Abstract
Strains of indigenous Escherichia coli, Bacteroides, and Lactobacillus were isolated from the gastrointestinal tracts of specific pathogen-free (SPF) mice. Nonvaccinated SPF mice exhibited in their spleens low numbers of plaque-forming cells (PFC) and rosette-forming cells reacting with antigens of these andigenous bacteria. PFC reacting with these bacterial antigens were not detected in infant SPF mice until 7 days after birth. Compared with nonvaccinated controls, SPF mice vaccinated parenterally with indigenous E. coli or Bacteroides produced a moderate increase in the numbers of specific PFC. Thus, the SPF mouse is capable of responding immunologically after vaccination with microbes indigenous to its intestinal tract. However, more PFC reacting with homologous vaccine antigens were detected after parenteral vaccination of SPF mice with nonindigenous E. coli O127:B8, E.coli O14, or B. fragilis than after parenteral vaccination with indigenous E. coli or Bacteroides. Gnotobiotic mice orally monoassociated with these nonindigenous bacteria exhibited greater immune responses to antigens of the bacteria used to monoassociation than did gnotobiotes monoassociated with the indigenous microbes. The results are consistent with the hypothesis that mice are more responsive immunologically to antigens of nonindigenous bacteria than they are to antigens of certain microbes indigenous to their gastrointestinal tracts.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balish E., Yale C. E., Hong R. Serum proteins of gnotobiotic rats. Infect Immun. 1972 Aug;6(2):112–118. doi: 10.1128/iai.6.2.112-118.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
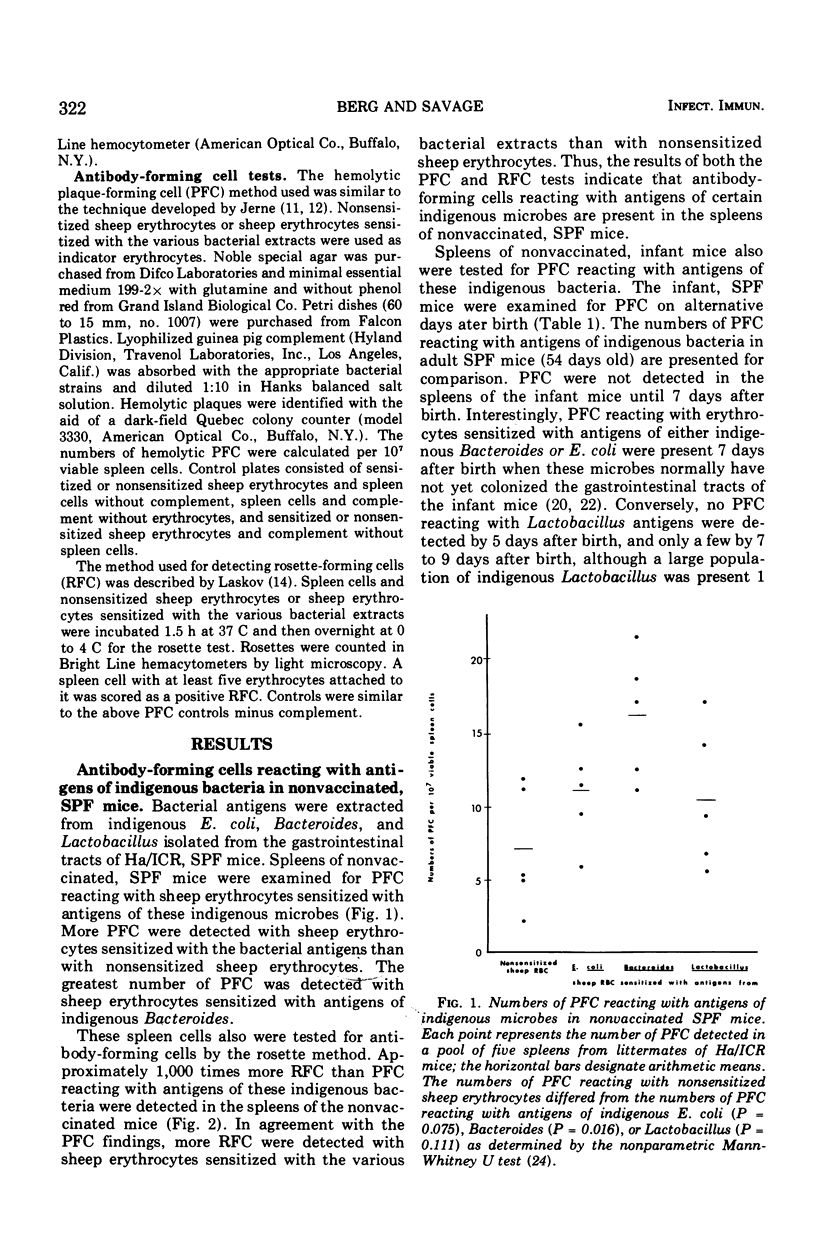
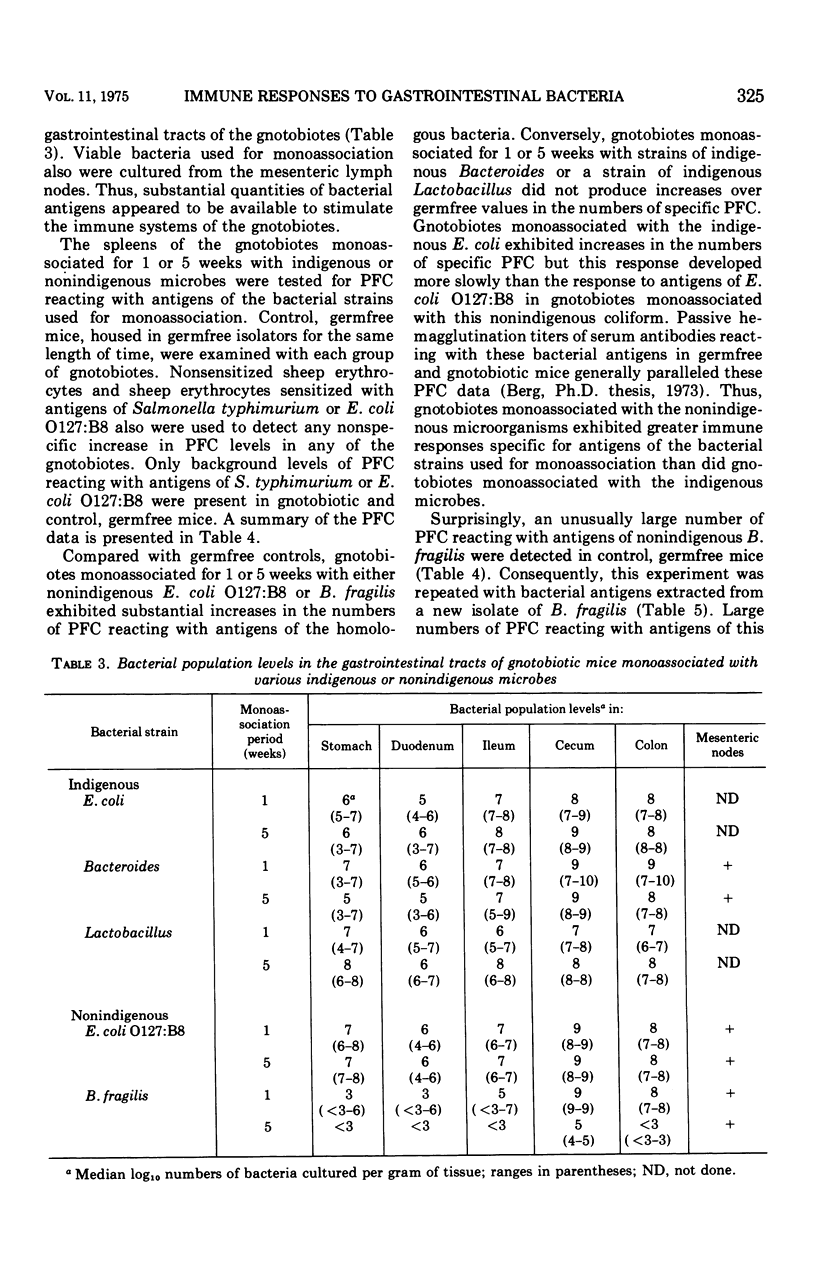
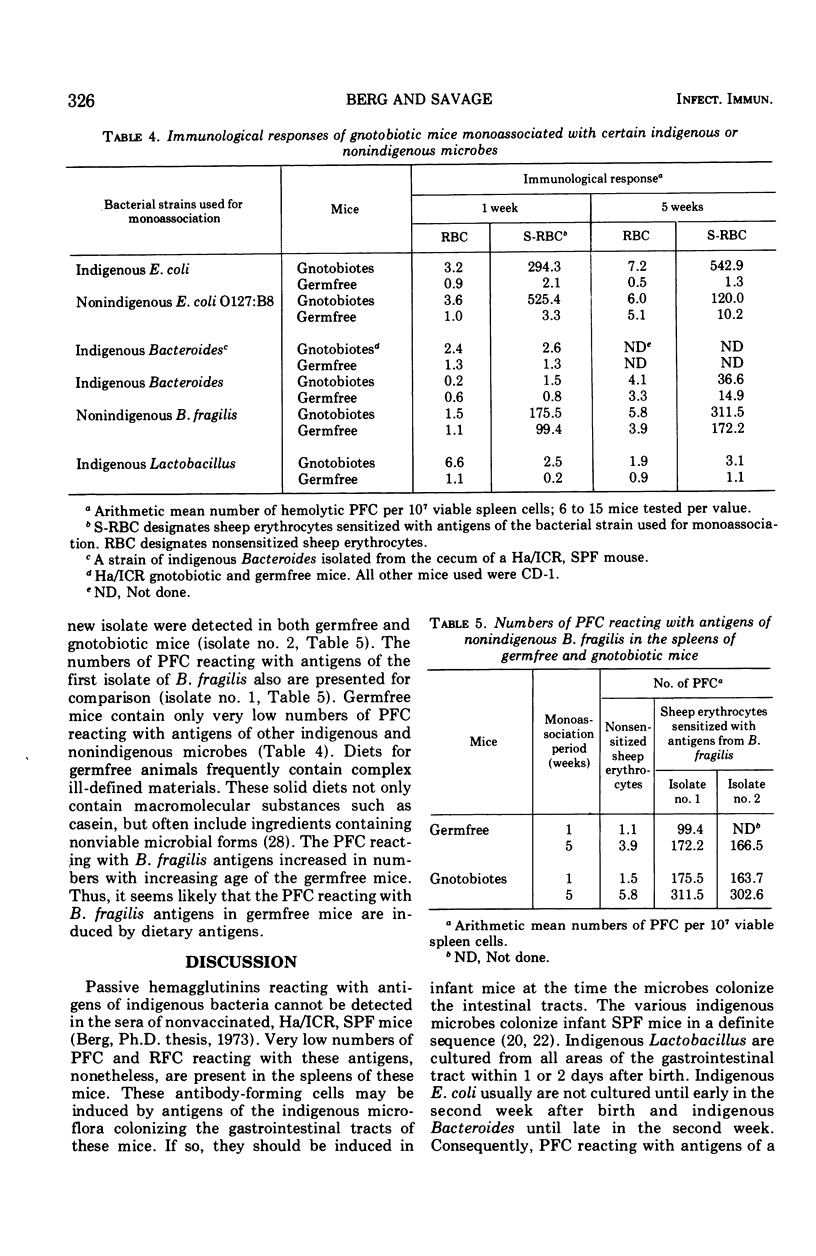
- Berg R. D., Savage D. C. Immunological responses and microorganisms indigenous to the gastrointestinal tract. Am J Clin Nutr. 1972 Dec;25(12):1364–1371. doi: 10.1093/ajcn/25.12.1364. [DOI] [PubMed] [Google Scholar]
- Carter P. B., Pollard M. Host responses to "normal" microbial flora in germ-free mice. J Reticuloendothel Soc. 1971 Jun;9(6):580–587. [PubMed] [Google Scholar]
- Foo M. C., Lee A. Immunological response of mice to members of the autochthonous intestinal microflora. Infect Immun. 1972 Oct;6(4):525–532. doi: 10.1128/iai.6.4.525-532.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon H. A., Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 1971 Dec;35(4):390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANKS J. H., WALLACE J. H. Determination of cell viability. Proc Soc Exp Biol Med. 1958 May;98(1):188–192. doi: 10.3181/00379727-98-23985. [DOI] [PubMed] [Google Scholar]
- Hechtel M., Dishon T., Braun W. Hemolysin formation in newborn mice of different strains. Proc Soc Exp Biol Med. 1965 Dec;120(3):728–732. doi: 10.3181/00379727-120-30638. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Krüger G., Stolpmann H. J. Postnatal development of lymph nodes in mice after prenatal antigenic stimulation. Z Immunitatsforsch Exp Klin Immunol. 1971 Aug;142(2):115–121. [PubMed] [Google Scholar]
- Laskov R. Rosette forming cells in non-immunized mice. Nature. 1968 Aug 31;219(5157):973–975. doi: 10.1038/219973a0. [DOI] [PubMed] [Google Scholar]
- Luckey T. D. Introduction to the ecology of the intestinal flora. Am J Clin Nutr. 1970 Nov;23(11):1430–1432. doi: 10.1093/ajcn/23.11.1430. [DOI] [PubMed] [Google Scholar]
- Miller C. E., Wong K. H., Feeley J. C., Forlines M. E. Immunological conversion of Vibrio chorlerae in gnotobiotic mice. Infect Immun. 1972 Nov;6(5):739–742. doi: 10.1128/iai.6.5.739-742.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann P., Hammarström S., Lagercrantz R., Campbell D. Autoantibodies to colon in rats and human ulcerative colitis: cross reactivity with Escherichia coli O:14 antigen. Proc Soc Exp Biol Med. 1967 Jul;125(3):975–980. doi: 10.3181/00379727-125-32253. [DOI] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARPE M. E. A serological classification of lactobacilli. J Gen Microbiol. 1955 Feb;12(1):107–122. doi: 10.1099/00221287-12-1-107. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Miller C. E. Progressive changes of Vibrio serotypes in germ-free mice infected with Vibrio cholerae. J Bacteriol. 1969 Sep;99(3):688–695. doi: 10.1128/jb.99.3.688-695.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Associations and physiological interactions of indigenous microorganisms and gastrointestinal epithelia. Am J Clin Nutr. 1972 Dec;25(12):1372–1379. doi: 10.1093/ajcn/25.12.1372. [DOI] [PubMed] [Google Scholar]
- Savage D. C. Associations of indigenous microorganisms with gastrointestinal mucosal epithelia. Am J Clin Nutr. 1970 Nov;23(11):1495–1501. doi: 10.1093/ajcn/23.11.1495. [DOI] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREXLER P. C., REYNOLDS L. I. Flexible film apparatus for the rearing and use of germfree animals. Appl Microbiol. 1957 Nov;5(6):406–412. doi: 10.1128/am.5.6.406-412.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]



