Abstract
Activation of the innate immune system by DNA containing hypomethylated CpG motifs has been implicated in the pathogenesis of systemic lupus erythematosus (SLE). Here, we examined the consequences of immunostimulatory CpG-oligodeoxynucleotide (ODN) and inhibitory GpG-ODN treatment in the NZB × NZW F1 (NZB/W) murine model of SLE. Beginning at 5 months of age, we administered CpG-ODN or GpG-ODN at regular intervals to female NZB/W animals. We also determined the effects of ODN administration on NZB/W mouse lymphocyte function, and the specificity of ODN binding to Toll-like receptors (TLRs) other than TLR-9. While CpG-ODN treatment did not appear to have a major impact on disease severity, GpG-ODN treatment significantly delayed the onset of proteinuria in NZB/W mice. Interestingly, short-term GpG-ODN treatment promoted Th2-type T and B cell responses, and inhibited B lymphocyte proliferation in vitro. On the other hand, extended GpG-ODN treatment did not result in sustained Th2 responses or significantly reduced renal disease. Moreover, the binding of CpG-ODN and GpG-ODN was not restricted to TLR-9 as both ODNs also interacted with TLR-3, TLR-7, and TLR-8. Taken together, the data indicate that the protective mechanism of GpG-ODN treatment in the NZB/W model of lupus nephritis involves modulating T cell cytokine profiles and B lymphocyte activation through the inhibition of several TLRs, including TLR-7 and TLR-9.
Keywords: Systemic lupus erythematosus, Toll-like receptors, inhibitory oligos, therapy, Th2
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that is characterized by the production of antinuclear antibodies directed against a variety of autoantigens. The disease can involve multiple organs and organ systems, including the kidneys, skin, and blood elements. Immune system abnormalities are considered to be of central importance to the pathogenesis of SLE. For example, autoreactive T and B cell expansion has been implicated in immune complex (IC)-mediated glomerulonephritis and associated end-organ damage [1]. The development of SLE is influenced by many factors, as genetic, environmental, and hormonal contributions have been well-documented (reviewed in [2]). In particular, microbes such as Epstein-Barr virus have been linked with lupus [3], suggesting a pathogenic role for infectious agents.
Members of the mammalian Toll-like receptor (TLR) gene family have critical roles in the induction of innate immune responses to pathogens. TLRs are involved in the recognition of numerous microbial components and products, including lipopolysaccharide (TLR-4), bacterial flagellin (TLR-5), and viral products such as single-stranded (ss) RNA (TLR-7 and TLR-8) and double-stranded (ds) RNA (TLR-3) (reviewed in [4]). One important role for TLRs is to bridge innate and adaptive immune responses through the coordinated up-regulation of major histocompatibility complex class II (MHC II) and co-stimulatory molecules (i.e., CD40, CD80, and CD86) for efficient antigen presentation and lymphocyte activation. Moreover, TLR-induced production of IL-6, IL-12, tumor necrosis factor (TNF), and type I interferons (IFNs) directs the maturation of immune cells, including dendritic cells, B cells, and T cells [5]. TLR activation has been implicated in the pathogenesis of numerous inflammatory diseases, including sepsis and atherosclerosis (reviewed in [6]). It has also been shown that MyD88, an adaptor protein that mediates signaling through certain TLRs [7], is important for induction of CD4+T helper 1 (Th1) responses in mice [8].
Data from numerous studies suggest that TLR signaling pathways, particularly TLR-9, may be effective therapeutic targets for inflammatory autoimmune diseases such as SLE [9]. TLR-9 is engaged by hypomethylated CpG motifs, which are commonly found in bacterial DNA [10]. Notably, co-engagement of the B cell receptor (BCR) and TLR-9 by mammalian chromatin-containing ICs has been implicated in the activation of autoreactive B cells [11,12]. In addition, Christensen et al. [13] demonstrated that TLR-9 is necessary for the generation of anti-chromatin and anti-dsDNA autoantibodies in murine lupus. Thus, modulation of the TLR-9 pathway using inhibitory oligonucleotides (ODN) may prove useful in therapy of B cell-mediated diseases such as SLE.
We have previously shown that administration of an immunomodulatory GpG-ODN, with a single base switch from CpG to GpG, can suppress the severity of the Th1/Th17-mediated disease experimental autoimmune encephalomyelitis (EAE), a mouse model for human multiple sclerosis [14]. The GpG-ODN reduced expression of co-stimulatory and MHC molecules by antigen presenting cells (APCs), and inhibited Th1 cytokine production. Moreover, we showed that the GpG-ODN also served as an effective novel therapeutic adjuvant capable of boosting the efficacy of antigen-specific tolerizing DNA vaccines used for treating EAE [15]. The GpG-ODN shifted autoreactive Th1 cells to a protective Th2 phenotype, and altered isotype switching of autoreactive B cells to a protective IgG1 isotype.
Several investigators have demonstrated a dominant role for Th1 cytokines in murine SLE [16–19]. Given that activation of TLR-9 by CpG DNA is typically associated with the induction of inflammatory Th1-type immune responses [20], we postulated that administration of an inhibitory GpG-ODN would suppress Th1 responses and ameliorate disease in the NZB × NZW F1 (NZB/W) model of lupus nephritis. In this study, we evaluated the effects of CpG- and GpG-containing ODNs on disease progression and lymphocyte function in a spontaneous murine model of lupus nephritis.
Materials and methods
Mice
Female NZB/W and C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All animals were maintained under standard conditions in the Research Animal Facility at Stanford University. Experiments were conducted in accordance with approved Institutional Animal Care and Use Committee (IACUC) and NIH guidelines.
Reagents
CpG-ODN 5′-TGACTGTGAACGTTAGAGATGA-3′ and GpG-ODN 5′ -TGACTGTGAAGGTTAGAG-ATGA-3′ oligodeoxynucleotides (ODNs) were synthesized with a phosphorothioate backbone and purified by HPLC by Qiagen Operon (Alameda, CA, USA). The underlining represents the CpG and GpG motifs, respectively.Loxoribine,ODN1826 5′-TCCAT-GACGTTCCTGACGTT-3′, and ODN1826 control 5′-TCCATGAGCTTCCTGAGCTT-3′ were purchased from InvivoGen, Inc. (San Diego, CA, USA).
Treatment
Treatment of NZB/W mice began at 20 weeks of age, with 10 mice per group for Experiment #1, and 15 mice per group for Experiment #2. Phosphate-buffered saline (PBS), or 50 µg of CpG-ODN, or GpG-ODN in a final volume of 200 µl of PBS was administered intraperitoneally once weekly for the first 3 weeks, and subsequently at 4-week intervals for both 32 (Experiment #1) and 40 week (Experiment #2) treatments. The last treatment was given 10–12 before termination. For Experiment #3, 12-week-old NZB/W mice were given a single 200 µl injection of PBS or 50 µg of ODN in PBS on day 0. Three mice from each group were then sacrificed on days 1, 2, 3, and 7.
Proliferation assay
Splenocytes enriched for T lymphocytes by a CD3+T cell negative selection column (R&D Systems, Minneapolis, MN, USA) and then stimulated with plate-bound anti-CD3 and anti-CD28 antibodies (5 µg/ml each) or whole splenocytes were cultured in RPMI-1640 supplemented with l-glutamine (2 mM), sodium pyruvate (1 mM), non-essential amino acids (0.1 mM), penicillin (100 U/ml), streptomycin (0.1 mg/ml), 2-mercaptoethanol (5 × 10−5 M), and 10% fetal calf serum. CpG-ODN or GpG-ODN at 5 µg/ml, ODN1826 or ODN1826 control at 1 µM, or 1 mM Loxoribine were included in the cultures separately and cells were incubated for 24 or 48 h. Wells were pulsed with 1 µCi[3H]TdR (Amersham Pharmacia Biotech, Piscataway, NJ, USA) for the final 6 h of a 24 h culture or the final 16 h of a 48 h culture, and incorporated radioactivity was measured using a betaplate scintillation counter.
Enzyme-linked immunosorbent assays (ELISAs)
Anti-dsDNA antibody levels were determined using an ELISA kit (Alpha Diagnostic International, San Antonio, TX, USA). Mouse sera were diluted 1:100, followed by addition of horseradish peroxidase-conjugated goat anti-mouse antibodies specific for IgG, IgG1, and IgG2a (Southern Biotechnology Associates, Birmingham, AL, USA) diluted 1:5000. Culture supernatants from plates set up concurrently with proliferation assays were collected and assayed undiluted in triplicate for the production of IFN-γ and IL-4 and by sandwich ELISA using standard ELISA kits (BD PharMingen, San Diego, CA, USA). Serum was diluted 1:10 and assayed in duplicate for the production of IL-6, and IL-12p40 by standard ELISA kits (BD PharMingen).
TLR ligand screening
Assessment of NF-κB activation by CpG-ODN and GpG-ODN was performed by InvivoGen. Activity of each ODN was tested on HEK293 cells individually expressing six mouse TLRs (TLR-2, TLR-3, TLR-4, TLR-5, TLR-7, and TLR-9), and compared to control ligands at a concentration of 25, 2.5, and 0.25 µg/ml. Control ligands included: heat-killed Listeria monocytogenes at 108 cells/ml (TLR-2), Poly(I:C) at 1 µg/ml (TLR-3), Escherichia coli K12 LPS at 1 µg/ml (TLR-4), Salmonella typhimurium flagellin at 1 µg/ml (TLR-5), Loxoribine at 1mM (TLR-7), and CpG ODN 1826 at 1 µg/ml (TLR-9). This screening was performed in triplicate and repeated once with similar results.
Renal pathology
Proteinuria was assessed once weekly using Albustix reagent strips for urinalysis (Bayer, Elkhart, IN, USA). Severe proteinuria was defined as a reading of 300 mg/dl or greater. At the end of the experiment, kidneys were harvested from surviving mice and fixed in 10% buffered formalin. Periodic acid Schiff (PAS) staining was performed on paraffin-embedded sections, and the extent of kidney damage was scored according to standard NIH activity and chronicity indices [21] in a blinded manner by one of the authors (J.P. Higgins).
Results
Treatment with GpG-ODN delays the development of proteinuria in NZB/W mice
Treatment schedules for two independent NZB/W experiments are shown in Figure 1A. Treatment began at 20 weeks of age, when mild renal pathology (glomerular deposits) is typically present in NZB/W mice [22]. Surviving animals were then euthanized at either 32 or 40 weeks of age (Experiments 1 and 2, respectively). PBS, CpG-ODN, or GpG-ODN was administered intraperitoneally once weekly for the first 3 weeks, and subsequently at 4-week intervals. Colorimetric assessment of proteinuria was performed weekly beginning at 20 weeks of age. By the end of Experiment 1 (32 weeks), 30% of PBS- and CpG-ODN-treated NZB/W mice had severe proteinuria (defined as a reading of 300 mg/dl or greater), while severe proteinuria was absent in all GpG-ODN-treated mice (Figure 1B). Severe proteinuria in both PBS- and CpG-ODN-treated groups was detectable as early as 25 weeks.
Figure 1.
A GpG-ODN delays the development of proteinuria in NZB/W mice. (A) Treatment schedules for two independent experiments are shown. PBS, or 50 µg of CpG-ODN, or GpG-ODN was administered intraperitoneally at the indicated time points. Numbers represent age in weeks. Colorimetric assessment of proteinuria was performed weekly beginning at 20 weeks of age and continued through 32 (B) or 40 (C) weeks of age. *p < 0.05 for the GpG-ODN group (compared to PBS-treated animals in Experiment #1) and **p < 0.05 for GpG-ODN versus CpG and for GpG-ODN versus PBS (Experiment #1), as determined by ANOVA and Dunn’s multiple comparison’s post-test. ***p < 0.05 for GpG-ODN versus PBS (Experiment 2). Representative images of PAS-stained kidney sections from Experiment 2. The top three panels are 200 × magnifications from PBS-treated (D), CpG-ODN-treated (E) and GpG-treated (F) mice. The bottom three panels are 400 × magnifications of a representative glomerulus from PBS-treated (G), CpG-ODN-treated (H), and GpG-treated (I) mice.
By the end of Experiment 2, 80% of PBS- and CpG-ODN-treated mice had significant proteinuria, compared to only 55% of GpG-ODN-treated mice (Figure 1C). Moreover, only 10 of the 15 mice in both the PBS- and CpG-ODN treated groups were alive (67% survival), whereas 13 of the 15 mice in the GpG-ODN treated group remained (87% survival). As mentioned above, we did not observe severe proteinuria in any GpG-ODN-treated mice at the end of Experiment 1. The results were confirmed and extended in Experiment 2, as severe proteinuria was not detectable in the GpG-ODN-treated group until 34 weeks of age.
Treatment with GpG-ODN delays the onset of kidney pathology in NZB/W mice
Mortality in NZB/W mice is likely due to renal failure resulting from IC-mediated glomerulonephritis [22]. Given the effects of GpG-ODN treatment on proteinuria in NZB/W mice, we next evaluated whether CpG-ODN or GpG-ODN treatment had any impact on renal pathology in NZB/W lupus nephritis. At the end of Experiments 1 and 2, PAS-stained kidney tissue from surviving SLE mice was evaluated for pathological changes by one of the authors (J.P. Higgins), who had no knowledge of the treatment each mouse had received. Compared to PBS-treated controls, CpG-ODN and GpG-ODN treatment had no significant effect on kidney pathology at the end of Experiment 1 (data not shown). However, at 40 weeks of age (Experiment 2), we observed statistically significant differences in renal disease (reflected in mean lupus scores; p < 0.05 by Mann–Whitney U-test) between CpG-ODN-treated NZB/W mice (mean score of 3.1) and their GpG-ODN-treated counterparts (mean score of 2.1; Table I). GpG-ODN-treated mice had less renal disease than the PBS-treated group (mean score 2.4), but this difference did not reach statistical significance (p = 0.57 by Mann–Whitney U-test).
Table I.
Renal pathology in NZB/W mice.
| PBS (n = 10) | CpG (n = 10) | GpG (n = 13) | |
|---|---|---|---|
| Lupus score | 2.4 (0.37) | 3.1 (0.38)* | 2.1 (0.31) |
| Interstitial inflammation |
1.1 (0.38) | 1.5 (0.40)** | 0.23 (0.12)NS |
| Necrosis | 1.2 (0.44) | 3.2 (0.53)*** | 1.1 (0.36)+ |
| Proliferation | 2.0 (0.3) | 2.6 (0.31) | 2.0 (0.28) |
PAS-stained kidneys from 40-week-old NZB/W mice were evaluated for pathologic changes. Values are presented as mean (standard error of mean, SEM). Renal pathology scores were compared using the Mann–Whitney U-test.
p < 0.05 (CpG vs. GpG);
p < 0.05 (CpG vs.GpG);
p < 0.05 (PBS vs. CpG);
p < 0.05 (CpG vs. GpG);
p = 0.0759 (PBS vs. GpG).
NZB/W mice were also evaluated using parameters of renal pathology that are representative of the most significant pathologic abnormalities in NZB/W mice with advanced lupus nephritis. These indices included grading of interstitial inflammation, necrosis, and proliferation. As indicated in Table I, CpG-ODN-treated mice had statistically significant higher amounts of interstitial inflammation (1.5) than those of their GpG-ODN-treated counterparts (0.23). Renal necrosis was also significantly elevated in CpG-ODN treated mice (3.2) compared with both the PBS-treated (1.2) and GpG-ODN-treated (1.1) groups.
Representative images of kidney sections stained with PAS from each of the treatment groups from Experiment 2 are shown in Figure 1. PBS treatment resulted in enlarged glomeruli which contained bulky IC deposits that occlude their capillary lumens, indicative of hyaline thrombi (200× magnification, Figure 1D). In the 400× magnification, there is a small crescent in the urinary space, and the glomerulus shows both mesangial proliferation as well as necrosis with both karyorrhectic fragments and fibrin deposition (Figure 1G). CgG-ODN treatment produced more pronounced interstitial lymphocytic infiltrates, where the glomeriuli are hypercellular and contain IC deposits (200× magnification, Figure 1E).
As shown in Figure 1H, intracapillary proliferation and karyorrhectic fragments are also seen in the CpG-treated glomerulus; ICs are also readily apparent (400× magnification). In contrast, GpG-ODN treatment resulted in glomeruli with nearly normal cellularity and no significant inflammation in the interstitium (200× magnification, Figure 1F). Under further magnification, the glomerulus shows no necrosis and no clear IC deposition (400× magnification, Figure 2). Taken together, the kidney pathology results indicate that CpG-ODN treatment worsens while GpG-ODN treatment improves SLE renal pathology.
Figure 2.
CpG-ODN and GpG-ODN treatment modulates NZB/W mouse T lymphocyte responses ex vivo. Splenocytes from NZB/W mice were enriched for T lymphocytes at 32 weeks (left column) or 40 weeks (right column) of age. (A) T cells were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies, and proliferation was assessed by thymidine incorporation assay after 48 h of culture. Experiment 1: *p < 0.05. Experiment 2: *p < 0.05; **p < 0.0001. (B) T cell cytokine production in response to ODN treatment. T cells were stimulated with plate-bound anti-CD3 plus anti-CD28, and levels of IFN-γ in culture supernatants were measured by ELISA after 48h. Experiment 1: NS, not significant (p = 0.062). Experiment 2: *p < 0.05. (C) Levels of IL-4 in culture supernatants were measured as in (B). Experiment 1: *p < 0.05. Experiment 2: *p < 0.05. Data are presented as mean + standard deviation (SD). p values were calculated using the Student’s t-test, and are based on comparison to results from the PBS-treated group.
GpG-ODNs induce a Th2 lymphocyte response
It has been shown that CD4+T helper cells have a critical pathogenic role in NZB/W lupus nephritis [23,24]. We asked whether the protective effects of the GpG-ODN could be due in part to dampening or modulation of T cell responses. To this end, splenocytes from treated NZB/W mice were enriched for T lymphocytes. T cells were then stimulated with plate-bound anti-CD3 and anti-CD28 antibodies, and proliferation was assessed by thymidine incorporation assay. As shown in Figure 2A, treatment with either CpG-ODN or GpG-ODN produced a heightened T cell proliferative response compared to PBS-treated mice at 32 and 40 weeks of age.
Several studies have demonstrated a disease-promoting role for Th1 cytokines in lupus nephritis [16,18,25]. To examine the potential effects of CpG-ODN and GpG-ODN treatment on relative Th1 and Th2 responses in NZB/W mice, we measured IFN-γ and IL-4 levels in culture supernatants by ELISA. Consistent with observations from GpG-ODN treatment in the EAE model [14], anti-CD3/CD28-stimulated T cells derived from 32-week-old GpG-ODN-treated mice produced lower levels of IFN-γ in vitro than their PBS-treated counterparts (Figure 2B, left panel). This result did not reach statistical significance (p = 0.062).
Although levels of IFN-γ from all three treated groups decreased by 40 weeks, we noted an increase in IFN-γ production by T cells from GpG-ODN treated mice (Figure 2B, right panel, p < 0.05). This may correlate to the increase in proteinuria and kidney disease in the GpG-ODN treated mice. IFN-γ levels from CpG-ODN treated mice were comparable to PBS-treated mice at both 32 and 40 weeks. T cell production of IL-4 was significantly higher in the T cell supernatants of 32-week GpG-ODN-treated mice compared to PBS-treated and CpG-ODN treated mice (Figure 2C, left panel, p < 0.05). Interestingly, after 40 weeks, GpG-ODN treatment still induced a statistically significant increase in IL-4 production by NZB/W T cells compared to PBS-treatment. Of note, IL-4 production from stimulated T cells of CpG-ODN treated mice was also statistically increased at 40 weeks, even though disease was just as severe as the PBS-treated mice (Figure 2C, right panel, p < 0.05).
Short-term treatment with a GpG-ODN induces Th2 anti-dsDNA antibody responses in vivo
Anti-dsDNA antibodies are a hallmark of human and murine lupus, and may be directly pathogenic in disease. To examine the effects of ODN treatment on anti-dsDNA antibody levels, we collected sera from NZB/W mice from Experiment 1 (Figure 3, left panel) and Experiment 2 (Figure 3, right panel). Levels of IgG anti-dsDNA antibodies were elevated in CpG-ODN- and GpG-ODN-treated mice compared to PBS-treated mice after 32 weeks (Figure 3A, left panel). However, only the results from the CpG-ODN-treated group reached statistical significance (p < 0.05). Interestingly, while there was a trend toward decreased levels of IgG anti-dsDNA antibodies for both CpG-ODN- and GpG-ODN treated mice at 40 weeks of age (as compared to PBS-treated mice), these results were not statistically significant (Figure 3A, right panel).
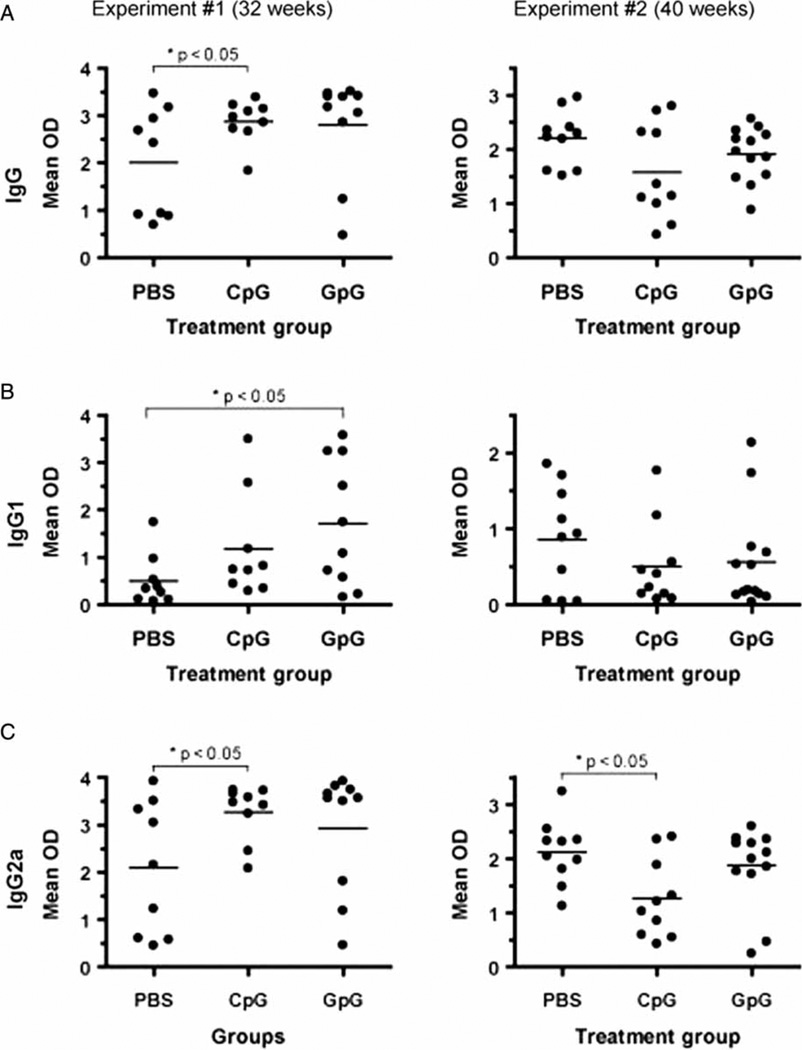
Figure 3.
GpG-ODN induces Th2 anti-dsDNA antibodies. Sera were collected from NZB/W mice at 32 (left panel) and 40 (right panel) weeks of age. Levels of IgG (A), IgG1 (B), and IgG2a (C) anti-dsDNA antibodies were determined by ELISA. *p < 0.05, as determined by Student’s t-test, where indicated.
Given the effects of ODN treatment on NZB/W T cell cytokine production, we next measured levels of Th1- and Th2-associated anti-dsDNA antibodies in CpG-ODN- and GpG-ODN-treated mice. Differences in the anti-dsDNA IgG1 and IgG2a antibody isotypes were more apparent in Experiment 1. Compared to PBS-treated mice, levels of anti-dsDNA IgG1 antibodies were elevated in both CpG-ODN- and GpG-ODN-treated mice (Figure 3B, left panel). However, only the GpG-ODN-treated group had statistically higher levels (p < 0.05) of anti-dsDNA IgG1 antibodies, which is consistent with our observation of increased IL-4 production by T cells from GpG-ODN-treated mice.
Levels of anti-dsDNA IgG2a antibodies were also elevated in both CpG-ODN- and GpG-ODN-treated mice (as compared to PBS-treated mice) after 32 weeks. However, only the CpG-ODN-treated group had statistically higher levels (p < 0.05) of anti-dsDNA IgG2a (Figure 3C, left panel). Taken together, the data indicate that short-term CpG-ODN and GpG-ODN treatment of NZB/W mice induces an overall increase in anti-dsDNA antibody levels. However, a Th2-associated B cell anti-dsDNA response (IgG1) was induced by GpG-ODN treatment, while a Th1-associated B cell response (IgG2a) was induced by CpG-ODN treatment. Notably, the complement-activating properties of IgG2a autoantibodies may render them more nephritogenic than IgG1 autoantibodies [26].
Although anti-dsDNA IgG2a is typically known as a major pathogenic autoantibody in mouse lupus, a potential explanation for the increased anti-dsDNA IgG2a responses observed in the short-term GpG-ODN treatment of mice could be that these IgG2a antibodies may be directed against other non-pathogenic antigens or epitopes. This could partly explain the lack of proteinuria (Figure 1B) in the 32-week GpG-ODN-treated group. We performed antigen microarray analysis, in an effort to delineate differences in the specificities of the autoantibody responses between the treatment groups, but these studies were unrevealing (data not shown). Although the microarrays contained proteins and synthetic overlapping peptides derived from several prominent lupus-associated autoantigens [27], it is possible that these antigens did not include targets of the serum antibodies.
In Experiment 2 (where we analyzed sera collected at 40 weeks of age), levels of IgG1 and IgG2a anti-dsDNA antibodies followed the same trend as the total IgG anti-dsDNA levels. Compared to PBS-treated mice, levels of anti-dsDNA IgG1 antibodies were lower in both CpG-ODN- and GpG-ODN-treated mice (Figure 3B, right panel). Compared to their PBS-treated counterparts, levels of anti-dsDNA IgG2a antibodies were also lower in both CpG-ODN-and GpG-ODN-treated mice. However, only the CpG-ODN-treated group had statistically lower amounts (p < 0.05) of anti-dsDNA IgG2a as compared to the PBS-treated group (Figure 3C, right panel).
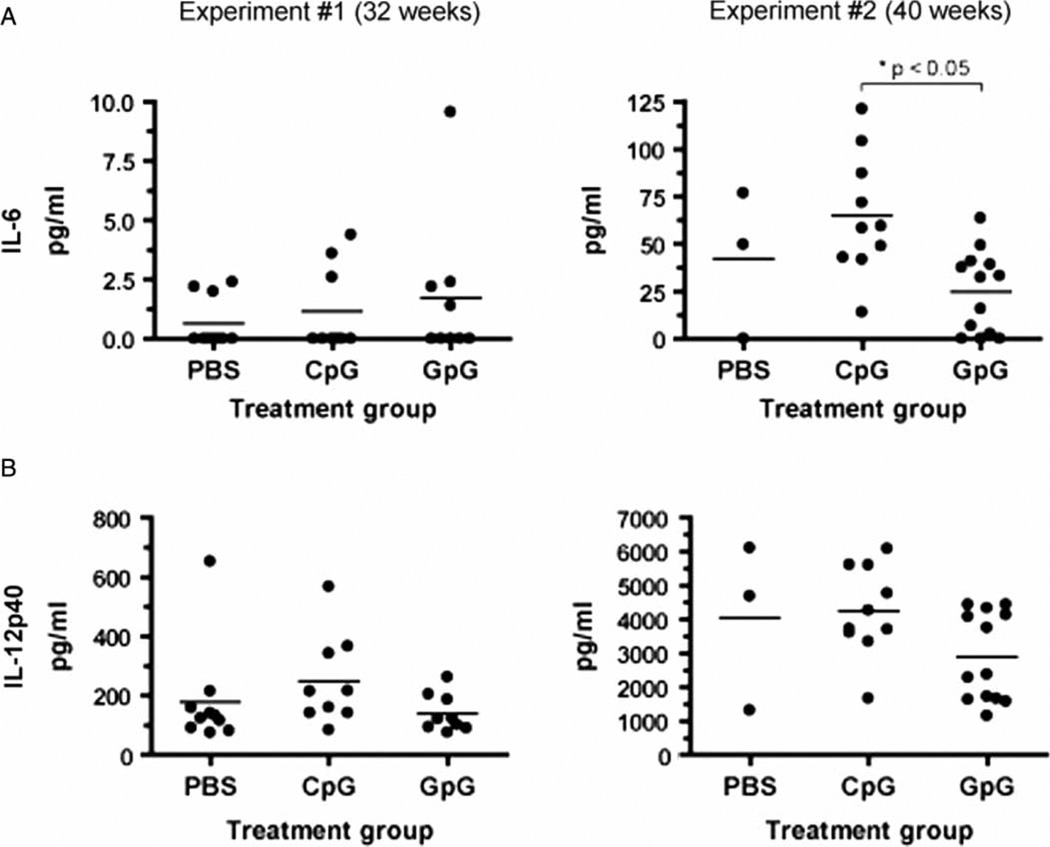
We also analyzed levels of IL-6 and IL-12(p40) in sera derived from each treatment group. At 32 weeks of age, there were no significant differences in serum IL-6 levels between PBS-, CpG-ODN-, and GpG-ODN-treated mice (Figure 4A, left panel). Conversely, when compared to PBS-treated mice, there was a trend towards increased IL-12(p40) levels in sera derived from CpG-ODN-treated mice and decreased IL-12(p40) levels in sera derived from GpG-ODN-treated mice. (Figure 4B, left panel). At 40 weeks of age, sera derived from CpG-ODN-treated mice had elevated amounts of IL-6 compared to the PBS- and GpG-ODN-treated groups (Figure 4A, right panel). Of note, GpG-ODN-treated mice had significantly decreased levels of IL-6 compared to the CpG-ODN-treated group (p < 0.05). In addition, levels of IL-12(p40) in sera derived from GpG-ODN-mice at 40 weeks of age were lower than the other two treatment groups (Figure 4B, right panel).
Figure 4.
Prolonged GpG-ODN treatment suppresses proinflammatory cytokine production in NZB/W mice in vivo. Sera were harvested from NZB/W mice at 32 weeks (left column) or 40 weeks (right column) of age, and levels of IL-6 (A) and IL-12(p40) (B) were assayed by ELISA.
Treatment with GpG-ODNs can induce a Th2 B cell response
Given that GpG-ODN treatment appeared to promote Th2-associated B cell responses in vivo, we further analyzed B cell function in NZB/W mice. At the termination of Experiment #1, B cells were isolated from whole splenocytes and stimulated in culture with plate-bound anti-IgM and/or anti-CD40 antibodies. Stimulation with anti-IgM and anti-CD40 resulted in higher B cell proliferative responses in PBS- and CpG-ODN-treated mice as compared to GpG-ODN-treated mice (Figure 5A). We also measured Th1- and Th2-associated antibody isotype levels in vitro. Compared to PBS-treated mice, B cells from CpG-ODN-treated mice produced higher levels of IgG1 (Figure 5B) and IgG2a antibodies (Figure 5C), as determined by analysis of culture supernatants. Conversely, GpG-ODN-treated B cells produced lower levels of IgG2a antibodies than B cells from their PBS-treated counterparts. Thus, 32-week GpG-ODN treatment appears to preferentially induce Th2-type B cell responses ex vivo.
Figure 5.
GpG-ODN induces a Th2 B cell response. (A) Purified splenic B cells (derived from animals analyzed in Experiment 1) were stimulated in vitro with anti-IgM, anti-CD40, or anti-IgM plus anti-CD40, and proliferation was assessed after 48 h in culture. Results are expressed as a stimulation index (mean cpm in presence of stimulus ÷ mean cpm without stimulus). Symbols indicate statistically significant values as follows: For the CpG-ODN group, +p < 0.05 (vs. PBS group, anti-IgM stimulation). For the GpG-ODN group, *p < 0.05 (vs. PBS); #p < 0.05 (vs. CpG-ODN). Levels of IgG1 (B) or IgG2a (C) in supernatants from unstimulated, purified B cells (Experiment 1) were assayed by ELISA after 48h in culture. For (B) and (C), *p < 0.05 (CpG-ODN vs. PBS); **p < 0.05 (GpG-ODN vs. PBS).
The cultured B cells originated from spleen and were further stimulated, both of which could be potential explanations for the discordance in antibody isotype usage in serum (Figure 3C) as compared to the stimulated splenic B cells (Figure 5B,C). It is possible that the splenic B cells are not representative of the B cells producing the majority of circulating antibodies. In addition, in vitro stimulation with anti-IgM and anti-CD40 could have modulated isotype class switching and Ig production.
CpG-ODN and GpG-ODN bind TLR-9 and other TLRs
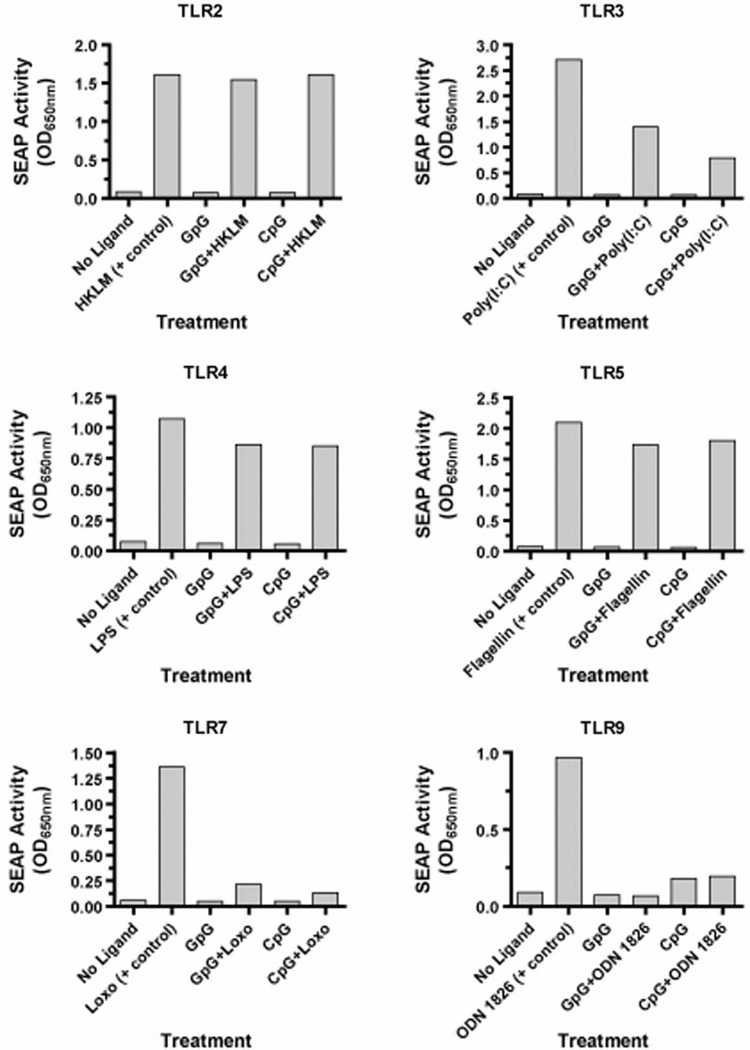
To confirm the binding specificity of the CpG-ODN and GpG-ODN to TLR-9, both ODNs were screened for NF-κB activation in HEK293 cells expressing a given TLR. ODN activity was tested against six mouse (m)TLRs—TLR-2, TLR-3, TLR-4, TLR-5, TLR-7, and TLR-9, and compared to control ligands specific for each TLR. The assay measures two distinct outcomes: (1) TLR-induced NF-κB activation in response to ODN binding and (2) the inhibitory effect of each ODN against the respective TLR control ligand.
The GpG-ODN did not stimulate any of the mTLRs, as indicated by the lack of NF-κB activation (Figure 6). Unexpectedly, the GpG-ODN had no inhibitory effect on mTLR-2, minimal inhibition of mTLR-4 and mTLR-5, significant inhibition of mTLR-3, and strong inhibition of mTLR-7 and mTLR-9. Likewise, except for the expected stimulatory effect (albeit weak) on mTLR-9, the CpG-ODN also did not stimulate any of the other mTLRs. Surprisingly, like the GpG-ODN, our CpG-ODN also had no inhibitory effect on mTLR-2, minimal inhibition of mTLR-4 and mTLR-5, significant inhibition of mTLR-3, and strong inhibition of mTLR-7 and mTLR-9. One explanation for the discrepancy of our CpG-ODN and the control CpG-ODN 1826 ligand is that the TLR ligand screening assay is optimized for the production of NF-κB by the control CpG-ODN 1826 ligand. Our CpG-ODN is still capable of binding mTLR-9 and activating NF-κB, albeit at a weaker capacity than the CpG-ODN 1826 (Supplemental Figure 1; online version only).
Figure 6.
GpG-ODN and CpG-ODN bind TLR-9 and other TLRs. Mouse TLR stimulation was tested by assessing NF-κB activation in HEK293 cells transfected to express the indicated TLR. CpG-ODN or GpG-ODN was incubated alone or in the presence of the indicated positive control ligand for TLR activation. Data presented are the average of two independent experiments with comparable results. HKLM, heat-killed L. monocytogenes; Poly(I:C), polyinosinic: polycytidylic acid; LPS, E. coli K12 lipopolysaccharide; Flagellin, S. typhimurium flagellin; Loxo, loxoribine; and CpG ODN 1826.
Both ODNs were also tested on a panel of human (h)TLRs that included all six of the respective mouse TLRs mentioned here, as well as hTLR-8. For both ODNs, performance in the human TLR-induced NF-kB activation assays was similar to the mouse TLR assays (Supplemental Figure 2; online version only). Both the CpG-ODN and the GpG-ODN had no inhibitory effect on hTLR-2, minimal inhibition of hTLR-4 and hTLR-5, significant inhibition of hTLR-3 and strong inhibition of hTLR-7 and hTLR-9. Of note, both ODNs also had strong inhibition of NF-κB activation mediated by hTLR-8.
A single injection of a GpG-ODN suppresses IL-12 production in vivo
The CpG-ODN and GpG-ODN utilized in this study were synthesized with a phosphorothioate backbone, which promotes resistance to nuclease digestion and prolongs cellular uptake and half-life [28,29]. The failure of the GpG-ODN to suppress proteinuria in NZB/W mice beyond 34 weeks of age (Figure 1) led us to postulate that the in vivo activity of the ODNs is relatively short-lived. To determine how long the CpG-ODN and GpG-ODN can remain active in vivo, pre-nephritic female 12-week-old NZB/W mice were treated with a single injection of PBS, CpG-ODN, or GpG-ODN on day 0 (Figure 7A). Serum IL-12(p40) levels were then measured on days 1, 2, 3, and 7 after treatment. As shown in Figure 7B, GpG-ODN treatment suppressed IL-12(p40) levels on days 1, 2, and 7 post-injection (compared to PBS-treated controls). Interestingly, when compared with PBS-treated mice, GpG-ODN treatment did not induce lower levels of IL-12(p40) on day 3 post-injection. Conversely, CpG-ODN treatment induced significantly higher levels of IL-12(p40) on day 3.
Figure 7.
GpG-ODN suppresses both in vivo IL-12(p40) production and in vitro proliferative responses to a TLR-7 agonist. (A) Female 12-week old NZB/W mice (3 per group) were treated with PBS, CpG-ODN, or GpG-ODN on day 0 (grey arrow). At the times indicated (black arrows), splenocytes were harvested and stimulated in vitro with CpG-ODN or GpG-ODN at 5 µg/ml, or 1 mM loxoribine for 24h. (B) Sera were collected at the indicated times, and levels of IL-12(p40) were assayed by ELISA. Proliferation of cells harvested 3 days (C) or 7 days (D) after initial treatment was assessed by thymidine incorporation assay. *Data from experiments performed at 1 and 2 days did not indicate any statistically significant differences, and thus are not shown. Results are expressed as a stimulation index. Bars represent mean + SD. For panel (B), symbols represent statistically significant differences (p < 0.05) as follows: +CpG versus PBS; *GpG versus PBS; #GpG versus CpG. For panels (C) and (D), symbols above the bars indicate significant inhibition of splenocyte proliferation in response to in vitro loxoribine stimulation: +CpG versus PBS; *GpG versus PBS; #GpG versus CpG.
A GpG-ODN suppresses proliferative responses to a TLR-7 agonist
The results of the TLR ligand screening assay (Figure 6) suggested that the CpG-ODN and GpG-ODN are capable of inhibiting the activity of several TLRs in vitro. Moreover, the finding that TLR-7 deficient lupus-prone MRL lpr/lpr mice have less severe disease, while their TLR-9 deficient counterparts have exacerbated disease, suggests that TLR-7 may play a more important regulatory role in lupus [30]. Given the effects of the CpG- and GpG-ODNs on TLR-7 and TLR-9 activity in vitro, we sought to further investigate the in vivo effects of the CpG-ODN and GpG-ODN on TLR-7 and TLR-9 responses. To this end, pre-nephritic female 12-week-old NZB/W mice were treated with a single injection of PBS, CpG-ODN, or GpG-ODN on day 0 (Figure 7A).
Splenocytes were harvested on days 1, 2, 3, and 7, and stimulated in vitro with CpG-ODN or GpG-ODN at 5 µg/ml, or 1 mM loxoribine (a TLR-7 agonist) for 24 h. Proliferation of cells harvested 3 days (Figure 7C) or 7 days (Figure 7D) after initial treatment was assessed by thymidine incorporation assay. Splenocytes harvested from PBS-, CpG-ODN-and GpG-ODN-treated mice at 3 and 7 days post-injection all proliferated similarly upon in vitro CpG-ODN stimulation. As expected, splenocytes from all three treated groups had minimal proliferative responses to in vitro stimulation with GpG-ODN at 3 and 7 days’ post-injection. Of particular interest is the response to loxoribine, a TLR-7 agonist, since both ODNs strongly inhibit TLR-7-induced NF-κB activation (Figure 6).
Compared to their PBS-treated counterparts, both CpG-ODN- and GpG-ODN-treated splenocytes proliferated less in response to loxoribine stimulation. However, treatment with GpG-ODN in vivo appeared to suppress in vitro proliferative responses to loxoribine more effectively than in vivo treatment with CpG-ODN at 3 days post-injection. Collectively, these data indicate that administration of a GpG-ODN inhibits systemic production of a Th1-driving cytokine for at least 1 week. Furthermore, the GpG-ODN significantly suppressed the proliferative response to a TLR-7 agonist for up to 3 days after initial treatment. However, this difference in inhibition was indistinguishable from CpG-ODN treatment by 7 days post-treatment.
In order to confirm that the GpG-ODN was able to suppress the stimulatory effects of loxoribine more effectively than the CpG-ODN, we performed in vitro competition assays (Supplemental Figure 3A; online version only). We added increasing amounts of GpG-ODN to determine its effects on loxoribine-induced splenocytes proliferation (Supplemental Figure 3B; online version only). At 5, 10 and 25 µg/ml amounts, GpG-ODN was able to suppress the stimulatory effects of 1 mM of loxoribine on splenocyte proliferation between 30 and 50%. At 50 and 100 µg/ml, GpG-ODN was able to suppress up to 80% stimulation by loxoribine. In contrast, the CpG-ODN at 5, 10, and 25 µg/ml amounts, could suppress only 25% of the stimulatory effects of loxoribine on splenocyte proliferation (Supplemental Figure 3C; online version only). At 50 µg/ml, the CpG-ODN suppressed about 50% stimulation by loxoribine, and at the highest concentration of 100 µg/ml, CpG-ODN suppressed only 70% stimulation of loxoribine. Thus, although both ODNs could inhibit the stimulation of splenocytes by the TLR-7 specific ligand, loxoribine, the GpG-ODN was able to inhibit loxoribine stimulation more effectively.
Discussion
In this study, we found that treatment with a TLR-9 ligand inhibitor delays and attenuates lupus nephritis in NZB/W mice. We discuss here seven observations made in this study:
We noted that treatment with the GpG-ODN suppressed development of severe proteinuria in NZB/W mice, but only through 33 weeks age (Figure 1B,C). Severe proteinuria developed most rapidly in GpG-ODN-treated NZB/W mice at a time when GpG-ODN treatment was given more infrequently than it was in earlier phases of the experiment (between 34 and 38 weeks of age; Figure 1A,C).
The GpG-ODN preferentially induced Th2-associated T and B cell responses in vitro and in vivo through 32 weeks of age. However, trends in renal pathology, as well as readouts of T and B cell function, were generally either ambiguous or indistinguishable from PBS-treated mice compared with GpG-ODN by 40 weeks of age. Nevertheless, we observed a consistent 7–8-week delay in proteinuria in the GpG-ODN treatment group in both the 32- and 40-week experiments, compared to the CpG-ODN treatment and PBS-treatment groups. Together, these results suggest the existence of a “window” during which it is possible to tip the Th1/Th2 balance and ameliorate disease in NZB/W lupus nephritis. As a corollary to this, we reasoned that the limited half-life of the GpG-ODN may explain the lack of significant long-term protection in the NZB/W model.
The proinflammatory cytokines, IL-6 and IL-12p40, were measured in the sera of each group at the end of 32 and 40 weeks. It has been reported that these Th1 cytokines are typically secreted by lymphocytes upon activation of CpG motifs [31]. Serum IL-6 levels were statistically decreased in the GpG-ODN treated mice by 40 weeks of age, and serum IL-12p40 levels trended lower in the GpG-ODN treated mice in both the 32- and 40-week experiments. Overall reduced proteinuria and reduced renal pathology, together with increased survival in the GpG-ODN-treated mice, implicates the importance of modulating IL-6 and IL-12p40 cytokine levels in NZB/W lupus neprhitis.
Work from a number of groups indicates an important role for the TLR-9 pathway in the etiology of lupus. Mammalian dsDNA-containing ICs have been proposed to trigger lupus through the binding of TLR-9 in plasmacytoid dendritic cells (pDCs) and B cells [11,32]. However, other TLRs have also been implicated in the pathogenesis of SLE. For example, Vollmer et al. [33] demonstrated that U1 small nuclear ribonucleo-proteins (snRNPs) found in apoptotic cell debris are capable of stimulating pDCs through both TLR-7 and TLR-8. The data suggest that autoantibodies directed against snRNPs may initiate SLE through TLR-7 and TLR-8. Moreover, snRNP-containing ICs are found in 30–40% of lupus patients, and the U1snRNA component of U1snRNP ICs has been shown to act as an endogenous self ligand for TLR-7, triggering the production of IFN-α and IL-6 [34]. Recent data also show the ability of human IgG-RNA-containing ICs to effectively activate mouse dendritic cells through TLR-7 [35]. Patole et al. [36] showed that exposure to pI:C RNA, a structural analog of viral dsDNA, could aggravate lupus nephritis through TLR-3, which is expressed by murine glomerular mesangial cells, as well as APCs (B cells, macrophages, and dendritic cells).
Autoantibodies produced in SLE are targeted to intracellular antigens located in the cell nucleus, particularly histones and extractable nuclear antigens. Antibodies to double stranded DNA (dsDNA) and chromatin are the more common autoantibodies in SLE, whereas antibodies to RNA-binding proteins such as Smith, Ro, La, U1RNP, or ribosomal components tend to be associated with the activation of the IFN-α pathway and define a patient subgroup with more severe SLE [37]. Christensen et al. [13] previously reported that TLR-9 is necessary for the spontaneous generation of DNA autoantibodies, but was not involved in the development of lupus nephritis. More recently, Christensen et al. [30] reported that TLR-9 deficient mice had more aggressive lupus. Conversely, TLR-7 deficient mice did not develop lupus. These mice had both decreased lymphocyte activation and decreased serum IgG. The development of a pathogenic autoantibody TLR-7 dependent knock-in mouse by Berland et al. [38] demonstrated the ability of TLR-7 to drive the production of nuclear antibodies and produce kidney disease similar to lupus. Together, these data suggest a dominant role for the TLR-9 pathway in the production of anti-dsDNA antibodies, while TLR-7 appears to play a prominent role in renal pathology, as well as for the production of autoantibodies directed against RNA-containing autoantigens. More importantly, Lau et al. [39] reported the absence of autoantibody production in SLE-prone mice lacking the TLR adaptor protein MyD88.
-
We found unexpectedly, an in vitro TLR ligand screening assay revealed that the CpG-ODN and GpG-ODN bind not only to mTLR-9, but also to mTLR-3 and mTLR-7 (Figure 6). When we tested the same CpG-ODN and GpG-ODN on a human TLR panel that included TLR-8, the results were similar to the mouse TLR panel. Moreover, hTLR-8 (which, like TLR-7, also recognizes ssRNA) was also inhibited by both ODNs (Supplemental Figure 2; online version only). Of note, several TLRs are unified by their ability to recognize nucleic acid sequences derived from bacteria and viruses including TLR-3 (dsRNA), TLR-7 and TLR-8 (ssRNA), and TLR-9 (dsDNA). TLR-7 is an intracellular receptor that recognizes ss U-rich viral ssRNA [40–42]. TLR-9 is an intracellular receptor that recognizes unmethylated CpG motifs in viral or bacterial DNA [8,43]. Because TLR-9 is phylogenetically similar to TLR-7 and TLR-8 [44,45], and all three TLRs are restricted intracellularly to the endosome, it is not completely surprising that we observed cross recognition of the CpG-ODN and GpG-ODN between the TLRs (Figure 6). Interestingly, it has been reported that several other immunostimulatory CpG DNAs were able to induce normal cytokine production by TLR-7-deficient macrophages, implicating a lack of cross-recognition by these specific CpG DNA sequences [43].
Several recent reports have highlighted the importance of TLR-7 in autoimmunity and SLE. The absence of TLR-7 in lupus-prone mice dramatically decreased disease [32]. Also, the Y-linked autoimmune accelerator (Yaa) locus contains a 4Mb duplication of the pseudoautosomal region that encompasses several immune response genes including TLR-7, which results in B cells becoming intrinsically biased toward nuclear antigens [46,47]. Since both ODNs in our study were capable of inhibiting loxoribine induced TLR-7 stimulation, we studied the in vivo long-term effects of ODN treatment. Splenocytes removed from mice both 3 and 7 days after injection of CpG-ODN or GpG-ODN revealed that in vitro stimulation by loxoribine was suppressed by both ODNs compared to splenocytes from PBS-treated mice (Figure 7). We further compared the ability of the ODNs to inhibit loxoribine stimulation by competition assays whereby increasing concentrations of GpG-ODN were more effective in suppressing loxoribine stimulation than similarly increasing concentrations of CpG-ODN (Supplemental Figure 3; online version only).
We had initially hypothesized that treatment with CpG-ODN would accelerate NZB/W lupus nephritis (compared to PBS-treated mice). However, there was no significant difference between the CpG-ODN- and PBS-treated groups with respect to frequency or onset of severe proteinuria. Consistent with this, only renal necrosis was significantly enhanced in CpG-ODN-treated mice when compared to PBS treatment (Table I). To show that the CpG-ODN is in fact stimulatory, we compared its proliferative response to ODN1826, a commonly used mouse CpG sequence. As shown in Supplemental Figure 4 (online version only), the CpG-ODN at the optimal 5 µg/ml (0.697 µM) concentration is capable of stimulating whole splenocytes like ODN1826, at the recommended 1 µM concentration. We show here that the combination of CpG-ODN and GpG-ODN suppresses the CpG-ODN proliferative response, whereas the combination of ODN1826 and its ODN1826 control sequence do not suppress proliferation. In fact the ODN 1826 control alone appears to provide a moderate amount of stimulation, unlike the GpG-ODN.
We propose that the GpG-ODN used in this study acts by inhibiting the activity of several TLRs, including TLR-9. The fact that both the GpG-ODN and CpG-ODN can bind and inhibit the same group of TLRs (TLR-3, TLR-7, and TLR-8), but only the GpG-ODN appears to be a potent inhibitor of TLR-9 activation may explain why treatment with the GpG-ODN was able to delay significant proteinuria through 33 weeks. Indeed, while total IgG anti-dsDNA antibodies were elevated in both the CpG-ODN-and GpG-ODN-treated groups relative to the PBS-treated group at 32 weeks, we found that the GpG-ODN-treated mice had elevated IgG1 anti-dsDNA antibodies while CpG-ODN-treated mice had higher levels of IgG2a anti-dsDNA antibodies (Figure 3).
Dong et al. [48] has previously described the benefits of a suppressive ODN sequence in SLE. Known as A151, this ss phosphorothioate molecule contains four repetitive TTAGGG motifs to mimic the telomeric regions of mammalian DNA. Treatment of NZB/W mice at 7 months of age with active renal disease (2 + proteinuria) with twice-weekly injections of 300 µg of suppressive A151 ODN for 3 months delayed the rise in proteinuria and prolonged survival but could not reverse the progression of renal disease. Suppressive ODN therapy with A151 ODN had similar effects in lupus disease as our inhibitory GpG-ODN motif, although their study required 6 times the amount of suppressive ODN (300 µg vs. 50 µg) and more frequent injections (twice weekly injections versus once weekly for the first 3 weeks, and subsequently at 4-week intervals). Moreover, there is no indication that A151 is able to suppress other TLRs as we have shown here with our inhibitory GpG-ODN.
The effects of inhibitory ODNs have also been studied in another spontaneous SLE model in MRL.lpr mice. Patole et al. [49] reported the efficacy of a G-rich ODN 2114 that was specific for blocking the TLR-9 pathway. In this study, MRL.lpr mice were treated with 40 µg of ODN on alternate days from weeks 11 to 24 of age. Damage to the lungs and kidneys were reduced, serum levels of IFN-α were reduced, IgG2a anti-dsDNA autoantibodies were reduced, and proteinuria was also reduced. A later study from the same group, by Pawar et al. [50], treated MRL.lpr mice with ODNs IRS 661, a TLR-7-inhibitory sequence, or IRS 954, a TLR-7 and TLR-9 inhibitory sequence, that were previously identified by Barrat et al. [32]. Here in this study, MRL.lpr mice were also treated with 40 µg of ODN on alternate days from age 11 to 24 weeks.
The authors found that inhibition with the TLR-7-specific IRS 661 ODN effectively reduced kidney and lung injury, serum levels of IgG2a and IgG2b anti-dsDNA autoantibodies were reduced, and intrarenal production of CC-chemokines were reduced. Interestingly, inhibition with IRS 954 was actually less effective than IRS 661 in reducing kidney and lung injury and did not reduce anti-dsDNA autoantibodies as ODN 2114 did. Nevertheless, all of these studies together provide a strong rationale for utilizing suppressive ODN as TLR-targeting therapy in the prevention and treatment of SLE and other autoimmune diseases.
Overall, it is becoming increasingly clear that multiple TLR pathways are likely to be involved in SLE. Indeed, a review by Krieg and Vollmer [51] suggests a more prominent role for TLR7 in the pathogenesis of SLE, while TLR9 appears to play an immune regulatory role. The relative importance of specific TLRs in SLE may vary according to a number of factors, including, but certainly not limited to, temporal and spatial control of TLR expression, the source and availability of autoantigen, and the local cytokine environment.
Inhibitory GpG-ODN therapy holds promise for the modulation of a broad array of inflammatory disorders due to its ability to antagonize several TLRs (TLR-3, TLR-7, TLR-8, and TLR-9) concurrently. One area of particular interest is the exact mechanism(s) whereby ODNs exert their immunomodulatory effects, especially the events that occur downstream of TLR binding including NF-kB activation. GpG-ODN treatment appears to ameliorate NZB/W lupus nephritis via modulation of T cell cytokine profiles and B lymphocyte activation. However, the inhibitory GpG-ODN may also have direct and indirect effects on other immune system cells, including macrophages and dendritic cells.
Endogenous TLR ligands generated by apoptotic cellular debris (i.e., dsDNA, chromatin, snRNPs, or ribosomal components) may be recognized by autoantibodies to form ICs that activate TLR-3, TLRs-7/8 or TLR-9, resulting in the initiation of SLE. Our data show the ability of an inhibitory GpG-ODN to antagonize and inhibit the activation of these TLR pathways in vitro, which suggests a mechanism for its ability to delay and attenuate SLE. Thus, an inhibitory oligonucleotide that can suppress multiple TLRs, such as the GpG-ODN that we describe here, may be a practical therapeutic for inflammatory autoimmune diseases where TLR pathways are critical for pathogenesis.
Supplementary Material
Acknowledgements
We thank Pearline Teo for technical support on unpublished data and Michael Leviten for critical scientific discussions. K.L.G. is supported by a NIH NRSA fellowship AI10663-02. L.S. is supported by NIH and National Multiple Sclerosis Society grants. P.J.U. is the recipient of a Donald E. and Delia B. Baxter Foundation Career Development Award, and was supported by the Dana Foundation, the Floren Family Trust, the Northern California Chapter of the Arthritis Foundation, NIH Grants DK61934, AI50854, AI50865, AR49328, and NHLBI Proteomics Contract N01-HV-28183. P.P.H. is the recipient of an Arthritis Foundation Northern California Chapter Grant. K.L.G. and P.P.H. conceived of the study, participated in its design and coordination, participated in the acquisition, analysis and interpretation of the data, and helped with the draft of the manuscript. L.Y.L. participated in the acquisition, analysis and interpretation of the data. J.P.H. participated in the analysis and interpretation of the data. L.S. and P.J.U. conceived of the study, participated in the analysis and interpretation of the data, and helped with the draft of the manuscript.
Abbreviations
- SLE
systemic lupus erythematosus
- ODN
oligodeoxynucleotide
- NZB/W
NZB × NZW F1
- TLRs
Toll-like receptors
- MHC II
major histocompatibility complex class II
- TNF
tumor necrosis factor
- IFNs
interferons
- ss
single-stranded
- ds
double-stranded
- Th1
CD4+T helper 1
- ICs
immune complexes
- EAE
experimental autoimmune encephalomyelitis
- APCs
antigen presenting cells
- PAS
periodic acid Schiff staining
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Tsao BP. The genetics of human systemic lupus erythematosus. Trends Immunol. 2003;24:595–602. doi: 10.1016/j.it.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 3.James J, Harley J, Scofield R. Epstein-Barr virus and systemic lupus erythematosus. Curr Opin Rheumatol. 2006;18:462–467. doi: 10.1097/01.bor.0000240355.37927.94. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K, Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 5.Lenert P. Targeting toll-like receptor signaling in plasmacytoid dendritic cells and autoreactive B cells as a therapy for lupus. Arthritis Res Ther. 2006;8:203. doi: 10.1186/ar1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook D, Pisetsky D, Schwartz D. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway C. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 8.Schnare M, Barton G, Holt A, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 9.Deane J, Bolland S. Nucleic acid-sensing TLRs as modifiers of autoimmunity. J Immunol. 2006;177:6573–6578. doi: 10.4049/jimmunol.177.10.6573. [DOI] [PubMed] [Google Scholar]
- 10.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 11.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 12.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 13.Christensen C, Kashgarian M, Alexopoulou L, Flavell R, Akira S, Shlomchik M. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho P, Fontoura P, Ruiz P, Steinman L, Garren H. An immunomodulatory GpG oligonucleotide for the treatment of autoimmunity via the innate and adaptive immune systems. J Immunol. 2003;171:4920–4926. doi: 10.4049/jimmunol.171.9.4920. [DOI] [PubMed] [Google Scholar]
- 15.Ho PP, Fontoura P, Platten M, Sobel RA, DeVoss JJ, Lee LY, Kidd BA, Tomooka BH, Capers J, Agrawal A, Gupta R, Zernik J, Yee MK, Lee BJ, Garren H, Robinson WH, Steinman L. A Suppressive oligodeoxynucleotide enhances the efficacy of myelin cocktail/IL-4-tolerizing DNA vaccination and treats autoimmune disease. J Immunol. 2005;175:6226–6234. doi: 10.4049/jimmunol.175.9.6226. [DOI] [PubMed] [Google Scholar]
- 16.Jacob C, Van Der Meide P, McDevitt H. In vivo treatment of (NZB × NZW)F1 lupus-like nephritis with monoclonal antibody to gamma-interferon. J Exp Med. 1987;166:798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakajima A, Hirose S, Yagita H, Okumura K. Roles of IL-4 and IL-12 in the development of lupus in NZB/W F1 mice. J Immunol. 1997;158:1466–1472. [PubMed] [Google Scholar]
- 18.Peng S, Moslehi J, Craft J. Roles of interferon-gamma and interleukin-4 in murine lupus. J Clin Invest. 1997;99:1936–1946. doi: 10.1172/JCI119361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balomenos D, Rumold R, Theofilopoulos A. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–371. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1(Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin HAIII, Muenz LR, Joyce KM, Antonovych TT, Balow JW. Diffuse proliferative lupus nephritis: Identification of specific pathologic features affecting renal outcome. Kidney Int. 1984;25:689–695. doi: 10.1038/ki.1984.75. [DOI] [PubMed] [Google Scholar]
- 22.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wofsy D, Seaman W. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985;161:378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connolly K, Roubinian J, Wofsy D. Development of murine lupus in CD4-depleted NZB/NZW mice. Sustained inhibition of residual CD4 + T cells is required to suppress autoimmunity. J Immunol. 1992;149:3083–3088. [PubMed] [Google Scholar]
- 25.Richards H, Satoh M, Jennette J, Croker B, Yoshida H, Reeves W. Interferon-γ is required for lupus nephritis in mice treated with the hydrocarbon oil pristane. Kidney Int. 2001;60:2173–2180. doi: 10.1046/j.1523-1755.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- 26.Santiago M-L, Fossati L, Jacquet C, Muller W, Izui S, Reininger L. Interleukin-4 protects against a genetically linked lupus-like autoimmune syndrome. J Exp Med. 1997;185:65–70. doi: 10.1084/jem.185.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham K, Vaysberg M, Kuo A, Utz P. Autoantigen arrays for multiplex analysis of antibody isotypes. Proteomics. 2006;6:5720–5724. doi: 10.1002/pmic.200600345. [DOI] [PubMed] [Google Scholar]
- 28.Stein C, Subasinghe C, Sinozuka K, Coehn J. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucl Acids Res. 1988;16:3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wickstrom E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J Biochem Biophys Methods. 1986;13:97–102. doi: 10.1016/0165-022x(86)90021-7. [DOI] [PubMed] [Google Scholar]
- 30.Christensen C, Shupe J, Nickerson K, Kashgarian M, Flavell R, Shlomchik M. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vollmer J, Tluk S, Schmitz C, Hamm S, Jurk M, Forsbach A, Akira S, Kelly KM, Reeves WH, Bauer S, Krieg AM. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savarese E, Chae O-W, Trowitzsch S, Weber G, Kastner B, Akira S, Wagner H, Schmid RM, Bauer S, Krug A. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood. 2006;107:3229–3234. doi: 10.1182/blood-2005-07-2650. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda K, Richez C, Maciaszek JW, Agrawal N, Akira S, Marshak-Rothstein A, Rifkin IR. Murine dendritic cell type I IFN production induced by human igg-rna immune complexes is IFN regulatory factor (IRF)5 and IRF7 dependent and is required for IL-6 production. J Immunol. 2007;178:6876–6885. doi: 10.4049/jimmunol.178.11.6876. [DOI] [PubMed] [Google Scholar]
- 36.Patole PS, Grone H-J, Segerer S, Ciubar R, Belemezova E, Henger A, Kretzler M, Schlondorff D, Anders H-J. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J Am Soc Nephrol. 2005;16:1326–1338. doi: 10.1681/ASN.2004100820. [DOI] [PubMed] [Google Scholar]
- 37.Kirou K, Lee C, George S, Louca K, Peterson M, Crow M. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 38.Berland R, Fernandez L, Kari E, Han J, Lomakin I, Akira S, Wortis H, Kearney J, Ucci A, Imanishi-Kari T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25:429–440. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Lau C, Broughton C, Tabor A, Akira S, Flavell R, Mamula M, Christensen S, Shlomchik M, Biglianti G, Rifkin I, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diebold S, Kaisho T, Hemmi H, Akira S, Sousa CE. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 41.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 42.Lund J, Alexopoulou L, Sato A, Karow M, Adams N, Gale N, Iwasaki A, Flavell R. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 44.Du X, Poltorak A, Wei Y, Beutler B. Three novel mammalian toll-like receptors: Gene structure, expression, and evolution. Eur Cytokine Netw. 2000;11:362–371. [PubMed] [Google Scholar]
- 45.Chuang T, Ulevitch R. Cloning and characterization of a sub-family of human toll-like receptors hTLR-7, hTLR-8, and hTLR-9. Eur Cytokine Netw. 2000;11:372–378. [PubMed] [Google Scholar]
- 46.Pisitkun P, Deane J, Difilippantonio M, Tarasenko T, Saterthwaite A, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 47.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou ZJ, Schultz RA, Wakeland EK. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong L, Ito S, Ishii K, Klinman D. Suppressive oligodeoxynucleotides delay the onset of glomerulonephritis and prolong survival in lupus-prone NZB × NZW mice. Arthritis Rheumat. 2005;52:651–658. doi: 10.1002/art.20810. [DOI] [PubMed] [Google Scholar]
- 49.Patole PS, Zecher D, Pawar RD, Grone H-J, Schlondorff D, Anders H-J. G-rich DNA suppresses systemic lupus. J Am Soc Nephrol. 2005;16:3273–3280. doi: 10.1681/ASN.2005060658. [DOI] [PubMed] [Google Scholar]
- 50.Pawar RD, Ramanjaneyulu A, Kulkarni OP, Lech M, Segerer S, Anders H-J. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J Am Soc Nephrol. 2007;18:1721–1731. doi: 10.1681/ASN.2006101162. [DOI] [PubMed] [Google Scholar]
- 51.Krieg A, Vollmer J. Toll-like receptors 7, 8, and 9: Linking innate immunity to autoimmunity. Immunol Rev. 2007;220:251–269. doi: 10.1111/j.1600-065X.2007.00572.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.