Abstract
Inclusion of artesunate in the cavity of β-cyclodextrin (β-CD) as well as its methyl and hydroxypropyl derivatives was investigated experimentally and by molecular modeling studies. The effect of PEG on the inclusion was also studied. A 1:1 stoichiometry was indicated by phase-solubility studies both in the presence and absence of PEG and suggested by the mass spectrometry. The mode of inclusion was supported by 2D NMR and results were further verified by docking studies utilizing Fast Rigid Exhaustive Docking acronym. The thermodynamic parameters were determined for both binary and ternary systems using solution calorimetry and were found to be best for the methyl-β-cyclodextrin (Me-β-CD) system. However, the presence of PEG improves the complexation ability as evident from elevation in the numerical value of the stability constant (K). Solubility and dissolution profile of binary complex is enhanced in the presence of PEG, which is approximately at par with drug Me-β-CD complexes. In vivo studies showed 100% survivability in artesunate–Me-β-CD complexes.
Keywords: Artesunate, β-Cyclodextrin and its derivatives, Inclusion complexes, Thermodynamic parameters, In vivo studies, Molecular modeling
1. Introduction
Despite the fact that we live in an era of advanced technology and innovation, infectious diseases, like malaria, continue to be one of the greatest health challenges worldwide. The main drawbacks of conventional malaria chemotherapy are the development of multiple drug resistance and the non-specific targeting of intracellular parasites, resulting in high dose requirements and subsequent intolerable toxicity [1,2]. Artemisnin derivatives are one of the few antimalarial drugs that remain effective against multidrug resistant strains of Plasmodium falciparum malaria [3–5]. Unfortunately, artesunate, a potent blood schizonticidal belonging to Class II of Biopharmaceutical Classification System, shows poor water solubility and low bioavailability following oral administration. This causes formulation problems and limits its therapeutic applications and bioavailability [6–8]. Various approaches such as liposomes [9] and nanoparticles [10] have been employed to enhance its solubility and bioavailability. However, no detailed study is reported on the cyclodextrin complexes except one report where the authors have reported the NMR data on artesunate–β-CD complexes [11]. Therefore, the present study is undertaken to utilize the CDs for improving the physicochemical properties as well as for therapeutic index of this poorly soluble drug. The focus of the work is to characterize the host–guest interactions by determining the stability constant and the other thermodynamic parameters associated with complexation. However, high molecular weight, cost, production capability and possible parenteral toxicity have hindered the use of CD's in the formulations [12]. A useful strategy to overcome these difficulties is the use of the third component such as water-soluble polymers [13–19], an emulsifying agent [20], a surfactant [21], a hydroxyacid [22,23]. These can enhance the CD complexing ability and result in the reduction of the above mentioned drawbacks associated with CD complexes. Consequently, the present work is extended by incorporating water-soluble polymer in drug–β-CD complexes to improve the complexing abilities leading to the better solubilizing efficiency by multicomponent complex formation. Out of all the three CD's, only β-CD is used for further studies because of its low price, easy availability, highly suitable cavity dimensions and the least toxicity [24]. The pharmacological activity of binary and ternary complexes was performed to evaluate their efficacy, which has been correlated with the complexing abilities of different CDs. The inclusion mode and stoichiometry of the reaction are supplemented by molecular modeling.
2. Material and methods
2.1. Materials
Artesunate was obtained as gift sample from Ipca Laboratories Ltd. Mumbai, India. β-CD, Me-β-CD and HP-β-CD were obtained from Sigma Aldrich. The other analytical grade chemicals such as sodium hydroxide, potassium hydrogen phosphate, sodium dihydrogen phosphate and polymers were procured from SD fine Chemicals, Chandigarh.
2.2. Methods
2.2.1. Preparation of binary complexes
Artesunate with β-CD, Me-β-CD or HP-β-CD was mixed in 1:1 molar ratio by different methods as given below:
Physical mixing (PM): Physical mixtures were prepared by simple mixing of drug with the selected CD in a mortar and to ensure uniform mixing. The vials filled with the mixtures were subjected to vortex mixing for 5 min.
Kneading (KN): Drug and CD's were blended together in a mortar with sufficient water to produce a paste and was kneaded for 90 min. The product was then dried under vacuum at 40 °C for 48 h and passed through a 150 μm mesh and stored in a glass vial in a vacuum desiccator.
Freeze-drying method (LY): The required 1:1 stoichiometric quantity of drug was added to an aqueous solution of the selected CD and was agitated on a magnetic stirrer for 24 h. The resulting solutions were frozen at (−80 °C) in a deep freezer for overnight. This was then lyophilized under 17.2 mTorr for 48 h. The samples were transferred immediately into a vacuum desiccator and dried over silica gel under vacuum for at least 24 h.
2.2.2. Preparation of ternary cyclodextrin complexes
Ternary complexes were prepared by physical mixing, Kneading, Lyophilized suspension method (Ly Susp) and Coevaporation method.
Coevaporated (CoE) solid system method: Equimolar amounts of artesunate and β-CD systems with 0.25% PEG were dissolved in water. The resulting solution was evaporated on a rotary evaporator at 60 °C to get a solid residue. The prepared system was dried in vacuum desiccators.
Characterization: Binary and ternary inclusion complexes of artesunate were characterized in the solid phase by DSC, PXRD, FT-IR, mass spectrometry and in solution phase by solution calorimetry and NMR.
2.3. Equilibrium solubility studies
Phase-solubility diagrams of artesunate for both binary and ternary systems with various CDs in phosphate buffer (pH 6.8) were obtained according to Higuchi and Connors [25]. An excess amount of the sample was added to 10 mL buffer or CD buffered solutions with a concentration of w/v (2–20 mM) in 20 mL glass vials with or without a polymer (0.25% PEG). The suspensions were sealed and shaken in water-bath shaker (MSW-275, Macroscientific Works Pvt. Ltd., Delhi) at 37±0.5 °C for 24 h to ensure equilibrium. After equilibration, aliquots of the supernatant were withdrawn, filtered through 0.45 μm Millipore filter paper, and the artesunate content was determined spectrophotometerically at λ=240 nm (UV/vis Spectrophotometer (Perkin Elmer Lamda 15, USA). The presence of CDs did not interfere with the spectrophotometric assay of the drug. The standard plot of artesunate was prepared by dissolving a weighed amount of the drug in phosphate buffer pH 6.8, suitably diluted and absorbance taken at wavelength 240 nm on a spectrophotometer.
2.4. Mass spectrometry
ESI-MS studies were performed using a Q-ToF quadruple time of flight mass spectrometer (Waters Pvt. Ltd., USA) equipped with an electrospray source. The sample was introduced via a syringe pump at a flow rate of 5 μL/min. High flow rate nitrogen gas was employed as the nebulizing gas as well as the drying gas to aid desolvation. The sheath gas flow rate was 0.5 μL/min. After optimization of the MS parameters, the spray voltage was set to 2.5 kV in the positive mode, and the heated metal capillary temperature was set at 80 °C. The mass scale was calibrated using the standard calibration procedure and compounds provided by manufacturer.
2.5. Differential scanning calorimetry (DSC)
DSC thermograms were obtained on DSC (Q20, TA Instruments-Waters LLC, USA). The calorimeter was calibrated for temperature and heat flow accuracy using the melting of pure indium (mp 156.6 °C and ΔH of 25.45 J/g). The temperature range was from 50 to 350 °C with a heating rate of 10 °C per minute.
2.6. X-ray powder diffraction
Powder diffraction patterns were recorded on an X-ray diffractometer (XPERT-PRO, PANalytical, Netherlands, Holand) with Cu as tube anode; the diffractograms were recorded under following conditions: voltage 40 kV, 35 mA, angular range 5 and fixed divergence slit.
2.7. Fourier transform infrared spectrometry (FT-IR)
The FT-IR spectra were obtained on an FT-IR spectrometer, Mode spectrum RXI, Perkin Elmer, England, over the range 400–4000 cm−1. Dry KBr (50 mg) was finely ground in an agate mortar and samples of drug and their complexes (1–2 mg) were subsequently added and mixed gently. A manual press was used to form the pellets.
2.8. 2D COESY, proton nuclear magnetic resonance (1H NMR) and 13C NMR spectroscopy
1H NMR, 13C NMR and 2D COESY spectra in d6DMSO of artesunate and inclusion complexes were recorded with a Brucker AC 300 °C NMR spectrometer apparatus operating at 300 MHz using tetramethylsilane as an internal standard. For 2D COESY experiments, samples were equilibrated for at least 24 h.
2.9. Solution calorimetry study
Isoperibol solution calorimeter (ISC) (Calorimetry Science Corporation, UTAH, USA) model 4300 was used for enthalpy of solution measurements. The calorimeter consists of a constant temperature bath held at 37 °C (±0.005 °C) and heater assembly. The drug was filled into batch adapter of volume 0.9 mL, sealed on both sides with ‘O’ rings and cover glass. The batch adapter holding the drug was inserted into the Dewar flask containing buffer (25 mL). The combined unit was then lowered in the calorimeter bath. The glass stirrer was rotated at 100 revolutions/min and was allowed to equilibrate for 90 min. The ampoule was shattered automatically by means of a plunger and temperature change was noted. The performance of the system was checked using KCl, which has known enthalpy of solution (±0.03 kJ/mol).
2.10. Dissolution study
The dissolution studies of the artesunate and its binary and ternary complexes were performed in 900 mL of phosphate buffer (pH 6.8 using) USP [12] apparatus at pre-equilibrated temperature 37±0.5 °C and at a stirring rate of 50 rpm. Drug and its inclusion complexes containing 100 mg of drug were filled in hard gelatin capsules. Samples were withdrawn at different intervals for a period of 6 h and analyzed spectrophotometrically at λ=240 nm.
2.11. Molecular modeling studies
2.11.1. Computational details
The computational studies were carried out on an Intel Xeon based system with the Linux OS (CentOS 5.4). Structure preparation, simulations and analysis were carried out with Maestro version 9.1 (Schrödinger LLC, New York, NY, 2010). The docking studies were carried out with Fast Rigid Exhaustive Docking acronym (FRED version 2.2.5, OpenEye Scientific Software, Santa Fe, USA) [26,27] while the Molecular Dynamics simulations was performed using Desmond (version 2.4, DE Shaw Research, NY, USA).
2.11.2. Structure preparation
The 3D structures of β-cyclodextrin (β-CD) and artesunate were retrieved from the Protein Data Bank [28] and PubChem (CID 5464098). The structures were ‘cleaned’ w.r.t. geometries, atom types and charges based on the OPLS2005 forcefield in Schrödinger Suite.
2.11.3. Docking studies
The β-CD molecule was subsequently imported into the program FRED-RECEPTOR (version 2.2.5) for docking. During the rigid body docking of the guest molecule into the host, the intrinsic scoring function Chemguass2 was utilized for identification of the docking solutions. From a set of 1000 solutions the best 100 were saved. On visual assessment the best solution was subsequently taken up for MD simulation using Desmond.
2.11.4. MD simulations
Initially the complex of β-CD–artesunate was solvated with TIP3P waters [29] to form a water shell 10 Å thick around the β-CD–artesunate complex. Na+ ions were added to attain a net charge of zero on the system. The solvated host–guest system was simulated for a period of 5 ns with the ‘NPTrelaxprotocol’ in Desmond. The protocol involves an initial minimization of the solvent with the solute restrained. The minimization is followed by short MD simulations of 12–24 ps in sequential NVT and NPT ensembles with the Langevin thermostat and barostat [30]. The temperature was maintained by coupling to an external 300 K bath based on the Langevin algorithm. The pressure was isotropically restrained to 1 bar with the Langevinbarostat. High-frequency vibrations were removed by applying the SHAKE algorithm [31] by constraining all bonds to their equilibrium values. Initial velocities were generated randomly from a Maxwell distribution at 300 K in accordance with the masses assigned to the atoms. The trajectories and corresponding energies were sampled every 5 ps. No constraints were applied on the β-CD–artesunate system during the simulations, so as to avoid introduction of any artifacts in the ligand conformation in the binding site.
2.12. In vivo studies: evaluation of pharmacological antimalarial activity of artesunate, its binary and ternary complexes in mice
Four to five weeks old BALB/c mice (25–30 g) were procured and maintained in the Central Animal House. They were provided with standard pellet diet and water ad libtum. Experiments were performed as per guidelines of Control and Supervision on Experiments on Animals (CPC-SEA) Committee. The experimental protocol was approved by the Institutional Animal Ethics Committee (A.I.E.C.). Plasmodium berghei (NK 65) strain was used for evaluation of antimalarial activity in vivo studies and was maintained in the mice. All the mice belonging to control group were challenged with 106 P. berghei infected red blood cells (RBCs) intraperitonial (i/p). After challenge mean percent parasitemia, percent activities of various complexes of artesunate along with animal survivality were monitored. Mean percent parasitemia was calculated for each group on every alternate day up to 30 days by tail blood smear, fixed in methanol and stained in Giemsa stain by counting at least 500 cells
Animals were divided into 6 groups and each group comprised of 6 animals (n=6). These were treated orally with single dose therapy (6 mg/kg of artesunate) two times a day on 1 day of post inoculation (PI) for 7 days to monitor the efficacy and potency of prepared lyophilized binary and ternary complexes. Each animal was treated orally with 100 μL artesunate and its various lyophilized complexes.
-
1.
Control group—treated with 0.5% carboxymethyl cellulose (CMC) suspension;
-
2.
Standard group—administered artesunate in 0.5% CMC suspension;
-
3.
Test group 1—treated with binary As–β-CD complex in 0.5% CMC suspension;
-
4.
Test group 2—treated with binary As–Me-β-CD complex in 0.5% CMC suspension;
-
5.
Test group 3—treated with binary As–HP-β-CD complex in 0.5% CMC suspension and
-
6.
Test group 4—treated with ternary As–β-CD–PEG complex in 0.5% CMC suspension.
2.12.1. Statistical analysis
Data of parasitemia of animals treated with different inclusion complexes were statistically assessed by one-way ANOVA and Turkey's test using Jandel sigma stat 2.0 version. Differences were considered significant at P<0.05.
The value of equilibrium constant (K) and enthalpy of binding (ΔHo) were determined by our computer program utilizing an iterative non-linear least square regression method to minimize the value of ∑(ΔHint(exp)−ΔHint(calc))2 where ΔHint(exp) is the experimentally determined enthalpy of interaction per liter of solution, ΔHint(calc) is the calculated enthalpy of interaction per liter of solution using iterative method.
3. Result and discussion
3.1. Equilibrium phase-solubility studies
The equilibrium phase-solubility study diagrams were found to be linear characterizing their AL type nature and suggest the formation of first order soluble complexes for artesunate with β-CD, HP-β-CD and Me-β-CD (Fig. 1). The increment in the solubility of drug seems to depend upon inclusion ability of cyclodextrin molecules with the solubilization strength increasing in the order: β-CD<HP-β-CD<Me-β-CD.
Fig. 1.
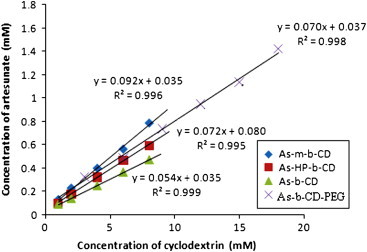
Phase-solubility diagram of artesunate with β-CD, Me-β-CD and HP-β-CD as well as with β-CD in the presence of 0.25% PEG at 37 °C (As–β-CD–PEG).
3.1.1. Selection of third component
As discussed above, the study involves the use of third component for improving the solubilizing efficiency as well as to decrease the bulk of formulation. Increment in the solubility of the drug with β-CD was measured in the presence from selected polymers (Fig. 2). The most effective third component for the ternary system was selected on the basis of solubilizing efficiency of the corresponding drug–cyclodextrin and drug–polymer systems. Various additives such as polyvinylpyrrolidine (PVP), hydroxylpropyl methyl cellulose (HPMC), polyethylene glycol (PEG) and poloxamer were used and the effect of these polymers on the solublizing efficiency is shown in Fig. 2 and 0.25% PEG was found to be the best of all the polymers. The addition of PEG resulted in 20 times increase in solubility as compared to the 13.4-fold increase in its absence at the same concentration of binary complex (Fig. 1). Only β-CD was used for the preparation of ternary complexes because of its lower cost and less parental toxicity compared to Me-β-CD and HP-β-CD. Interestingly, the enhancement in solubility of drug in the presence β-CD and PEG is approximately equal to that in the presence of Me-β-CD alone. The addition of water-soluble polymers to the CD solution did not change the type of phase-solubility diagrams obtained for binary systems (1:1 stoichiometry).
Fig. 2.
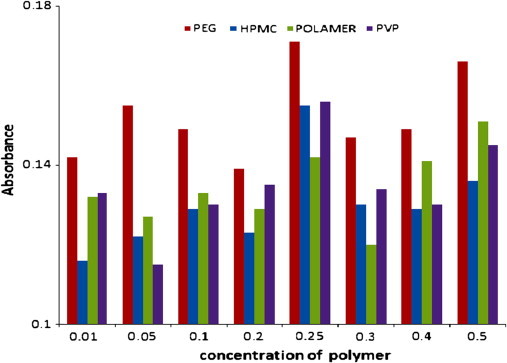
Selection of third component of different additives on β-CD concentration.
3.2. Electrospray ionization mass spectrometry (ESI-MS)
The use of ESI-MS for characterizing the stoichiometry and strength of interactions between synthetic or biological hosts and guests is a growing area of research [32,33]. It is clear from the figure that peaks observed in mass spectra at m/z 1157, 1543, 1717 and 1787 correspond to the charged [β–CD+Na]+, [As+β–CD+Na+H]+, [As+Me-β–CD+Na]+ and [As+HP-β–CD+Na]+, respectively, indicating 1:1 stoichiometry (Fig. 3).
Fig. 3.
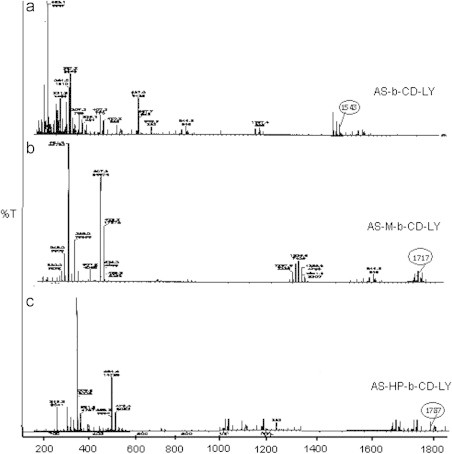
Mass spectra of artesunate with (a) β-CD, (b) Me-β-CD and (c) HP-β-CD.
3.3. Differential scanning calorimetry (DSC)
Differential scanning calorimetry (DSC) provides evidence for differences between the physical mixtures and the putative inclusion complex. The complete disappearance of the fusion endotherm was observed for binary lyophilized complexes indicating formation of a true inclusion complex. In the physical mixtures of drug with β-CD, Me-β-CD and HP-β-CD, the phase transition thermal profile of artesunate (140.2 °C) remained recognizable with the reduction and the broadening of drug fusion peak, with concomitant shift to lower temperature (Fig. 4).
Fig. 4.
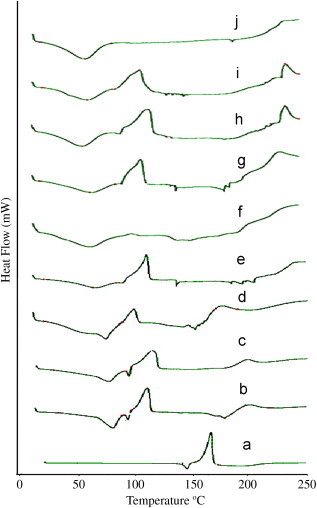
DSC thermograms of (a) artesunate, (b) As–β-CD PM, (c) As–β-CD KN, (d) As–β-CD LY, (e) As–Me-β-CD PM, (f) As–Me-β-CD KN, (g) As–Me-β-CD LY, (h) As–HP-β-CD PM, (i) As–HP-β-CD KN and (j) HP-β-CD LY.
Similarly, the ternary systems prepared by lyophilized suspension method showed complete absence of melting endotherm of the drug. Whereas, the kneaded complexes as well as in coevaporated system exhibited the melting endotherm with reduced intensity suggesting a weak interaction between the components (Fig. 5).
Fig. 5.
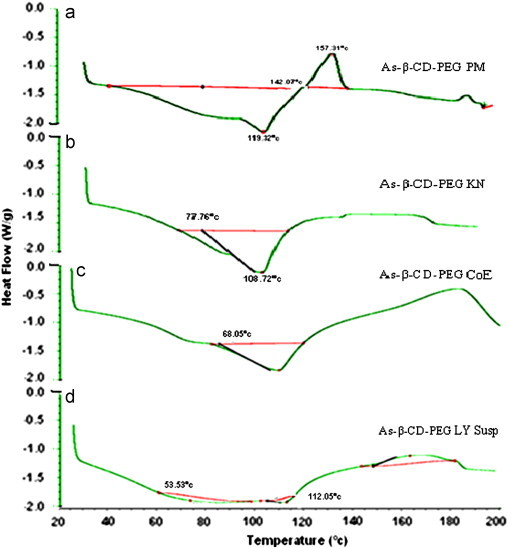
DSC thermograms of artesunate with (a) β-CD–PEG PM, (b) β-CD–PEG KN, (c) β-CD–PEG CoE and (d) β-CD–PEG LY Susp.
The interesting feature of the ternary complexes is the absence of the decomposition peak in lyophilized suspension system and in coevaporated ternary system supporting the fact that the inclusion of the drug has enhanced its physical stability.
3.4. X-ray powder diffraction (XRPD)
The diffraction patterns of the complexes should be clearly distinct from that of superimposition of each component if a real inclusion has taken place. The presence of drug characteristic peaks with reduced intensity in physical mixtures and kneaded binary complexes of all three CDs indicates incomplete inclusion phenomenon (Fig. 6). It is no longer possible to distinguish the characteristic peaks of the drug in the lyophilized system, which showed a modified and hollow pattern suggesting the formation of amorphous inclusion complex with Me-β-CD and HP-β-CD.
Fig. 6.
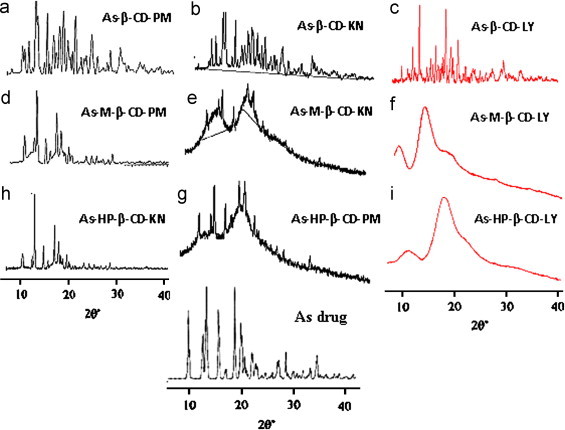
PXRD pattern of artesunate with (a) β-CD PM, (b) β-CD KN, (c) β-CD LY, (d) Me-β-CD PM, (e) Me-β-CDKN, (f) Me-β-CDLY, (g) HP-β-CDPM, (h) HP-β-CDKN and (i) HP-β-CD LY.
The ternary systems of all the formulations with β-CD showed some diffraction 2θ peaks with little intensity, which is attributed to a crystalline nature of β-CD (Fig. 7).
Fig. 7.
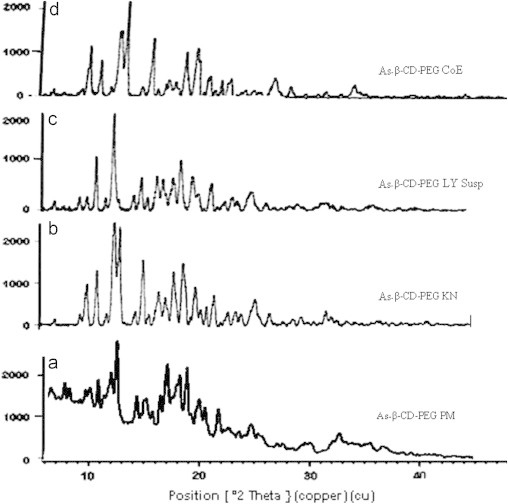
PXRD pattern of artesunate with (a) β-CD–PEG PM, (b) β-CD–PEG KN, (c) β-CD–PEG LY Susp and (d) β-CD–PEG CoE.
3.5. Fourier transform infrared spectroscopy (FT-IR)
FT-IR spectra of the binary and ternary inclusion complexes are quite similar to the corresponding CDs because of the coincidental absorption of both the host and guest molecules in the same spectral regions. However, the small shifts in characteristic bands of drug at 1719, 1370 and 1029 cm−1 undoubtedly confirm the presence of drug in all the complexes.
3.6. Proton NMR spectroscopy
Inclusion of drug protons resulted in modification of NMR frequencies of both the guest (artesunate) and the host (CD). A downfield shift in the cycloheptane protons l, k, j, i, m and h of drug revealed the presence of artesunate molecule into the cyclodextrins cavity. Insertion is favored towards the cycloheptane ring with endoperoxide group due to its narrower dimension (2.89 Å) as compared to the opposite end of the drug molecule, consisting of two cyclohexane rings (6.9 Å) (Fig. 8). Insertion of side chain of artesunate molecule is ruled out due to more hydrophilic nature. A downfield displacement in protons c and o indicate that these protons are closer to the electronegative atom (oxygen) on the exterior of the CD cavity. Two-dimensional (2D) COESY spectra were used further to get a better insight into the geometry of the complex. It provides the information about the spatial proximity between host and guest atoms by observing intermolecular cross-relations. The appearance of cross peaks (Fig. 9) between H-5 and H-3 protons of CD and H-l, H-j and H-g protons supports our proposed inclusion mode involving insertion of cycloheptane ring with endoperoxide bridge (trioxane ring) deep into the cavity. No data is available for the direct comparison of NMR studies except one work reported by Hartell et al. In this work the authors reported that trioxane ring as well as aromatic ring of artesunic acid is complexed with the CDs, thus supporting our results. However, authors also suggested the possibility of 2:1 stoichiometry whereas our studies reports 1:1 stoichiometry.
Fig. 8.
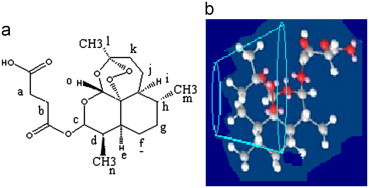
(a) The chemical structure of artesunate and (b) inclusion mode of drug into cyclodextrin cavity.
Fig. 9.
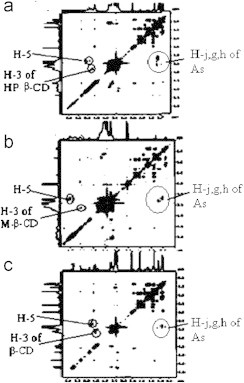
1H COESY of artesunate with (a) β-CD, (b) Me-β-CD and (c) HP-β-CD.
3.7. Thermodynamic parameters of inclusion complexes in the presence or absence of PEG
Solution calorimetry is used to quantify the binding interactions between the drugs with CD's forming noncovalent complexes in aqueous solution and to determine the relationship between noncovalent structure and free energy of binding including the roles of enthalpy and entropy of association.
Stability constant and other thermodynamic parameters were calculated by determining the enthalpy of solution of the drug in the absence and presence of CDs as well as PEG. The molar enthalpy of solution of drug (ΔsolH(M)) was found to be exothermic (−0.09 kJ/mol) in phosphate buffer (pH 6.8). Enhanced exothermic behavior was exhibited by the drug in the presence of CDs and further enhancement was observed when both CDs and 0.25% PEG were present. This is attributed to synergetic interaction between drug and the cyclodextrin in the presence of PEG. The enthalpy of interaction was calculated by the following equation:
| (1) |
where ΔHint(exp) is the enthalpy of interaction between drug and cyclodextrin per liter of solution, ΔsolH and ΔsolH(CD) are the enthalpies of solution of drug in buffer and in buffered aqueous solution of cyclodextrin, respectively, v (l)=volume of sample cell in liters (0.025 L).
Enthalpy of interaction per mole of drug and β-CD (ΔHint(M)) were calculated from
| (2) |
where a and b are the initial molar concentrations of drug and cyclodextrin, x1 and x2 are the apparent mole fractions of the drug and cyclodextrin ignoring the concentration of other ingredients in the buffer.
The detailed calorimetric data for artesunate and Me-β-CD are given in Table 1.
Table 1.
Interaction enthalpy of inclusion complexes artesunate with Me-β-CD at pH 6.8.
| x2 | MAs(a) ((M)×10−4) | Mm-b-CD(b) ((M)×10−4) | ΔsolH(CD)((J)×10) | ΔHint(exp)(J/L) | ΔHint(M)(kJ/mol) |
|---|---|---|---|---|---|
| 0.902 | 3.26 | 30.49 | −1.80 | −3.47 | −1.03 |
| 0.851 | 3.92 | 22.35 | −2.17 | −4.09 | −1.56 |
| 0.801 | 3.79 | 15.22 | −2.09 | −3.63 | −1.91 |
| 0.754 | 3.68 | 11.29 | −2.04 | −3.36 | −2.25 |
| 0.688 | 3.25 | 7.15 | −1.79 | −2.36 | −2.27 |
| 0.606 | 1.97 | 3.03 | −1.09 | −1.14 | −2.28 |
| 0.515 | 2.87 | 3.05 | −1.59 | −1.38 | −2.33 |
| 0.458 | 3.63 | 3.07 | −2.01 | −1.52 | −2.28 |
| 0.397 | 4.71 | 3.09 | −2.61 | −1.70 | −2.18 |
| 0.315 | 7.08 | 3.26 | −3.92 | −2.20 | −2.13 |
| 0.259 | 8.88 | 3.10 | −4.92 | −2.50 | −2.09 |
| 0.202 | 11.94 | 3.07 | −6.62 | −2.78 | -1.85 |
| 0.157 | 14.12 | 2.63 | −7.82 | −2.50 | −1.49 |
| 0.096 | 14.31 | 1.52 | −7.93 | −1.48 | −0.93 |
The stoichiometry of the complex was ascertained utilizing continuous variation method (Job's plot) [34] by plotting (ΔHint(M)) versus (x2) (Fig. 10). It can be seen that the minimum occurs at x2=0.5, which indicates that the complex has 1:1 stoichiometry and supports its determination by other techniques.
Fig. 10.
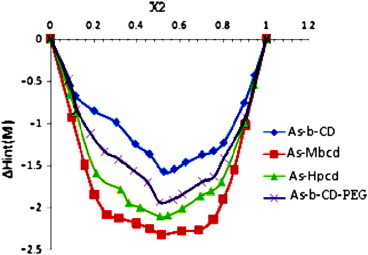
Plot of ΔsolHint(M) versus mole fraction (x2) of artesunate with β-CD, Me-β-CD, HP-β-CD and As–β-CD–PEG at pH 7.
Similarly the enthalpy of interaction for the ternary system was calculated by subtracting the enthalpy of solution of drug in the presence of cyclodextrin and 0.25% PEG from that in pure buffer (ΔHo(M)).
The thermodynamic constants are calculated assuming the following equilibria:
| CD+artesunate↔CD:artesunate | (3) |
The experimentally calculated enthalpy of interaction (ΔHint(exp)) is proportional to the product of molar concentration of CD:artesunate complex (c) in the solution at equilibrium and enthalpy of binding per mole of drug (ΔHo)
| (4) |
The binding constant K and enthalpy of binding (ΔHo) for both binary and ternary systems were computed from the experimentally determined enthalpy of interaction (ΔHint(exp)). The calculations were done by our computer program utilizing an iterative non-linear least square regression method to minimize the value of ∑(ΔHint(exp)−ΔHint(calc))2 and are given in Table 2.
Table 2.
Thermodynamic parameters of artesunate with β-CD, Me-β-CD and HP-β-CD as well as in the presence of PEG at pH 6.8 in phosphate buffer, determined using solution calorimetry.
| System | K (M−1) | ΔHo (kJ/mol) | ΔGo (kJ/mol) | ΔSo (J/mol/K) |
|---|---|---|---|---|
| As+β-CD | 1227±5.0 | –9.10±0.005 | –18.33±0.008 | 29.78±0.005 |
| As+HP-β-CD | 1852±8.0 | –11.00±0.006 | –19.39±0.007 | 27.07±0.006 |
| As+Me-β-CD | 2410±10.0 | –12.02±0.006 | –20.07±0.006 | 25.97±0.006 |
| As+β-CD+PEG | 2250±16.0 | –11.8±0.013 | –19.89±0.008 | 26.11± 0.013 |
The values of free energy of inclusion (ΔGo) and entropy of inclusion (ΔSo) were calculated from the following equations:
| (5) |
| (6) |
The table shows that the inclusion of drug is exothermic process (ΔHo is negative) while entropy of reaction is positive in all these cases leading to values of Gibbs free energy (ΔGo) between −18.33 and −20.7 kJ/mol. The favorable enthalpy and entropy changes indicate proper fit of artesunate into the CD cavity. Table 2 shows that the numerical value of ΔHo is the highest in As–Me-β-CD complex. The enthalpic gain is obtained predominantly through van der Waals interaction of methyl group introduced in M-βCD. However, this decrease in ΔHo in case of Me-β-CD is more or less compensated by lesser positive entropy change (Table 2). In the case of HP-β-CD, the hydroxyl groups make the CD cavity partially hydrophilic and the complexation reaction between HP-β-CD and artesunate is less enthalpically driven but are accompanied by a more positive entropy change.
The magnitudes of stability constant (K) reveals that host–guest affinity is found to be maximum (2410 M−1) for artesunate Me-β-CD complex. Interestingly, it compares well with K value for ternary complexes (As–β-CD in the presence of PEG), which have nearly the same magnitude (2250 M−1). This shows that β-CD in the presence of PEG has approximately the same complexation efficiency as Me-β-CD alone has towards artesunate. Polymer establishes different interactions with CD and drug molecules such as hydrophobic bonds, Van der Waals dispersion forces or hydrogen bonds. Besides this, strong interaction between CD and artesunate is reflected in less positive entropy in the presence of PEG (Table 2). Addition of PEG to As–β-CD binary complex resulted in marked enhancement in the complexation and solubilizing efficiencies of β-CD and this approach seems useful for improving the performance of β-CD.
3.8. Dissolution studies
All the binary systems show significantly improved dissolution rate as compared to the pure drug. It is also clear that release rate is fastest for Me-β-CD followed by HP-β-CD and β-CD complexes. In the case of ternary system, dissolution is fastest in lyophilized complexes as to PM, KN and CoE systems. The increase in dissolution rate in lyophilized binary and ternary complexes may be due to the true inclusion as well as due to the high energy amorphous state of lyophilized products (Fig. 11). In the presence of hydrophilic polymers a smaller amount of CD is used to obtain the desired dissolution profile. Significant enhancements in dissolution rate of freeze dried product of ternary complex may be attributed to an increase in solubility upon complexation of β-CD as well as due to polymer. Thus, addition of hydrophilic polymers could be a strategy for improving the usefulness of CDs. The lyophilized complex with the highest dissolution rate is most suitable product for the animal studies.
Fig. 11.
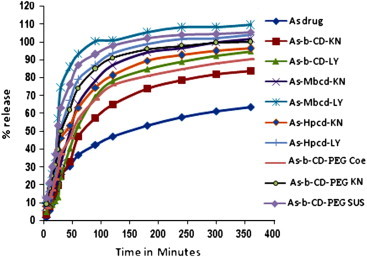
Dissolution studies in artesunate in binary and ternary systems.
3.9. Molecular modeling studies
During the trajectory analysis, it was seen that the β-CD–artesunate complex retained its structure and was stable during the entire time period of the simulation. The average root-mean-square deviation (RMSD) for the complex over the entire trajectory of 5 ns was computed as 1.33, while that of the final frame was 1.56 (Fig. 12). This shows that the β-CD–artesunate complex does not separate out and remains steady throughout the time period of simulation, which is acceptable in simulations. The interaction energies (Coulombic, van der Waals) between β-CD and artesunate were computed to be −20.31 and −30.93 kcal/mol (Table 3) and are further used for calculating the binding energy for the entire trajectory:
The mean binding energy computed for β-CD–artesunate complex is −4.89 kcal/mol (−20.46 kJ/mol), which is close to the experimentally determined values. The visual inspection of the complex presents that on an average there are two H-bond interactions between β-CD and artesunate. The first H-bond occurs between proton-o (Fig. 8a) and the primary OH group of β-CD, while the second is seen between the carboxlyate group and secondary OH groups (varying in between the 2′-OH and 3′-OH groups) of the β-CD. The artesunate core is snugly fitted into the host cavity exhibiting an excellent interaction with the pyran rings of the sugar residues (Fig. 13).
Fig. 12.
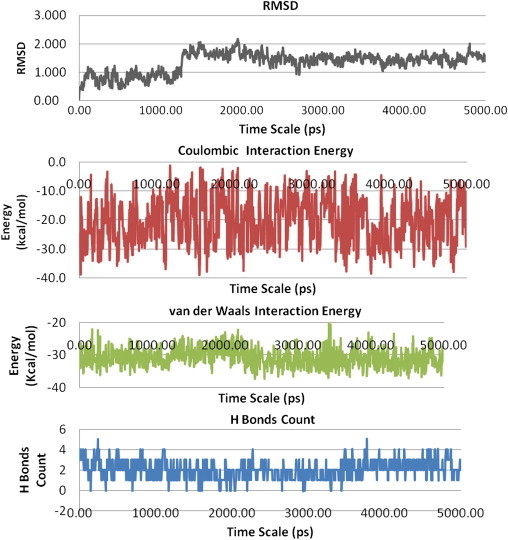
RMSD, Coulombic, van der Waals and H-bond interactions mapped over a 5 ns MD trajectory of the As–β-CD complex.
Table 3.
Interaction energies (Coulombic, van der Waals), H-bond counts and RMSD (w.r.t. frame zero in simulation) computed over the MD trajectory of the BCD–artesunate complex.
| Sl. no. | Structure | Interaction energies (kcal/mol) | Binding energies (kcal/mol) |
|---|---|---|---|
| 1. | Max. total energy | – | 37.22 |
| 2. | Min. total energy | – | −40.72 |
| 3. | Mean. total energy | – | −4.89 |
| 4. | Max. Coulombic energy | −20.3 | 31.0 |
| 5. | Min. Coulombic energy | −1.3 | −48.3 |
| 6. | Mean Coulombic energy | −39.0 | −7.9 |
| 7. | Max. van der Waals energy | −30.9 | 33.3 |
| 8. | Min. van der Waals energy | −19.8 | −21.7 |
| 9. | Mean. van der Waals energy | −37.3 | 3.1 |
| 10. | Max. no. of H bonds | 5 | – |
| 11. | Min. no of H bonds | 0 | – |
| 12. | Average no. of H bonds | 2 | – |
| 13. | Max. RMSD | 1.338 | – |
| 14. | Min. RMSD | 2.171 | – |
Energy values in kcal/mol.
Fig. 13.
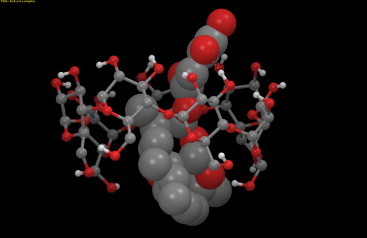
The space-fill model of artesunate (guest) and β-cyclodextrin (host) depicting the intermolecular interactions.
3.10. In vivo antimalarial activity of artesunate and its binary and ternary inclusion complexes
The effectiveness of the binary and ternary complexes was confirmed by performing in vivo antimalarial activity against P. berghei infection. Suspensions containing artesunate, binary and ternary inclusion complexes were tested with respect to parasitemia progression and survival period. It is clear from the Table 4 that mice treated with artesunate dose (Standard Group) significantly prolonged their survival period (day 15–18) compared to control (day 9), but is insufficient to prevent the mortality. Test Group 1, Test Group 2 and Test Group 4 treated mice died between 15–25 days, 20–26 and 27–30 days, respectively, whereas Test Group 3 (Ternary lyophilized system) resulted in a 100% survival of infected mice even after 30 days. Survival rate of the infected mice increases from 16.7% to 50%, 67%, 83.3% for standard group and binary complexes of artesunate with β-CD, HP-β-CD and ternary complexes of β-CD, respectively. Binary Me-β-CD lyophilized suspensions are found to be more effective against P. berghei malaria with 0% mortality. Significantly less (P<0.001) mean percent parasitemia was observed in the Test Group 3 (0.002±0.0016) compared to all test groups. ANOVA have also shown significant (P<0.05) antimalarial activity of all binary and ternary complexes as to artesunate (Fig. 14).
Table 4.
Antimalarial activity of binary and ternary lyophilized complexes of artesunate in P. berghei infected mice.
| Sl. no. | Groups | Treatment | Mean % parasitemia ⁎ on day 8th PI | % Survivality (n=6, t=30 days) |
|---|---|---|---|---|
| 1 | Control group | 0.5% CMC solution | 49.23±12.34 | 0 |
| 2 | Standard group | Artesunate ∼(6 mg/kg) | 8.48±3.21 | 16.7 |
| 3 | Test group 1 | As−β-CD ∼(6 mg/kg of artesunate) | 6.25±6.82 | 50 |
| 4 | Test group 2 | As–HP-β-CD ∼(6 mg/kg of artesunate ) | 4.27±9.43 | 67.7 |
| 5 | Test group 3 | As–Me-β-CD ∼(6 mg/kg of artesunate ) | 0.002±0.0016 | 100 |
| 6 | Test group 4 | As–β-CD–PEG ∼(6 mg/kg of artesunate) | 1.025±1.04 | 83.3 |
t=no. of days.
n=no. of animals per group.
PI=post inoculation.
As–β-CD=artesunate–β-cyclodextrin complexes.
As–Me-β-CD=artesunate–methyl-β-cyclodextrin complexes.
As–HP-β-CD=artesunate–hydroxypropyl-β-cyclodextrin complexes.
As–β-CD–PEG=artesunate–β-cyclodextrin–PEG complexes.
Values were expressed as mean±SD (standard deviation).
Fig. 14.
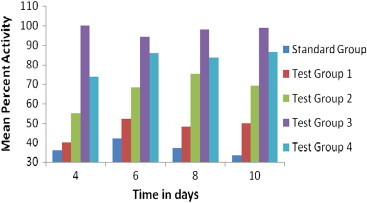
Antimalarial activity of Me-β-CD lyophilized complexes of artesunate in P. berghei infected mice “(n=6)” as compared to drug.
4. Conclusion
The mean binding energy computed by molecular modeling for β-CD–artesunate complex is correlated well with the experimentally determined values. The ternary systems clearly signify superiority over binary complexes in terms of solubility and reduction in the formulation bulk. PEG was found to be the most suitable auxiliary substance in terms of superior complexation efficiency and stability constant. Higher stability constant values in the presence of PEG suggest a significant improvement in the complexation efficiency between artesunate and β-CD. This is supported by the in vitro dissolution rate, which was found to be maximum for Me-β-CD lyophilized complexes. Enhanced in vivo antimalarial activity and protective efficacy against P. berghei infection was observed for the complexes. However, increment is more in the presence of PEG. Best survival rate was observed for binary complexes with Me-β-CD, which is comparable to the ternary complexes of β-CD in the presence of PEG. Thus, encapsulation of artesunate by cyclodextrins in the presence of PEG is a good alternative to enhance the bioavailability of the drug as well as to enhance its antimalarial activity.
Acknowledgment
The financial assistance provided by Indian Council of Medical Research (BMS; 45/49/2006), New Delhi, India, and Instrumentation assistance by Department of Science Technology (DST), New Delhi, is gratefully acknowledged. The computational facilities supported by the Department of Biotechnology (DBT; BT/TF-8/BRB/2009) and Department of Science and Technology (DST; SR/FST/LSI-163/2003), New Delhi, are gratefully acknowledged.
References
- 1.Lee I., Hufford C.D. Pharmacol Ther. 1990;48:345–355. doi: 10.1016/0163-7258(90)90053-5. [DOI] [PubMed] [Google Scholar]
- 2.Benakis A., Paris M., Plessas C., Hien T.T., Waller D., White N.J. Am J Trop Med Hyg. 1993;49:293. [Google Scholar]
- 3.Balint G.A. Pharmacol Ther. 2001;90:261–265. doi: 10.1016/s0163-7258(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 4.Lin A.J., Miller R.E.J. Med Chem. 1995;38(5):764–770. doi: 10.1021/jm00005a004. [DOI] [PubMed] [Google Scholar]
- 5.Ferone R. Bull WHO. 1977;55:291–298. [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q.G., Peggins J.O., Fleckenstein L.L., Masonic K., Heiffer M.H., Brewer T.G. J Pharm Pharmacol. 1998;50:173–182. doi: 10.1111/j.2042-7158.1998.tb06173.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X., Liu J., Qiao H., Liu H., Ni J., Zhang W. Powder Technol. 2010;197:120–128. [Google Scholar]
- 8.Hein T.T., White N.J. Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- 9.Gabriels M., Plaizier-Vercammen J.J. Pharm Biomed Anal. 2003;31:655–667. doi: 10.1016/s0731-7085(02)00678-7. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Xin-Cai., Hong Zong-Guo. Int J Nanomed. 2010;5:483–486. doi: 10.2147/ijn.s10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartell M.G., Hicks R., Bhattacharjee A.K., Koser B.W., Carvalho K., Van Hamont J.E.J. Pharm Sci. 2004;93:2076–2089. doi: 10.1002/jps.20106. [DOI] [PubMed] [Google Scholar]
- 12.Loftsson T., Brewster M.J. Pharm Sci. 1996;85:1017–1025. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 13.Loftsson T. Pharmazie. 1998;53:733–740. [PubMed] [Google Scholar]
- 14.Asbahr A.C.C., Franco L., Barison A., Silva C.W.P., Rodrigues L.N.C., Ferraz H.G. Bioorg Med Chem. 2009;17:2718–2723. doi: 10.1016/j.bmc.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 15.Zhao D., Zhao L., Zhu C., Tian Z., Shen X. Carbohydr Polym. 2009;78:125–130. [Google Scholar]
- 16.Fakayode S.O., Busch M.A., Busch K.W. Talanta. 2006;68:1574–1583. doi: 10.1016/j.talanta.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Santos-Magalhães N.S., Mosqueira. V.C.F. Adv Drug Deliv Rev. 2010;62(4–5):560–575. doi: 10.1016/j.addr.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Mura P., Cirri M., Maestrelli F. Int J Pharm. 2003;260:293–302. doi: 10.1016/s0378-5173(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 19.Mura P., Bettinetti G.P., Cirria M., Maestrelli F., Sorrenti M., Catenacci L. Eur J Pharm Biopharm. 2005;59:99–106. doi: 10.1016/j.ejpb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Patravale V.B., Date A.A., Kulkarni R.M.J. Pharm Pharmacol. 2004;56:827–840. doi: 10.1211/0022357023691. [DOI] [PubMed] [Google Scholar]
- 21.Chingunpitak J., Puttipipatkhachorn S., Chavalitshewinkoon-Petmitr P., Tozuka Y., Moribe K., Yamamoto K. Drug Dev Ind Pharm. 2008;34:314–322. doi: 10.1080/03639040701662388. [DOI] [PubMed] [Google Scholar]
- 22.Mura P., Faucci M.T., Manderioli A., Bramanti G. J Incl Phenom. 2001;39:131–138. [Google Scholar]
- 23.Redenti E., Szente L., Szejtli J.J. Pharm Sci. 2000;89:1–8. doi: 10.1002/(SICI)1520-6017(200001)89:1<1::AID-JPS1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 24.Loftsson T., Fridriksdottir H., Olafsdottir B.J. Acta Pharm Nordica. 1991;3:215–217. [Google Scholar]
- 25.Higuchi T., Connors K.A. Adv Anal Chem Instrum. 1965;4:117–212. [Google Scholar]
- 26.McGann M., Almond H., Nicholls A., Grant J.A., Brown F. Biopolymers. 2003;68:76. doi: 10.1002/bip.10207. [DOI] [PubMed] [Google Scholar]
- 27.McGaughey G.B., Sheridan R.P., Bayly C.I., Culberson J.C., Kreatsoulas C., Lindsley S. Chem Inf Model. 2007;47:1504. doi: 10.1021/ci700052x. [DOI] [PubMed] [Google Scholar]
- 28.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H. Protein Data Bank—Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mark P., Nilsson L.J. Phys Chem A. 2001;105:9954–9960. [Google Scholar]
- 30.Quigley Q., Prober M.I.J. Comput Phys Commun. 2005;169:322–325. [Google Scholar]
- 31.Ryckaert J.P., Ciccotti G., Berendsen H.J.C.J. Comput Phys. 1977;23:327–341. [Google Scholar]
- 32.Kobetic R., Jursic B.S., Bonnette S., Tsai S.-C., Salvotore S.J. Tetrahedron Lett. 2001;42:6077–6082. [Google Scholar]
- 33.Cai Y., Tarr M.A., Xu G.X., Yalcin T., Cole R.B.J. Am Soc Mass Spectrom. 2003;14:449–459. doi: 10.1016/S1044-0305(03)00136-3. [DOI] [PubMed] [Google Scholar]
- 34.Job P. Ann Chim. 1928;9:113–203. [Google Scholar]


