Abstract
Background
Self-injury and aggression have been reported in individuals with TSC (tuberous sclerosis complex), yet few data exist about treatment. Everolimus, an mTOR inhibitor, has been FDA-approved for subependymal giant cell astrocytomas (SEGAs) and renal angiomyolipomas in TSC. However, clinical use of everolimus with direct, real-time observations of self-injury and aggression in an individual with TSC has not been reported.
Methods
During an inpatient admission to a neurobehavioral unit, real-time measurements of behaviors and seizures were recorded. An interdisciplinary team used these data to make treatment decisions and applied behavioral and pharmacological treatments, one at a time, in order to evaluate their effects.
Results
Aggression and self-injury improved with applied behavioral analysis (ABA), lithium, and asenapine. Improvements in SEGA size, facial angiofibromas, seizures, and the most stable low rates of self-injury were observed during the interval of treatment with everolimus.
Conclusion
Mechanism-based treatments in the setting of an evidence-based behavioral and psychopharmacological intervention program may be a model with utility for characterization and treatment of individuals with severe behavior and TSC.
Abbreviations: ABA, applied behavioral analysis; CYP3A4, cytochrome p450 3A4; FDA, Food and Drug Administration; SEGA, subependymal giant cell astrocytoma; mTOR, mammalian target of rapamycin; mTORC, mammalian target of rapamycin complex; TSC, tuberous sclerosis complex
Keywords: Tuberous sclerosis complex, Self-injury, Aggression, Everolimus, Behavioral intervention and epilepsy
1. Case report
Tuberous sclerosis complex (TSC) is variably expressed based on the random distribution, number, size, and location of lesions. A wide variation in the extent and severity of clinical manifestations is present even between relatives. The phenotype may be mild in some patients with only minor cutaneous lesions and asymptomatic cardiac rhabdomyomas or highly disabling in others with seizures, intellectual disability, autism, and self-injurious or aggressive behavior. The source of this variability and best treatment for those with a severe phenotype are unknown.
We report the case of a 14-year-old young man with clinically definite tuberous sclerosis complex, epilepsy characterized primarily by focal motor seizures with altered awareness evolving to bilateral convulsive seizures (left head and eye deviation, drooling, grunting, facial twitching, and tonic–clonic extremity movements), autism spectrum disorder, and moderate intellectual disability who was transferred from a residential treatment facility to an inpatient neurobehavioral unit because of severe aggression. Aggressive behavior included kicking, biting, head-butting, hair-pulling, hitting, slapping, pushing, pinching, and scratching. Self-injurious behavior included self-biting or scratching. Elopement, fecal play, disrobing, and property destruction were also present.
As an infant, cardiac rhabdomyomas and pathognomonic brain lesions were observed. Infantile spasms observed without EEG recording at 6 months evolved into intractable epilepsy without remission despite multiple medications (phenobarbital, vigabatrin, carbamazepine, topiramate, valproic acid, lamotrigine, and oxcarbazepine) and 2 neurosurgical procedures. Subependymal giant cell astrocytomas, a shagreen patch, facial angiofibromas, a retinal phakoma, and an ungual fibroma also developed over time.
Hyperactivity following neurosurgery was the first behavioral concern. Despite behavioral treatment and multiple medications — methylphenidate, dextroamphetamine, sertraline, aripiprazole quetiapine, alprazolam, amitriptyline, clonidine, guanfacine, risperidone, paroxetine, and diazepam, severe aggression resulted in residential placement. After four years in residential treatment, the severity of aggression prompted a referral to a neurobehavioral unit where inpatient evaluation and treatment were recommended.
Real-time recording of behaviors and seizures as well as a structured daily routine including activities of daily living, academic tasks, and scheduled leisure time was implemented upon admission. Observations revealed aggression and self-injury that occurred throughout the day at an average rate of 68.0 per hour and 4.0 per hour, respectively. Aggression was likely to occur in bursts and last for long durations, whereas self-injury occurred as discrete instances or in short episodes. An extensive functional behavioral analysis was initiated immediately upon admission to determine what environmental variables were maintaining these behaviors. This process involves isolating possible motivational factors hypothesized to trigger the target behavior and manipulating responses during brief and repeated experimental sessions. The highest rates of aggressive behavior were observed during transitions and instructional demands or when adult attention was unavailable. Collective results identified the following functions: to escape from transitions, academic tasks, and daily living tasks and to gain access to adult attention. Self-injury was not reliably observed during the functional analysis; thus, conclusions about its function could not be made. Following the assessment period, the behavioral psychology team initiated evaluations of possible function-based behavioral interventions.
During the functional analysis process, medical attention focused primarily on management of epilepsy. Admission medications included oxcarbazepine, tranxene (a benzodiazepine derivative), vigabatrin, and melatonin. Oxcarbazepine and vigabatrin were continued, and abortive treatment (rectal diazepam or nasal midazolam) was added for single seizures lasting longer than 5 min or a cluster of three or more seizures lasting at least 30 s in a 24-hour period. These seizures requiring diazepam or midazolam either due to duration or total number in 24 h were classified as “severe seizures” and documented separately. The percentage of severe seizures during baseline was 23.8% (5/21) while taking vigabatrin and oxcarbazepine over 8 weeks. Ictal activity was suspected near the SEGA in the right frontal lobe; therefore, everolimus treatment was initiated to reduce the size of the SEGA. A confirmatory EEG was not feasible because of the severity of behaviors. Dose titration and serum level monitoring were conducted in accordance with FDA-approved guidelines [1,2]. Overall, data recorded during therapeutic serum levels of everolimus revealed a slight decrease in size of the SEGA (Fig. 1), decreased frequency of severe seizures, and improvement in facial angiofibromas. Severe seizures decreased to 8% (3/39) during 12 weeks of everolimus at therapeutic serum levels (5–15 ng/ml) and oxcarbazepine. After discontinuation of everolimus due to parental concern regarding an adverse event (pneumonia), frequency of severe seizures increased to 21% (6/29) over 8 weeks with oxcarbazepine alone. Other antiepileptic medication changes included weaning of vigabatrin and addition of rufinamide. These changes did not affect seizures, and rufinamide was discontinued after 2 weeks because of increased aggression and agitation.
Fig. 1.
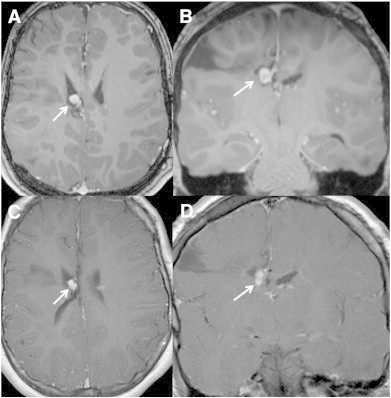
Magnetic resonance imaging of subependymal giant cell astrocytoma (SEGA) before and after treatment with everolimus. Pretreatment (A) axial and (B) coronal and (C) axial and (D) coronal 3D-T1-postcontrast-weighted images after 5-month treatment with everolimus show a mild reduction in size of the SEGA.
Lithium was initiated to target aggression, while the behavioral psychology team evaluated possible behavioral interventions. After initiation of lithium, an initial behavioral intervention was implemented 24 h daily. This intervention, behavioral extinction, was based on the finding of the functional analysis that aggressive behavior was maintained, in part, by access to attention. Behavioral extinction is a process that involves no longer providing reinforcement for target behaviors. Therefore, during this period, staff no longer provided statements of concern (e.g., “Stop doing that, it hurts.”) when aggressive behaviors were observed. In combination with extinction, a continued increase in lithium toward a therapeutic dose resulted in a steady decrease in aggressive behavior, achieving a 69% reduction relative to baseline. After approximately one month, additional behavioral intervention components were added to extinction and implemented 24 h daily based on data from intensive behavioral therapy sessions. These evidence-based behavioral treatments consisted of a structured schedule, functional communication, noncontingent reinforcement, differential reinforcement, and redirection. A structured schedule was used to enhance the predictability for required work, transitions, or down time. Functional communication involved teaching appropriate communication using a picture card to request a break from a transition or a task or for attention from adults. Noncontingent reinforcement involved providing free access to highly preferred leisure items that were also found to be associated with low levels of aggressive and self-injurious behavior during transitions and down time. Differential reinforcement involved providing preferred snacks and attention during compliance with a transition or task demand without engaging in problem behavior. Finally, redirection involved physically moving away from the target of aggression using a specified procedure.
Shortly after the initiation of full behavioral treatment, everolimus reached goal levels (5–15 ng/ml). Asenapine 5 mg twice daily was added to target residual aggression and further mood stabilization. Asenapine was chosen after past failed trials of multiple first- and second-generation antipsychotics over the course of several years. During this period of intensive behavioral therapy and medication management with lithium, asenapine, everolimus, and oxcarbazepine, aggressive and self-injurious behaviors were observed to be at the lowest and steadiest levels of the admission, achieving an 80% and 78% reduction from baseline, respectively. Low rates of aggressive behavior maintained after everolimus was discontinued and slight modifications were made to the behavioral treatment, with a 92% reduction relative to baseline. Rates of self-injury remained low but became more variable about one month after everolimus was discontinued, with a 65% reduction in self-injury relative to baseline during the last few weeks of the admission.
2. Discussion
Aggression, self-injury, seizures, and SEGA size were targets of treatment in this case. Aggression was decreased by behavioral intervention, lithium, and asenapine. Severe seizures were the lowest when treated with everolimus and oxcarbazepine, and the frequency increased to baseline levels when everolimus was discontinued. Everolimus and lithium, two of the treatments used in this case, are both directly involved in the underlying neurobiology of TSC. Everolimus directly inhibits abnormally elevated mTOR activity associated with mTORC1, and lithium inhibits inositol-1-phosphate, which would typically increase activity of mTORC2 (Fig. 2). Everolimus has been FDA-approved for treatment of SEGAs and renal angiomyolipomas (AMLs) in TSC; however, the effects of everolimus on behavior and seizures have not been systematically studied. In contrast, the efficacious usage of lithium in aggressive behavioral disorders has been reported for decades. Early studies of lithium's utility in manic–depressive illness concomitantly showed significant reduction of aggression in animal models [3], and the benefit of lithium in youth with severe aggression and intellectual disability quickly appeared in the literature [4,5]. Similarly, behavioral interventions have a large body of literature supporting their effectiveness in reducing problem behavior in individuals with autism spectrum disorders and intellectual disabilities [6].
Fig. 2.
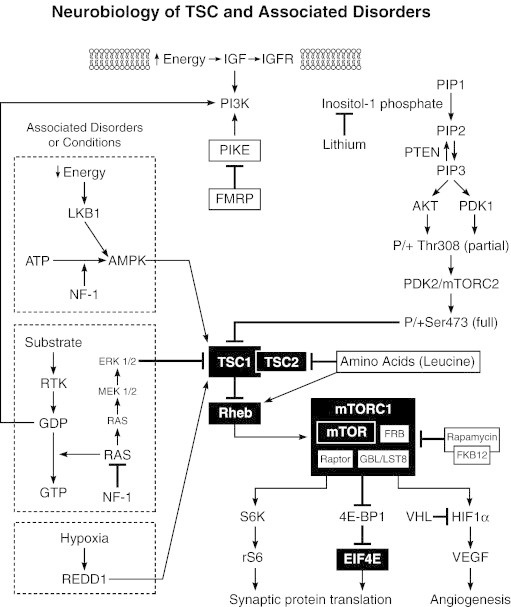
Schematic diagram of the mammalian target of rapamycin (mTOR) pathway and site of action of mechanism-based treatments.
Asenapine is a new second-generation antipsychotic indicated for the treatment of schizophrenia in the US and of mania associated with bipolar affective illness in both the US and Europe. Its sublingual formulation is unique among antipsychotics [7–10]. There is no current literature regarding the usage of asenapine in youth or in any patients with autism or other intellectual disabilities.
Aggression and self-injury have been reported in TSC; however, behavioral functions and response to treatment are rarely reported [11,12]. Previous case reports have described intellectual disability and seizures associated with self-injury in one clinical population of individuals with TSC. Frontal lobe ictal activity, the same location as the SEGA in this case, was also an association [13]. An integrated, interdisciplinary, evidence-based model was used for clinical care in this adolescent with TSC, SEGAs, epilepsy, cognitive limitations, severe aggression, and self-injury [14,15]. Multicenter studies are warranted to evaluate the utility and generalizability of this model for similarly affected individuals.
Conflict of interest statement
Dr. Gipson and Dr. Johnston have agreed to be site investigators for an upcoming clinical trial of everolimus sponsored by Novartis. Funds will be provided to Kennedy Krieger Institute for research expenses.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supported by Grant 2K12NS001696-11A1 (to TTG) from the National Institute of Neurological Disorders and Stroke (NINDS).
Approval and consent: The Johns Hopkins Institutional Review Board was consulted, and formal review was not required. The manuscript was reviewed and approved for publication by the parents.
References
- 1.Franz D.N. Everolimus: an mTOR inhibitor for the treatment of tuberous sclerosis. Expert Rev Anticancer Ther. 2011;11(8):1181–1192. doi: 10.1586/era.11.93. [DOI] [PubMed] [Google Scholar]
- 2.Curran M.P. Everolimus: in patients with subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Paediatr Drugs. 2012;14(1):51–60. doi: 10.2165/11207730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Sheard M.H. Lithium in the treatment of aggression. J Nerv Ment Dis. 1975;160(2–1):108–118. doi: 10.1097/00005053-197502000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Dostal T., Zvoltsky P. Antiaggressive effect of lithium salts in several mentally retarded adolescents. Int Pharmacopsychiatry. 1970;5:203–207. [Google Scholar]
- 5.Annell A.L. Manic-depressive illness in children and effect of treatment with lithium carbonate. Acta Paedopsychiatr. 1969;36(8):292–301. [PubMed] [Google Scholar]
- 6.Didden R., Duker P.C., Korzilius H. Meta-analytic study on treatment effectiveness for problem behaviors with individuals who have mental retardation. Am J Ment Retard. 1997;101(4):387–399. [PubMed] [Google Scholar]
- 7.Szegedi A., Calabrese J.R., Stet L., Mackle M., Zhao J., Panagides J. Asenapine as adjunctive treatment for acute mania associated with bipolar disorder: results of a 12-week core study and 40-week extension. J Clin Psychopharmacol. 2012;32(1):46–55. doi: 10.1097/JCP.0b013e31823f872f. [DOI] [PubMed] [Google Scholar]
- 8.McIntyre R.S., Cohen M., Zhao J., Alphs L., Macek T.A., Panagides J. A 3-week, randomized, placebo-controlled trial of asenapine in the treatment of acute mania in bipolar mania and mixed states. Bipolar Disord. 2009;11(7):673–686. doi: 10.1111/j.1399-5618.2009.00748.x. [DOI] [PubMed] [Google Scholar]
- 9.Samalin L., Charpeaud T., Llorca P.M. Asenapine in bipolar I disorder: evidence and place in patient management. Ther Adv Chronic Dis. 2013;4(1):5–14. doi: 10.1177/2040622312468933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagiolini A., Forgione R.N., Morana B., Maccari M., Goracci A., Bossini L. Asenapine for the treatment of manic and mixed episodes associated with bipolar I disorder: from clinical research to clinical practice. Expert Opin Pharmacother. 2013;14(4):489–504. doi: 10.1517/14656566.2013.765859. [DOI] [PubMed] [Google Scholar]
- 11.Hunt A. Development, behaviour and seizures in 300 cases of tuberous sclerosis. J Intellect Disabil Res. 1993;37(pt1):41–51. doi: 10.1111/j.1365-2788.1993.tb00868.x. [DOI] [PubMed] [Google Scholar]
- 12.Gillberg C., Uvebrant P., Carlsson G., Hedstrom A., Silfvenius H. Autism and epilepsy (and tuberous sclerosis?) in two pre-adolescent boys: neuropsychiatric aspects before and after epilepsy surgery. J Intellect Disabil Res. 1996;40(pt1):75–81. doi: 10.1111/j.1365-2788.1996.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 13.Staley B.A., Montenegro M.A., Major P., Muzykewicz D.A., Halpern E.F., Kopp C.M. Self-injurious behavior and tuberous sclerosis complex: frequency and possible associations in a population of 257 patients. Epilepsy Behav. 2008;13(4):650–653. doi: 10.1016/j.yebeh.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Wachtel L.E., Hagopian L.P. Psychopharmacology and applied behavioral analysis: tandem treatment of severe problem behaviors in intellectual disability and a case series. Isr J Psychiatry Relat Sci. 2006;43(4):265–274. [PubMed] [Google Scholar]
- 15.Hagopian L., Caruso-Anderson M. Integrating behavioral and pharmacological interventions for severe problem behavior displayed by children with neurogenetic and developmental disorders. In: Shapiro B.K., Accardo P.K., editors. Neurogenetic syndromes: Behavioral Issues and Their Treatment. Paul H. Brookes Publishing Co.; Baltimore: 2010. pp. 217–239. [Google Scholar]


