Abstract
Rasmussen's encephalitis (RE) is an inflammatory, probably autoimmune disorder manifested by refractory seizures and progressive deterioration of one cerebral hemisphere [1].
Here, we describe the unfortunate history of a girl with a progressive disorder which, upon clinical, neuroimaging, and histopathological evaluation, proved to be bilateral RE associated with type II focal cortical dysplasia. Whether the second pathology is relevant for the extent of the disease is discussed.
We demonstrated histopathological evidence of RE and type II FCD in the left hemisphere, which led to EPC on the right hemibody at presentation. In addition, there was unequivocal progressive cortical and subcortical atrophy of the right hemisphere, which accounted for the EPC on the left hemibody. This is highly compatible with RE (+/− FCD) in the right hemisphere as well. Although the association of FCD and RE – as well as the occasional occurrence of bilateral RE – has already been reported [3–5], this is the first such case in which bilateral RE and FCD co-occur.
Keywords: Epilepsy surgery, Dual pathology, Bilateral Rasmussen's encephalitis
1. Introduction
Rasmussen's encephalitis (RE) is an inflammatory, probably autoimmune disorder manifested by refractory seizures and progressive deterioration of one cerebral hemisphere [1]. Clinical suspicion arises when a patient with partial seizures or epilepsia partialis continua (EPC) also has (even mild) unilateral atrophy and no other lesion on MRI. The unilaterality of the pathological and clinical involvement has been a puzzle for epileptologists since the description of this disorder, and several theories have been proposed to accommodate this fact [2,3].
Increased awareness about RE led to reports of a few patients in whom this classically unilateral disease affected both hemispheres [5]. These bilateral cases are even more puzzling because they, in a sense, challenge any simplistic explanation for a unilateral pathogenesis [4]. One aspect which has not been specifically examined is whether bilateral RE may be more prone to be associated with another pathology (dual pathology).
Here, we describe the history of an unfortunate girl with a progressive disorder which, upon clinical, neuroimaging, and histopathological evaluation, proved to be bilateral RE associated with type II focal cortical dysplasia. Whether the second pathology is relevant for the extent of the disease is discussed.
2. Case report
This girl had an uneventful history until starting with partial motor seizures at age 4.5 years. The attacks involved the entire right hemibody and progressed to medically refractory epilepsia partialis continua (EPC). At age 6, her right arm and hand were nonfunctional, she could not walk, swallowing was difficult, and cognition was markedly affected by unstoppable seizures and recurrent ICU admissions. MRI showed a high-intensity lesion in the left precentral gyrus, suggestive of FCD, and also a more diffuse atrophy of the left hemisphere, including cortical (insula) and subcortical (caudate and hippocampus) structures (Fig. 1).
Fig. 1.
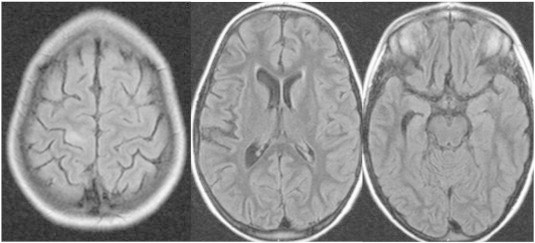
Preoperative MRI showing the alterations described above. Note that there is no hint of pathology on the contralateral hemisphere.
Continuous spiking, intermittently associated with bursts of high-frequency discharges, was present in the pre- and postcentral gyri on acute ECoG, leading to a large Rolandic resection (Fig. 2). Histopathology suggested RE and type II FCD (Fig. 3).
Fig. 2.
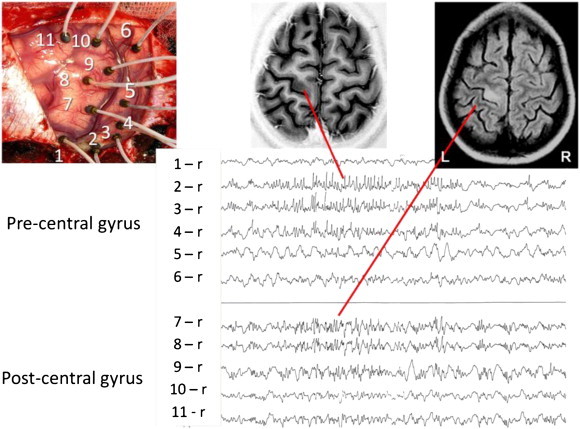
Intraoperative ECoG mapping the epileptogenic areas.
Fig. 3.
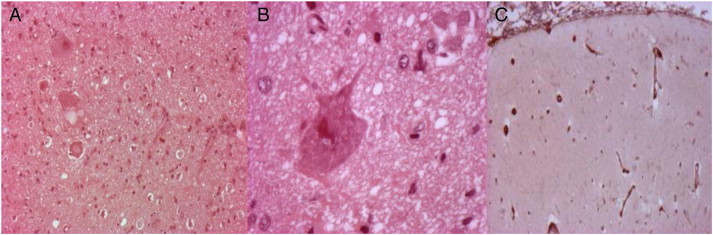
A and B: Pathological specimen showing dysmorphic neurons. C: Pathological evidence of neuronal loss, gliosis, and perivascular cuffing.
The girl became seizure-free for 1 year with remarkable improvement in global functioning, including walking and better cognition. One year later, in the context of high fever, this girl started with EPC in the other (left) hemibody. She quickly deteriorated, and a new MRI showed severe diffuse atrophy of the left hemisphere (where operation had been performed) with increased cortical and subcortical signal extending way beyond the margins of the previous resection. There was also cortical and subcortical atrophy of the right hemisphere, which was unequivocal upon comparison with the MRI performed a few years earlier (Fig. 4).
Fig. 4.
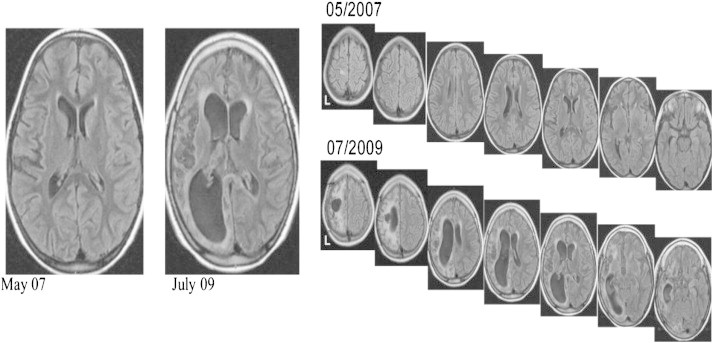
Control MRI showing progressive atrophy of the contralateral hemisphere (nonoperated).
In extremis, she underwent functional hemispherectomy of the left hemisphere, complementing the initial resection, in the hope that this might interfere with disease progression. Nonetheless, left-sided EPC and cognitive deterioration persisted. She is currently tetraparetic, bed-ridden, and does not communicate. Pathology again confirmed RE (Fig. 5).
Fig. 5.
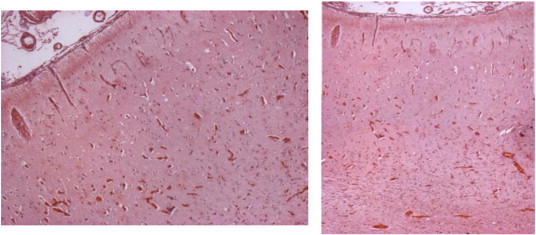
Histopathological analysis again confirming RE.
3. Conclusion
We demonstrated histopathological evidence of RE and type II FCD in the left hemisphere, which led to EPC on the right hemibody at presentation. In addition, there was unequivocal progressive cortical and subcortical atrophy of the right hemisphere, which accounted for the EPC on the left hemibody. This is highly compatible with RE (+/− FCD) in the right hemisphere as well. Although the association of FCD and RE – as well as the occasional occurrence of bilateral RE – has already been reported, this is the first such case in which bilateral RE and FCD co-occur.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Bien C.G., Elger C.E. Epilepsia partialis continua: semiology and differential diagnoses. Epileptic Disord. 2008;10(1):3–7. doi: 10.1684/epd.2008.0161. [DOI] [PubMed] [Google Scholar]
- 2.Cheong J.Y., Wongb C., Bleasel A., Varikatt W., Ng T., Dexter M.A. Late onset Rasmussen's encephalitis with triple pathology. J Clin Neurosci. 2009;16:1677–1681. doi: 10.1016/j.jocn.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 3.Bien C.G., Granata T, Antozzi C. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis — a European consensus statement. Brain. 2005;128:454–471. doi: 10.1093/brain/awh415. [DOI] [PubMed] [Google Scholar]
- 4.Bauer J., Bien C.G. Encephalitis and epilepsy. Semin Immunopathol. 2009;31:537–544. doi: 10.1007/s00281-009-0176-1. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes R.H., Lehman R.M., Wu B.Y., Roychowdhury S. Focal chronic inflammatory epileptic encephalopathy in a patient with malformations of cortical development, with a review of the spectrum of chronic inflammatory epileptic encephalopathy. Epilepsia. 2007;48(6):1184–1202. doi: 10.1111/j.1528-1167.2007.01034.x. [DOI] [PubMed] [Google Scholar]


