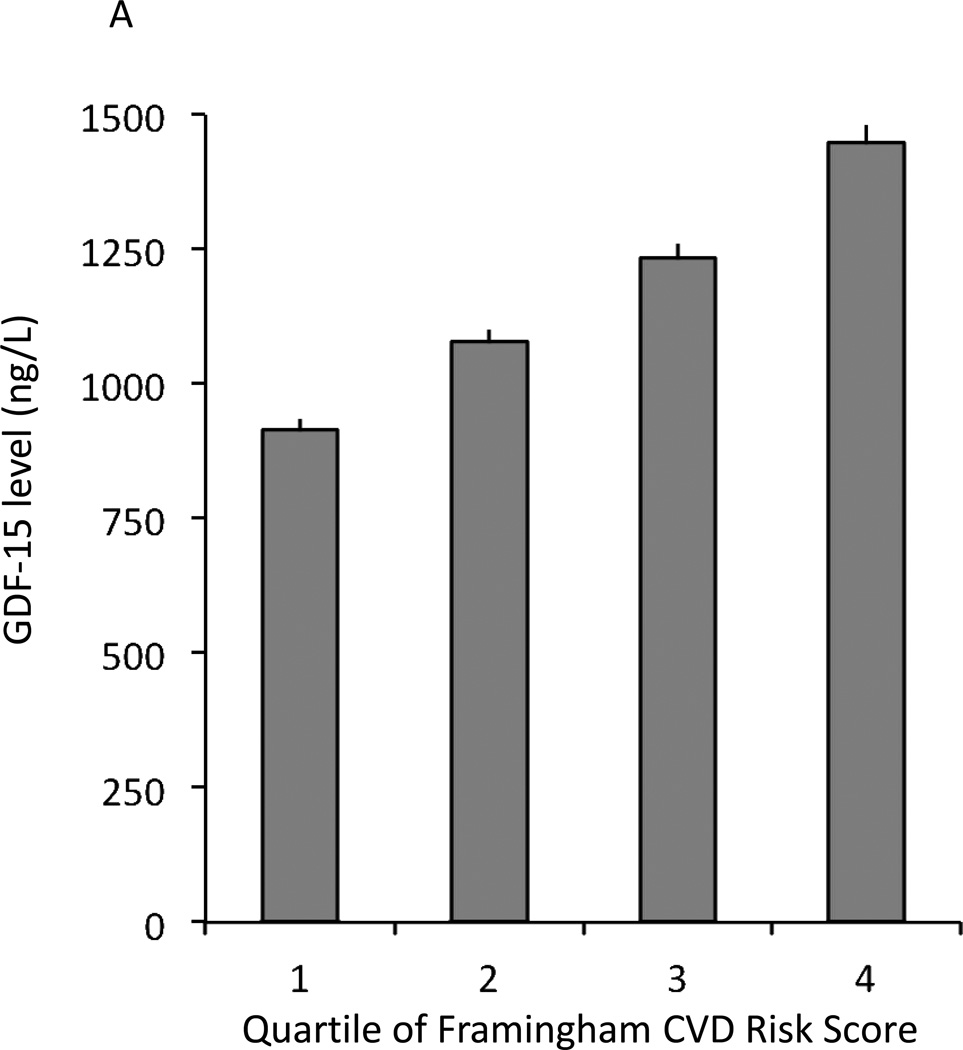
In secondary analyses, GDF-15 levels were significantly associated with the metabolic syndrome in age- and sex-adjusted analyses (P<0.0001). When examining cardiovascular risk factors in aggregate using the Framingham CVD risk score (26), GDF-15 levels increased across risk score quartiles (Figure 1A) and GDF-15 levels were positively correlated with the risk score in age- and sex-adjusted analyses (P<0.0001).
Figure 1.
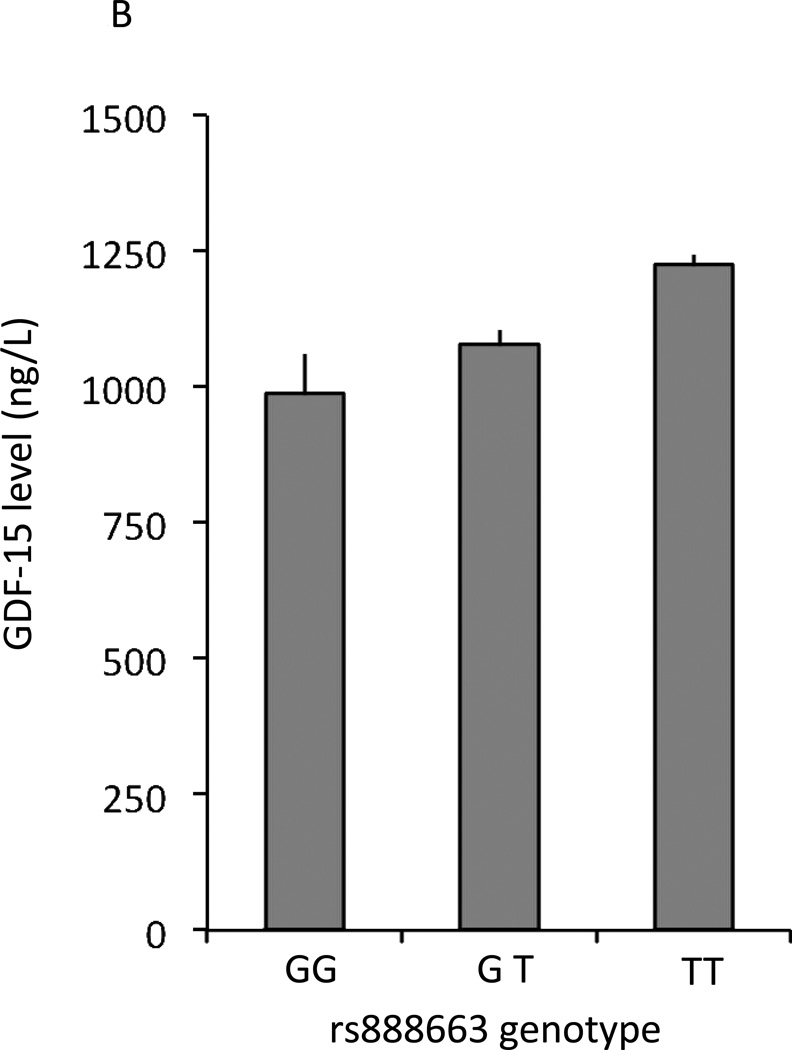
Association of mean levels of circulating GDF-15 and clinical and genetic determinants. Error bars in each panel represent standard errors. A. Circulating mean levels of GDF-15 by Framingham risk score quartiles. B. Circulating GDF-15 levels by rs888663 genotype. GDF-15 levels were estimated (back-transformed from log-transformed data) and estimated allele frequency within FHS, error bars represent standard errors.
In secondary analyses, the positive association of NSAID use and GDF-15 levels appeared to be independent of inflammation, as the correlation persisted within apparently healthy individuals (P=0.007), after exclusion of participants with inflammatory arthritis (P=0.01), and after adjustment for CRP (P=0.01). Secondary analyses adjusting for relatedness within our sample using GEE models did not appreciably change the results. Inclusion of high-sensitivity troponin I (hsTnI), BNP, or CRP in the clinical correlates analysis did not materially change our findings (Supplemental Data Table 1), although all three biomarkers were modestly correlated with GDF-15 levels (hsTnI: r=0.19, P<0.0001, BNP: r=0.26, P<0.0001; CRP: r=0.25, P<0.0001).
GENETIC CORRELATES OF GDF-15
The age- and sex-adjusted heritability of GDF-15 in FHS was 0.38 (P=2.5 × 10−11) and the multivariable-adjusted heritability was 0.30 (P=4.8 × 10−8).
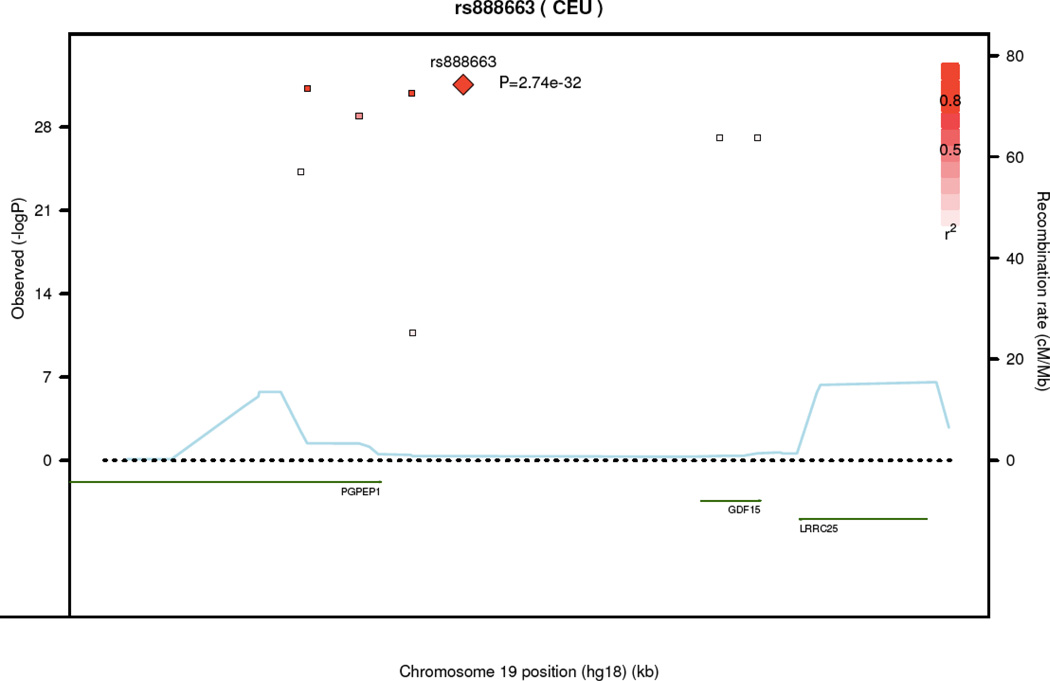
Nine SNPs had genome-wide significant associations (P<5 × 10−8) in FHS (Table 4, details in Supplemental Data Table 2). These SNPs were located in noncoding regions near GDF15/PGPEP1 (pyroglutamyl-peptidase 1) (pairwise linkage disequilibrium in Supplemental Data Table 3). The most significantly associated SNP (rs749451) had a P value of 8.1 × 10−25 and explained 2.5% of the residual phenotypic variance in GDF-15 levels. Three of these nine SNPs also had genome-wide associations with GDF-15 in the PIVUS sample (rs1054564, rs1227731, rs3195944). After meta-analysis, we found eight genome-wide significant SNPs near GDF15, with the most significantly associated SNP being rs888663 (P=2.7 × 10−32). GDF-15 concentrations by rs888663 genotype are displayed in Figure 1B. The regional association plot in Figure 2 demonstrates that all genome-wide significant SNPs were well within 100 kb of the GDF15 gene.
Table 4.
Genome-wide significant variants associated with GDF-15 in FHS and PIVUS*
| Chr | SNP | Position (NCBI 36.3) |
Location Relative to gene |
Nearest gene(s) |
Allele (major/ minor) |
MAF | FHS P |
PIVUS P |
Meta- analysis P |
|---|---|---|---|---|---|---|---|---|---|
| 19 | rs888663 | 18345922 | downstream | PGPEP1 | T/G | 0.19 | 2.54E-24 | 1.01E-03 | 2.74E-32 |
| 19 | rs3746181 | 18338017 | 3' UTR | PGPEP1 | G/A | 0.19 | 7.61E-24 | 4.08E-04 | 6.22E-32 |
| 19 | rs1363120 | 18343304 | downstream | PGPEP1 | G/C | 0.19 | 6.82E-24 | 9.23E-04 | 1.50E-31 |
| 19 | rs749451 | 18340647 | 3' UTR | PGPEP1 | C/T | 0.43 | 8.09E-25 | 5.17E-02 | 1.21E-29 |
| 19 | rs1054564 | 18360815 | 3' UTR | GDF15 | G/C | 0.14 | 7.98E-15 | 2.49E-13 | 8.17E-28 |
| 19 | rs1227731 | 18358903 | intronic | GDF15 | G/A | 0.14 | 8.57E-15 | 2.53E-13 | 8.22E-28 |
| 19 | rs3195944 | 18337711 | 3' UTR | PGPEP1 | A/G | 0.13 | 2.28E-14 | 2.24E-09 | 6.16E-25 |
| 19 | rs17725099 | 18343358 | downstream | PGPEP1 | G/A | 0.24 | 2.59E-13 | 1.74E-01 | 1.91E-11 |
| 19 | rs1043063 | 18341171 | 3' UTR | PGPEP1 | C/T | 0.35 | 4.58E-09 | 4.29E-01 | 3.12E-06 |
See Supplemental Data Table 2 for estimated coefficients/standard errors, Supplemental Data Table 3 for pairwise linkage disequilibrium between SNPs, Supplemental Data Figure 1 for quantile-quantile plot, and Supplemental Data Figure 2 for Manhattan plot of the genome-wide association study.
MAF indicates minor allele frequency; FHS, Framingham Heart Study; PIVUS, Prospective Investigation of the Vasculature in Uppsala Seniors study; UTR, untranslated region; PGPEP1, pyroglutamyl peptidase-I;
Figure 2.
Regional association plot of meta-analysis loci associated with circulating GDF-15 levels.
Secondary analyses adjusting for the first two principal components did not substantively alter meta-analysis results (Supplemental Data Table 4), and adjustment for BMI did not meaningfully change results. There was no statistically significant interaction between NSAID use or eGFR and the results for the genome-wide significant loci within FHS (P>0.05 for all). Adjustment for eGFR did not attenuate heritability estimates (h2 0.36, P=1.0×10−9) or genome-wide significant associations.
In conditional analyses accounting for rs888663, rs749451, and rs1054564, we observed two potentially independent signals: one signal from rs888663/rs749451 (r2 = 0.43 for the SNPs with each other), and one from rs1054564 (Supplemental Data Table 5). When conditioned on these two signals, no further SNPs reached genome-wide significance. Although the directionality of association between SNP genotype and GDF-15 concentrations was consistent between studies, there was nominal evidence (Cochran’s Q-statistic P<0.05) of heterogeneity in allelic effects between FHS and PIVUS results, despite similar allele frequencies between the two populations (see Supplemental Data Table 2 for allele frequencies; rs888663, P=0.04; rs749451, P=0.0005; rs1054564, P=0.001).
Putative functional variants
All three SNPs associated with GDF-15 (rs888663, rs749451 and rs1054564) were associated with cis-gene expression in lymphocyte, monocyte, and adipose tissue cell lines (30–32). Specifically, rs749451 and rs888663 were associated with PGPEP1 expression (P=1.32 × 10−11; P=4.86 × 10−4, respectively), a gene coding for pyroglutamyl peptidase I, residing on chromosome 9p13.11 abutting GDF15. Both rs1054564 and rs888663 were associated with cis-expression of LRRC25 also on 9p13.11 (leucine rich repeat containing 25, P=3.61 × 10−66 and P=7.81 × 10−7, respectively). In addition, rs1054564 was associated with trans-expression of CRLF2 (cytokine receptor-like factor 2, chromosome Xp22.3, P=1.61 × 10−11) and LRRC31 (leucine rich repeat containing 31, chromosome 3q26.2, P=2.87 × 10−11).
In silico association with clinical traits
Given the cross-sectional association of GDF-15 and both total and HDL cholesterol, an in silico investigation of the two independent GDF-15 variants was pursued within a published genome-wide association study of lipid traits (29). Our genome-wide significant GDF-15 SNP rs1054564 was associated with HDL cholesterol (P=0.025). Specifically, the allele related to higher GDF-15 concentrations was associated with lower HDL cholesterol, consistent with the association found in the clinical correlates of GDF-15.
Discussion
In this study, we report the clinical and genetic correlates of GDF-15 in the community. Our findings demonstrate that higher circulating GDF-15 levels are associated with increasing cardiometabolic risk factors in individuals without overt cardiovascular disease. Our findings also suggest that genetic factors play an important role in determining GDF-15 concentrations. Additive genetic effects may explain up to 38% of the phenotypic variation in GDF-15 concentrations, which is comparable to the proportion of variability explained by clinical factors. In genome-wide association studies, specific variants near the GDF15 gene on chromosome 19p13.11 were strongly associated with plasma GDF-15 concentrations. Furthermore, GDF-15 variants were associated with gene expression in published databases.
In experimental studies, GDF-15 is expressed in human atherosclerotic plaque (6, 33) and cardiac myocytes after an ischemic insult (4). Under these conditions, GDF-15 appears to protect against cardiac injury via anti-inflammatory (5), anti-apoptotic (4), and anti-hypertrophic pathways (2). Clinical studies in individuals with existing cardiovascular disease (7–13) and in community-based populations (14–16) have largely shown higher GDF-15 levels to be associated with adverse outcomes, although it remains unclear whether GDF-15 is a mediator or marker of cardiovascular disease. It may be that GDF-15 is similar to the natriuretic peptides, which have protective biological effects, but are elevated in individuals at risk for cardiovascular disease, presumably reflecting a response to increased hemodynamic stress (35). Accordingly, understanding the clinical and genetic correlates of GDF-15 may elucidate pathways underlying the association of GDF-15 and cardiovascular disease.
CLINICAL CORRELATES OF GDF-15
Similar to previous studies (21), we found a strong association with higher GDF-15 concentrations and older age, which was quite pronounced even in apparently healthy adults in our study. Using the previously studied upper reference limit of 1,200 ng/L (21), less than 10% of adults aged 40–49 years met criteria for ‘abnormal’ GDF-15 levels. In contrast, more than 50% of apparently healthy adults aged 70–79 years were classified as having an elevated GDF-15 level using the same cut-off. This marked increase in GDF-15 levels with older age may be related to a higher burden of subclinical cardiovascular disease even within an ostensibly healthy population. GDF-15 levels have also been elevated in a number of advanced cancers (36), but it is less like that occult malignancies could be related to the age-related increase in GDF-15 concentrations. Lastly, due to reduced renal clearance (21), age-related declines in kidney function may result in higher GDF-15 concentrations, although age was an independent predictor even after adjustment for eGFR in our study. In considering the potential clinical utility of GDF-15 as a biomarker, it will be important to account for the robust effect of age on GDF-15 concentrations, an effect greater than any other clinical trait in apparently healthy community-dwelling adults.
We found no gender difference in GDF-15 levels in contrast to prior studies examining populations of acute coronary syndrome,(8, 10, 11, 37–39) heart failure,(13) and older community-based cohorts(14, 15) which demonstrated higher GDF-15 levels in men compared with women. It is possible that gender differences in cardiovascular disease severity or subtype may have contributed to previously observed differences in GDF-15 levels.
We observed a strong association between higher GDF-15 levels and cardiometabolic risk factors, including diabetes, hypertension, smoking, and low HDL, which precedes the onset of overt cardiovascular disease. Our findings are similar to those in older participants of the PIVUS study (15), as well as the Rancho Bernardo Study (14). Although the mechanisms by which GDF-15 may modulate risk are not well understood, recent animal and clinical studies have shown that GDF-15 is expressed in adipocytes and may act as an adipokine (40). Circulating GDF-15 has been associated with insulin resistance in obese individuals (41). Interestingly, higher GDF-15 levels were associated with lower total and LDL cholesterol in the community, as others have found (14, 15). Experimental data suggest that oxidized LDL can induce GDF-15 in atherosclerotic lesions (6), and it may be that different LDL subtypes are differentially associated with GDF-15.
Lastly, we show that NSAID use is associated with higher circulating GDF-15 levels, an association which has not previously been described. GDF-15 is also known as NSAID-activated gene, and its expression is induced by NSAIDs in experimental studies, a process that appears to be independent of cyclo-oxygenase or prostanoids (25). Inflammatory conditions such as rheumatoid arthritis have been associated with elevations in GDF-15 (42), but the association of NSAID use and GDF-15 levels persisted even after exclusion of individuals with rheumatoid arthritis and adjustment for CRP as a marker of inflammation.
GENETIC CORRELATES OF GDF-15
Our study is the first study to report the heritability of GDF-15, and suggests that circulating levels of GDF-15 are at least in part genetically determined. We conducted a meta-analysis of 2 independent community-based cohorts, and report 8 SNPs in the region of the GDF-15 gene that are associated with circulating levels on a genome-wide significant basis, two of which appear to be independent signals in conditional analyses. A missense variant in the promoter region in pairwise linkage disequilibrium with one of our genome-wide significant loci (rs4808793 and rs1054564, respectively, r2 0.40) has previously been associated with increased transcriptional activity and higher circulating GDF-15 levels, as well as favorable echocardiographic traits in hypertensive Chinese individuals (43). Two of the genome-wide significant SNPs in our analysis (rs888663 and rs1054564) have previously been associated with circulating GDF-15 levels in a cohort of 1,442 prostate cancer patients (44), further lending support to our findings.
Although we show that genetic factors play an important role in circulating GDF-15 levels, the exact mechanism by which the two potentially independent signals identified modulate GDF-15 expression remains unknown. We observed nominal evidence of heterogeneity in the effect of genetic variants between the two studies, which could potentially be attributed to clinical differences in age and cardiovascular risk factors. Alternatively, this pattern of heterogeneity in allelic effects and independent association signals could reflect different underlying unobserved rare causal variants in the two populations. Further studies are needed to explore other environmental and genetic factors that might explain this heterogeneity. Interestingly, three genome-wide significant GDF-15 variants were strongly associated with cis-gene expression of PGPEP1 and LRRC25, genes within 100kb of GDF15. There is no known relationship between PGPEP1, LRRC25, and GDF-15. Because these genetic variants are intergenic, it may be that they upregulate expression via promoter or other distal elements, that may affect GDF-15 expression itself.
One notable finding was that the C allele of one of the GDF-15 loci (rs1054561) was associated with both higher GDF-15 and lower HDL cholesterol levels. It may be that genetically elevated GDF-15 levels could lead to lower HDL, however further prospective studies are needed to explore this association.
LIMITATIONS
Several limitations deserve mention. In our apparently healthy sample, GDF-15 concentrations were slightly higher than those reported by Kempf and colleagues, who studied a sample of elderly Swedish individuals. Several aspects of the Framingham cohort support the generalizability of our findings, including the community-based design and the rigorous characterization of participants who have been followed since the early 1970's. We cannot exclude the possibility that laboratory variation, unmeasured differences in the study cohorts, and/or residual, unmeasured disease contributed to differences in study findings. The cross-sectional nature of our study limits inferences of causality with respect to clinical correlates; thus, GDF-15 may both contribute to, and be a marker of cardiometabolic risk. While we were able to account for many measured clinical factors, cross-sectional associations may also be subject to residual confounding. With regard to genetic findings, the mechanism by which genetic variants identified in our study influence circulating GDF-15 levels is unknown. Future studies involving larger sample sizes may be able to identify genetic variants outside of the GDF15 locus that may play an important role in determining GDF-15 levels. Lastly, our sample consisted of white participants of European ancestry, limiting the generalizability of our findings to other racial and ethnic groups.
CONCLUSION
In summary, we demonstrate that GDF-15 is associated with cardiometabolic risk factors, and that genetic factors play an important role in determining GDF-15 concentrations. Importantly, using two independent community-based cohorts, we identified genetic variants in the GDF15 gene region on chromosome 19p13.11 that influenced circulating GDF-15 levels. Two GDF15 variants were associated with altered gene expression in different blood cell lines, and one was associated with lower HDL levels. Further studies are required to elucidate how genetic factors regulate GDF-15 expression, and how these mechanisms relate ultimately to the development of cardiovascular disease.
Supplementary Material
ACKNOWLEDGMENTS
None
Abbreviations
- GDF-15
growth differentiation factor-15
- PIVUS
Prospective Investigation of the Vasculature in Uppsala Seniors
- CRP
C-reactive protein
- FHS
Framingham Heart Study
- LV
left ventricular
- BP
blood pressure
- eGFR
estimated glomerular filtration rate
- BNP
B-type natriuretic peptide
- NSAID
non-steroidal anti-inflammatory drug
- CVD
cardiovascular disease
- eSNPs
expression SNPs
Footnotes
Human Genes: GDF15, growth differentiation factor 15; PGPEP1, pyroglutamyl-peptidase I; LRRC25, leucine rich repeat containing 25; CRLF2, cytokine receptor-like factor 2; LRRC31, leucine rich repeat containing 31;
References
- 1.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. Mic-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, et al. Gdf15/mic-1 functions as a protective and antihypertrophic factor released from the myocardium in association with smad protein activation. Circ Res. 2006;98:342–350. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 3.Kempf T, Wollert KC. Growth-differentiation factor-15 in heart failure. Heart Fail Clin. 2009;5:537–547. doi: 10.1016/j.hfc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–360. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 5.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, et al. Gdf-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med. 2011;17:581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 6.Schlittenhardt D, Schober A, Strelau J, Bonaterra GA, Schmiedt W, Unsicker K, et al. Involvement of growth differentiation factor-15/macrophage inhibitory cytokine-1 (gdf-15/mic-1) in oxldl-induced apoptosis of human macrophages in vitro and in arteriosclerotic lesions. Cell Tissue Res. 2004;318:325–333. doi: 10.1007/s00441-004-0986-3. [DOI] [PubMed] [Google Scholar]
- 7.Bonaca MP, Morrow DA, Braunwald E, Cannon CP, Jiang S, Breher S, et al. Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: Observations from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol. 2011;31:203–210. doi: 10.1161/ATVBAHA.110.213512. [DOI] [PubMed] [Google Scholar]
- 8.Kempf T, Bjorklund E, Olofsson S, Lindahl B, Allhoff T, Peter T, et al. Growth-differentiation factor-15 improves risk stratification in st-segment elevation myocardial infarction. Eur Heart J. 2007;28:2858–2865. doi: 10.1093/eurheartj/ehm465. [DOI] [PubMed] [Google Scholar]
- 9.Khan SQ, Ng K, Dhillon O, Kelly D, Quinn P, Squire IB, et al. Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur Heart J. 2009;30:1057–1065. doi: 10.1093/eurheartj/ehn600. [DOI] [PubMed] [Google Scholar]
- 10.Eggers KM, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Jantzen F, et al. Growth-differentiation factor-15 for long-term risk prediction in patients stabilized after an episode of non-st-segment-elevation acute coronary syndrome. Circ Cardiovasc Genet. 2010;3:88–96. doi: 10.1161/CIRCGENETICS.109.877456. [DOI] [PubMed] [Google Scholar]
- 11.Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, et al. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007;115:962–971. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]
- 12.Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 13.Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, et al. Serial measurement of growth-differentiation factor-15 in heart failure: Relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation. 2010;122:1387–1395. doi: 10.1161/CIRCULATIONAHA.109.928846. [DOI] [PubMed] [Google Scholar]
- 14.Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett-Connor E. Growth-differentiation factor-15 is a robust, independent predictor of 11-year mortality risk in community-dwelling older adults: The Rancho Bernardo Study. Circulation. 2011;123:2101–2110. doi: 10.1161/CIRCULATIONAHA.110.979740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: Results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Eur Heart J. 2009;30:2346–2353. doi: 10.1093/eurheartj/ehp261. [DOI] [PubMed] [Google Scholar]
- 16.Rohatgi A, Patel P, Das SR, Ayers CR, Khera A, Martinez-Rumayor A, et al. Association of growth differentiation factor-15 with coronary atherosclerosis and mortality in a young, multiethnic population: Observations from the Dallas Heart Study. Clin Chem. 2012;58:172–182. doi: 10.1373/clinchem.2011.171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 18.Desai AS, Toto R, Jarolim P, Uno H, Eckardt K-U, Kewalramani R, et al. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. Am J Kidney Dis. 2011;58:717–728. doi: 10.1053/j.ajkd.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 21.Kempf T, Horn-Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, et al. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007;53:284–291. doi: 10.1373/clinchem.2006.076828. [DOI] [PubMed] [Google Scholar]
- 22.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Abecasis GR. Mach 1.0: Rapid haplotype reconstruction and missing genotype inference. Am J Hum Genet. 2006:2290. [Google Scholar]
- 24.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baek SJ, Wilson LC, Lee C-H, Eling TE. Dual function of nonsteroidal anti-inflammatory drugs (NSAIDs): Inhibition of cyclooxygenase and induction of nsaid-activated gene. J Pharmacol Exp Ther. 2002;301:1126–1131. doi: 10.1124/jpet.301.3.1126. [DOI] [PubMed] [Google Scholar]
- 26.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 27.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen MH, Yang Q. Gwaf: An r package for genome-wide association analyses with family data. Bioinformatics. 2010;26:580–581. doi: 10.1093/bioinformatics/btp710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goring HH, Curran JE, Johnson MP, Dyer TD, Charlesworth J, Cole SA, et al. Discovery of expression qtls using large-scale transcriptional profiling in human lymphocytes. Nat Genet. 2007;39:1208–1216. doi: 10.1038/ng2119. [DOI] [PubMed] [Google Scholar]
- 31.Zeller T, Wild P, Szymczak S, Rotival M, Schillert A, Castagne R, et al. Genetics and beyond--the transcriptome of human monocytes and disease susceptibility. PLoS One. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenawalt DM, Dobrin R, Chudin E, Hatoum IJ, Suver C, Beaulaurier J, et al. A survey of the genetics of stomach, liver, and adipose gene expression from a morbidly obese cohort. Genome Res. 2011;21:1008–1016. doi: 10.1101/gr.112821.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Jager SC, Bermudez B, Bot I, Koenen RR, Bot M, Kavelaars A, et al. Growth differentiation factor 15 deficiency protects against atherosclerosis by attenuating ccr2-mediated macrophage chemotaxis. J Exp Med. 2011;208:217–225. doi: 10.1084/jem.20100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taddei S, Virdis A. Growth differentiation factor-15 and cardiovascular dysfunction and disease: Malefactor or innocent bystander? European heart journal. 2010;31:1168–1171. doi: 10.1093/eurheartj/ehq077. [DOI] [PubMed] [Google Scholar]
- 35.Ho JE, Wang TJ. Growth differentiation factor 15: A canary in a coal mine? Clinical chemistry. 2012;58:3–5. doi: 10.1373/clinchem.2011.175406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauskin AR, Brown DA, Kuffner T, Johnen H, Luo XW, Hunter M, Breit SN. Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res. 2006;66:4983–4986. doi: 10.1158/0008-5472.CAN-05-4067. [DOI] [PubMed] [Google Scholar]
- 37.Eggers KM, Kempf T, Allhoff T, Lindahl B, Wallentin L, Wollert KC. Growth-differentiation factor-15 for early risk stratification in patients with acute chest pain. Eur Heart J. 2008;29:2327–2335. doi: 10.1093/eurheartj/ehn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kempf T, Sinning J-M, Quint A, Bickel C, Sinning C, Wild PS, et al. Growth-differentiation factor-15 for risk stratification in patients with stable and unstable coronary heart disease: Results from the AtheroGene study. Circ Cardiovasc Genet. 2009;2:286–292. doi: 10.1161/CIRCGENETICS.108.824870. [DOI] [PubMed] [Google Scholar]
- 39.Wollert KC, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Allhoff T, et al. Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST-elevation acute coronary syndrome. Circulation. 2007;116:1540–1548. doi: 10.1161/CIRCULATIONAHA.107.697714. [DOI] [PubMed] [Google Scholar]
- 40.Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, Bing C. Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology. 2009;150:1688–1696. doi: 10.1210/en.2008-0952. [DOI] [PubMed] [Google Scholar]
- 41.Vila G, Riedl M, Anderwald C, Resl M, Handisurya A, Clodi M, et al. The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clin Chem. 2011;57:309–316. doi: 10.1373/clinchem.2010.153726. [DOI] [PubMed] [Google Scholar]
- 42.Brown DA, Moore J, Johnen H, Smeets TJ, Bauskin AR, Kuffner T, et al. Serum macrophage inhibitory cytokine 1 in rheumatoid arthritis: A potential marker of erosive joint destruction. Arthritis Rheum. 2007;56:753–764. doi: 10.1002/art.22410. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Yang X, Sun K, Chen J, Song X, Wang H, et al. The haplotype of the growth-differentiation factor 15 gene is associated with left ventricular hypertrophy in human essential hypertension. Clin Sci (Lond) 2010;118:137–145. doi: 10.1042/CS20080637. [DOI] [PubMed] [Google Scholar]
- 44.Brown DA, Lindmark F, Stattin P, Balter K, Adami HO, Zheng SL, et al. Macrophage inhibitory cytokine 1: A new prognostic marker in prostate cancer. Clin Cancer Res. 2009;15:6658–6664. doi: 10.1158/1078-0432.CCR-08-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.