Abstract
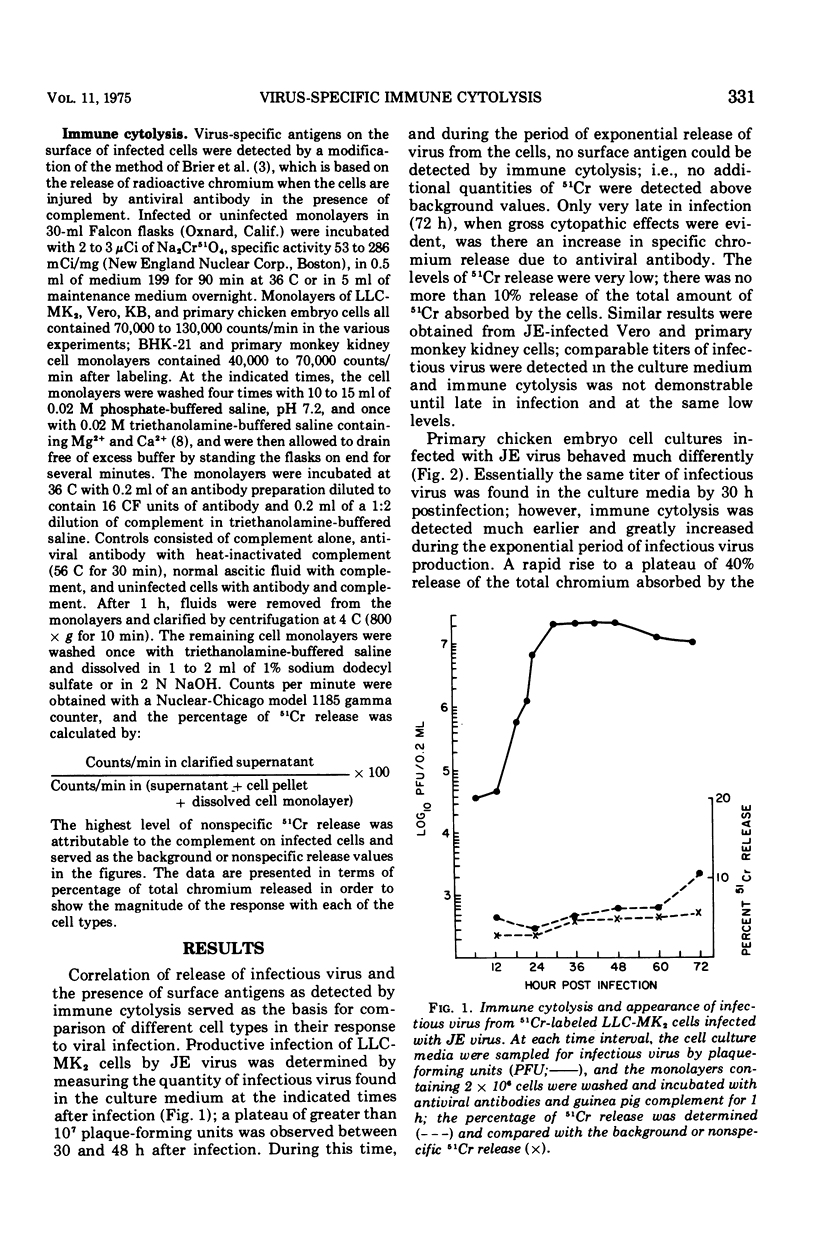
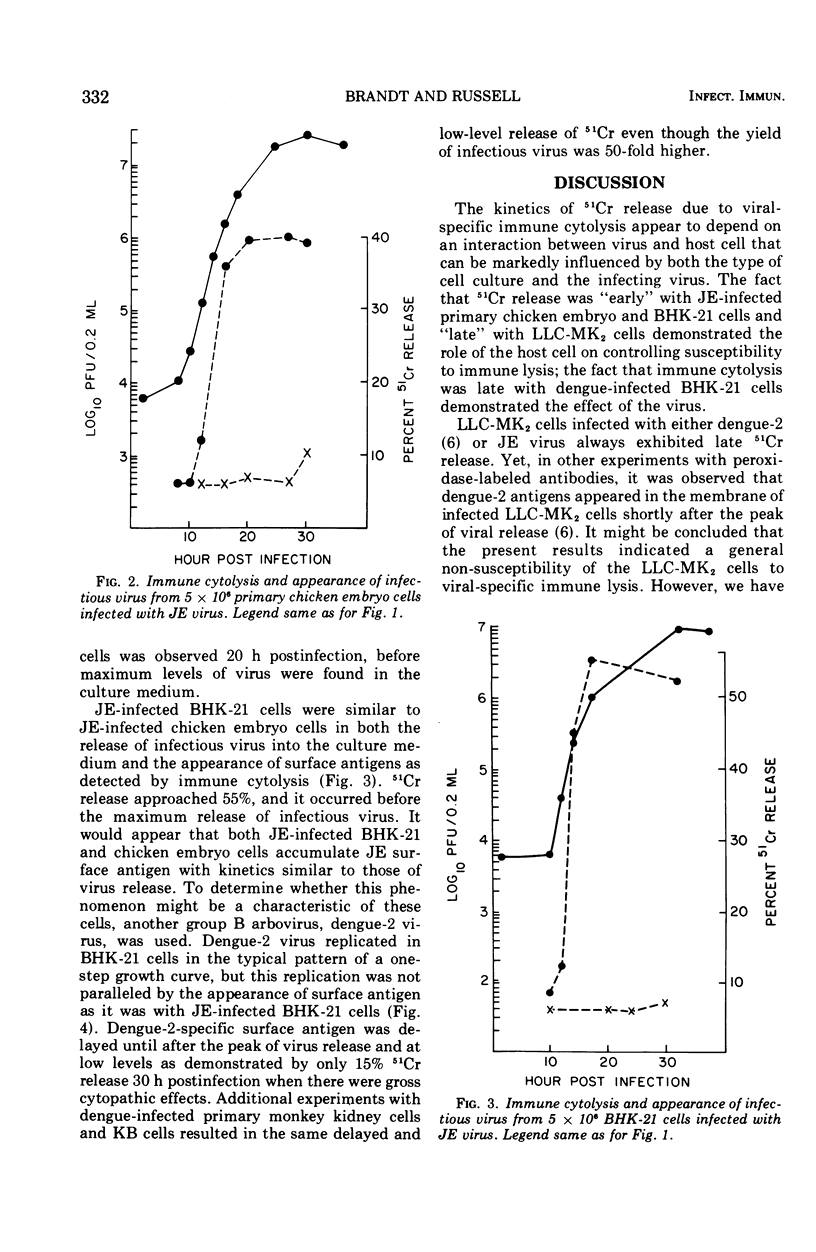
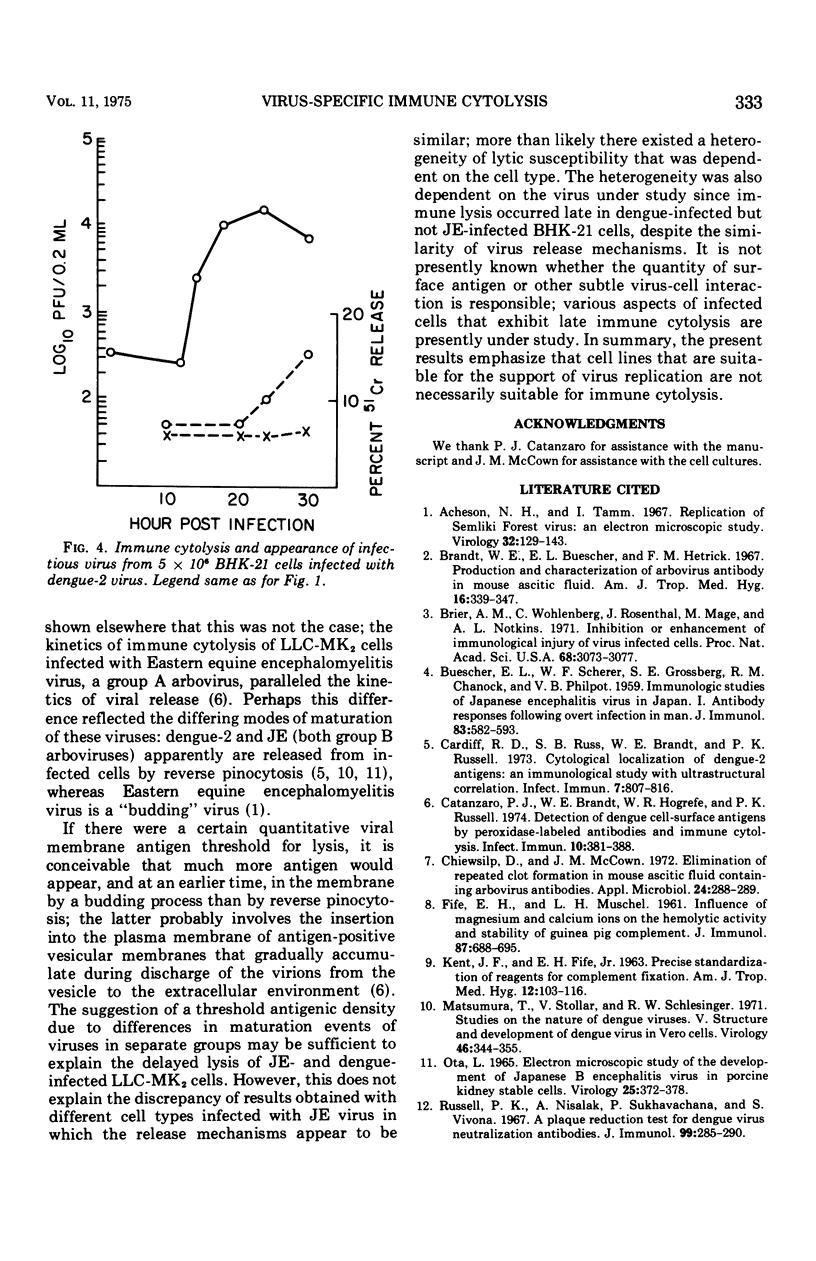
Immune cytolysis was measured by release of absorbed radioactive chromium from infected cells that were incubated with antiviral antibody and complement. The presence of virus-specific antigens detected in this manner on the surface of several types of cells infected with Japanese encephalitis (JE) virus did not correlate in each instance with the maturation of infectious virus. JE-infected LLC-MK-2 and Vero cells could not be lysed until long after the first appearance of released virus, and the lysis was minimal in that only a small amount of chromium was released. However, JE-infected BHK-21 and chicken embryo cells could be lysed as soon as new virus was detected in the culture medium, and the lysis reached maximum levels before the time that maximum levels of infectious virus were found in the culture fluids. This phenomenon was restricted to JE virus since BHK-21 cells infected with dengue-2 virus (another group B arbovirus) could not be lysed until well after the appearance of new virus in the culture medium.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Replication of Semliki Forest virus: an electron microscopic study. Virology. 1967 May;32(1):128–143. doi: 10.1016/0042-6822(67)90261-9. [DOI] [PubMed] [Google Scholar]
- BUESCHER E. L., SCHERER W. F., GROSSBERG S. E., CHANOCK R. M., PHILPOT V., Jr Immunologic studies of Japanese encephalitis virus in Japan. I. Antibody responses following overt infection of man. J Immunol. 1959 Dec;83:582–593. [PubMed] [Google Scholar]
- Brandt W. E., Buescher E. L., Hetrick F. M. Production and characterization of arbovirus antibody in mouse ascitic fluid. Am J Trop Med Hyg. 1967 May;16(3):339–347. doi: 10.4269/ajtmh.1967.16.339. [DOI] [PubMed] [Google Scholar]
- Brier A. M., Wohlenberg C., Rosenthal J., Mage M., Notkins A. L. Inhibition or enhancement of immunological injury of virus-infected cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3073–3077. doi: 10.1073/pnas.68.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff R. D., Russ S. B., Brandt W. E., Russell P. K. Cytological localization of Dengue-2 antigens: an immunological study with ultrastructural correlation. Infect Immun. 1973 May;7(5):809–816. doi: 10.1128/iai.7.5.809-816.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro P. J., Brandt W. E., Hogrefe W. R., Russell P. K. Detection of dengue cell-surface antigens by peroxidase-labeled antibodies and immune cytolysis. Infect Immun. 1974 Aug;10(2):381–388. doi: 10.1128/iai.10.2.381-388.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiewsilp D., McCown J. M. Elimination of repeated clot formation in mouse ascitic fluid containing arbovirus antibodies. Appl Microbiol. 1972 Aug;24(2):288–289. doi: 10.1128/am.24.2.288-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIFE E. H., Jr, MUSCHEL L. H. Influence of magnesium and calcium ions on the hemolytic activity and stability of guinea pig complement. J Immunol. 1961 Dec;87:688–695. [PubMed] [Google Scholar]
- KENT J. F., FIFE E. H., Jr Precise standardization of reagents for complement fixation. Am J Trop Med Hyg. 1963 Jan;12:103–116. doi: 10.4269/ajtmh.1963.12.103. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Stollar V., Schlesinger R. W. Studies on the nature of dengue viruses. V. Structure and development of dengue virus in Vero cells. Virology. 1971 Nov;46(2):344–355. doi: 10.1016/0042-6822(71)90036-5. [DOI] [PubMed] [Google Scholar]
- OTA Z. ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF JAPANESE B ENCEPHALITIS VIRUS IN PORCINE KIDNEY STABLE (PS) CELLS. Virology. 1965 Mar;25:372–378. doi: 10.1016/0042-6822(65)90057-7. [DOI] [PubMed] [Google Scholar]
- Russell P. K., Nisalak A., Sukhavachana P., Vivona S. A plaque reduction test for dengue virus neutralizing antibodies. J Immunol. 1967 Aug;99(2):285–290. [PubMed] [Google Scholar]



