Abstract
We report a case of sustained ventricular tachycardia following the initiation of lacosamide as adjunctive epilepsy treatment. A 49-year-old male with intractable frontal lobe seizures experienced severe ventricular tachycardia following the addition of 400 mg lacosamide to his existing regimen of carbamazepine, lamotrigine, clonazepam, and valproate. The tachycardia occurred during a cardiac stress test; stress tests prior to initiation of lacosamide were normal. Conduction defects, including QRS prolongation, persisted during hospitalization until lacosamide was discontinued. The patient had no prior history of cardiac arrhythmia but did possess cardiac risk factors, including hypertension, hypercholesterolemia, and low heart rate variability. This case represents one part of a growing body of literature suggesting a link between arrhythmia and use of lacosamide, which enhances slow inactivation of sodium channels in both the brain and the heart. We believe further study may be necessary to assess the safety of lacosamide in epilepsy patients with cardiac risk factors.
Keywords: Ventricular tachycardia, Arrhythmia, Lacosamide, Epilepsy
1. Introduction
Sudden unexplained death in epilepsy (SUDEP) is a leading cause of death among patients with severe, refractory epilepsy, accounting for between 10% and 50% of all deaths in these patients [1]. Several SUDEP risk factors have been identified, including polytherapy with a number of antiepileptic drugs (AEDs) [2]. However, the relationship between AED use and SUDEP remains unclear because the mechanism of SUDEP is poorly understood [1]. Data on the relationship between AED use and cardiac arrhythmias are particularly limited [3]. Much of the data linking AED use to arrhythmia and SUDEP are derived from small case series and video-telemetry studies in patients undergoing video-telemetry, where tachycardia, bradycardia, and conduction abnormalities (prolongation of the QT interval) may occur during and after seizures [3–5]. The present case describes severe arrhythmia in a patient treated with lacosamide, a sodium channel blocker with a novel mechanism: enhancement of sodium channel slow inactivation. This case is one of several recent cases linking lacosamide to severe cardiac events [6,7]. Taken together, these reports indicate the need for a more authoritative study of lacosamide and cardiac risk.
Lacosamide [(R)-2-acetamido-N-benzyl-3-methoxproionamide] is a novel antiepileptic drug that enhances slow inactivation of voltage-gated sodium channels [8]. It was approved as adjunctive therapy for epilepsy by the FDA in October 2008 [9]. Preclinical and clinical trials suggest that lacosamide acts upon both neurons and the heart and may increase the risk of cardiac arrhythmias [9]. In studies of isolated canine Purkinje fibers, lacosamide reduces action potential duration and maximal upstroke velocity in a dose-dependent manner [8]. Moreover, in human myocytes, therapeutic levels of lacosamide inhibit SCN5A, a cardiac sodium channel [8]. In-vivo canine studies confirm that lacosamide acts as a cardiac depressant, causing decreased systolic left ventricular pressure, decreased velocity of pressure change, reduced cardiac output, and transient increases in PR interval and QRS complex duration [8].
A Phase III study of lacosamide in diabetic peripheral neuropathy found that lacosamide was associated with cardiac conduction defects (AV block) in 0.5% of subjects and atrial fibrillation in 0.5% versus no cardiac events in the placebo cohort [9]. In Phase III epilepsy trials, conduction defects (AV block) occurred in 0.4% of lacosamide subjects versus none for placebo [9–11]. Post-marketing studies have observed a small median increase in PR interval of patients taking lacosamide [10,11]. Moreover, recent case reports have associated lacosamide treatment with conduction disturbances and arrhythmias [6,7]. We report a similar case of ventricular tachycardia associated with initiation of adjunctive lacosamide treatment.
2. Report of a case
A 49-year-old male with severe drug-resistant complex partial and generalized tonic-clonic seizures of frontal lobe onset was placed on 400 mg/day lacosamide to complement his existing AED regimen of carbamazepine, lamotrigine, clonazepam, and valproate. The patient had undergone a left orbital–frontal craniotomy in 1989 that failed to control his epilepsy. Moreover, the patient's seizures failed to improve with a number of AEDs, including phenytoin and levetiracetam. The patient had no prior history of cardiac events but was on atorvastatin, valsartan, and triamterene for well-controlled hypertension and hypercholesterolemia. In addition, the patient had low heart rate variability (HRV), detected during a clinical trial of n − 3 fatty acids for epilepsy. Heart rate variability has been associated with a higher risk of sudden cardiac death in healthy patients [12].
Prior to the initiation of lacosamide, the patient had undergone two cardiac treadmill stress tests while taking his normal AED regimen and experienced no recorded cardiac arrhythmias on electrocardiography (ECG). The patient did not report any significant lifestyle or health changes following these two stress tests that may have contributed to a cardiac event. Of note, no arrhythmias were reported during prior seizures captured during video-EEG monitoring, and multiple Holter ECG monitoring for a clinical trial also failed to identify any arrhythmia. Four months after initiating lacosamide, during a third treadmill stress test, the patient developed sustained ventricular tachycardia (Fig. 1). The ventricular tachycardia resolved acutely after cessation of the stress test, and no cardiac enzyme elevation was noted to suggest myocardial infarction as the cause. He was admitted to Glendale Medical Center emergently and underwent continuous cardiac ECG telemetry. During the hospitalization, his physicians rapidly lowered the lacosamide dose by 100 mg/day until lacosamide termination. During hospitalization, the ECG demonstrated first-degree AV block, posterior left fascicular block, and severe widening of the QRS complex, but following discontinuation of lacosamide, the patient's ECG immediately returned to baseline (Fig. 2). An automatic defibrillator was implanted with an event recorder. Over one year after termination of lacosamide, the patient had reported no cardiac complications, and the event monitor has not identified any episodes of ventricular tachycardia or arrhythmias.
Fig. 1.
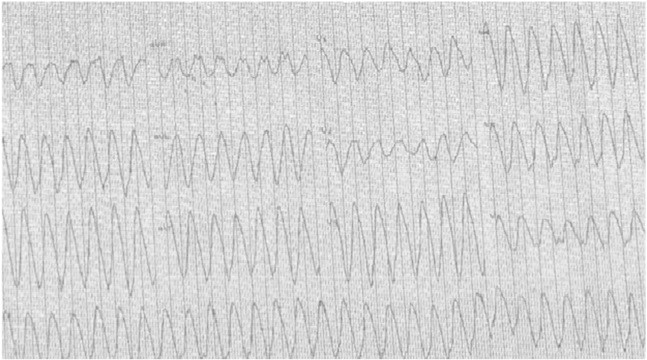
Electrocardiography recording from the patient's cardiac stress test while taking lacosamide 400 mg/day, illustrating ventricular tachycardia.
Fig. 2.
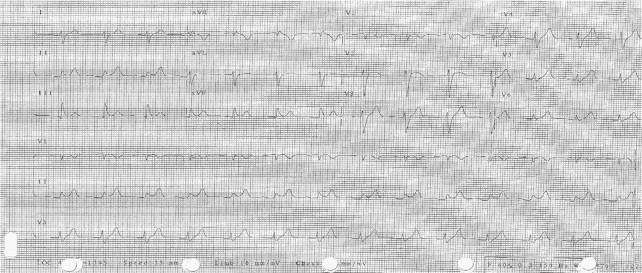
Electrocardiogram after stabilization of ventricular tachycardia. The ECG demonstrated ST elevation, early repolarization, borderline AV conduction delay, and left posterior fascicular block.
3. Discussion
We report a patient with drug-resistant epilepsy who suffered sustained ventricular tachycardia and a ventricular conduction disturbance during a stress test while exposed to adjunctive lacosamide as part of his AED regimen. Though ventricular tachycardia resolved spontaneously, the conduction defects resolved only after discontinuation of lacosamide following which all cardiac conduction abnormalities resolved. Extended implantable cardiac monitor failed to identify any cardiac arrhythmias after the lacosamide termination. The onset of the arrhythmia during the period while the patient was exposed to lacosamide, the acute abnormality of cardiac conduction which did not resolve until lacosamide was discontinued, and the absence of arrhythmia in the long-term follow-up provide evidence for a possible role of lacosamide in the patient's ventricular tachycardia.
The case is complicated by the patient's concurrent treatment with several AEDs, including two sodium channel blockers, lamotrigine and carbamazepine. Lamotrigine has been associated with several cases of SUDEP, while carbamazepine has been associated with a number of arrhythmias [4,13]. Both drugs may exert pro-arrhythmic influence by inhibition of IKr, a cardiac potassium channel, which lacosamide does not affect [13–16]. However, this mechanism of action remains controversial, largely because prolonged QTc, a consequence of IKr inhibition, has not been observed with lamotrigine or carbamazepine in humans [4,14,15].
Lacosamide, carbamazepine, and lamotrigine all inhibit the cardiac sodium channel SCN5A in a concentration-dependent manner [8,15–17]. Disruption of the SCN5A channel has been associated with severe arrhythmia, including ventricular tachycardia, in patients with Brugada syndrome, a channelopathy associated with an increased risk of ventricular tachycardia and sudden death [18]. Unfortunately, a direct comparison of SCN5A inhibition curves for lacosamide, carbamazepine, and lamotrigine cannot be made because of the limited preclinical lacosamide data available [7]. In-vitro lacosamide elicits 10–20% SCN5A inhibition at upper therapeutic concentrations, so it is reasonable to believe that lacosamide would be at least partially involved in any SCN5A-mediated arrhythmia [8]. Moreover, lacosamide is believed to inhibit SCN5A by enhancing slow inactivation, a novel mechanism [8]. Several SCN5A mutations that amplify intermediate or slow inhibition have been associated with Brugada syndrome and sudden death [18–21]. Lacosamide's mechanism may complement that of other sodium blockers and amplify the cardiac effects associated with them [13–15].
This case report provides additional evidence for a possible role of lacosamide in cardiac arrhythmias in patients with drug-resistant epilepsy. The lethal potential of the arrhythmia (sustained ventricular tachycardia) is of particular concern. The timing of the ventricular tachycardia after initiation of lacosamide and resolution after its discontinuation suggests a possible pro-arrhythmic role for lacosamide. It is our hope that there will be further investigation into the cardiac effects of lacosamide and the role of slow sodium inactivation in arrhythmias in patients with epilepsy.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Tomson T., Walczak T., Sillanpaa M., Sander J.W.A.S. Sudden unexpected death in epilepsy: a review of incidence and risk factors. Epilepsia. 2005;46:54–61. doi: 10.1111/j.1528-1167.2005.00411.x. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson L., Farahmand B.Y., Persson P.G., Thiblin I., Tomson T. Risk factors for sudden unexpected death in epilepsy: a case–control study. Lancet. 1999;353:888–893. doi: 10.1016/s0140-6736(98)05114-9. [DOI] [PubMed] [Google Scholar]
- 3.Tomson T., Kenneback G. Arrhythmia, heart rate variability, and antiepileptic drugs. Epilepsia. 1997;38:S48–S51. doi: 10.1111/j.1528-1157.1997.tb06128.x. [DOI] [PubMed] [Google Scholar]
- 4.Saetre E., Abdelnoor M., Amilie J.P. Cardiac function and antiepileptic drug treatment in the elderly: a comparison between lamotrigine and sustained-release carbamazepine. Epilepsia. 2009;50:1841–1849. doi: 10.1111/j.1528-1167.2009.02069.x. [DOI] [PubMed] [Google Scholar]
- 5.Surges R., Scott C.A., Walker M.C. Enhanced QT shortening and persistent tachycardia after generalized seizures. Neurology. 2010;74:421–426. doi: 10.1212/WNL.0b013e3181ccc706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeGiorgio C.M. Atrial flutter/atrial fibrillation associated with lacosamide for partial seizures. Epilepsy Behav. 2010;18:322–324. doi: 10.1016/j.yebeh.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 7.Nizam A., Mylavarapu K., Thomas D. Lacosamide-induced second-degree atrioventricular block in a patient with partial epilepsy. Epilepsia. 2011;52:153–155. doi: 10.1111/j.1528-1167.2011.03212.x. [DOI] [PubMed] [Google Scholar]
- 8.Beyreuther B.K., Freitag J., Heers C. Lacosamide: a review of preclinical properties. CNS Drug Rev. 2007;13:21–42. doi: 10.1111/j.1527-3458.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UCB, Inc. April 2011. Vimpat prescribing information. [Google Scholar]
- 10.Rosenfeld W., Fountain N.B., Kaubrys G. Abstract: lacosamide: an interim evaluation of long-term safety and efficacy as oral adjunctive therapy in subjects with partial-onset seizures. Epilepsia. 2007;48(Suppl. 6):318. [Google Scholar]
- 11.Chung S., Sperling M., Bilton V. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia. 2010;51:958–967. doi: 10.1111/j.1528-1167.2009.02496.x. [DOI] [PubMed] [Google Scholar]
- 12.Stein P.K., Kleiger R.E. Insights from the study of heart rate variability. Annu Rev Med. 1999;50:249–261. doi: 10.1146/annurev.med.50.1.249. [DOI] [PubMed] [Google Scholar]
- 13.Aurlien D., Tauboll E., Gjerstad L. Lamotrigine in idiopathic epilepsy — increased risk of cardiac death? Acta Neurol Scand. 2007;115:199–203. doi: 10.1111/j.1600-0404.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 14.Danielsson B.R., Lansdell K., Patmore L., Tomson T. Effects of the antiepileptic drugs lamotrigine, topiramate and gabapentin on hERG potassium currents. Epilepsy Res. 2005;63:17–25. doi: 10.1016/j.eplepsyres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Danielsson C., Azarbayjani F., Sköld A.C., Sjögren N., Danielsson B.R. Polytherapy with hERG-blocking antiepileptic drugs: increased risk for embryonic cardiac arrhythmia and teratogenicity. Birth Defects Res A Clin Mol Teratol. 2007;79:595–603. doi: 10.1002/bdra.20378. [DOI] [PubMed] [Google Scholar]
- 16.Dixon R., Job S., Oliver R. Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects. Br J Clin Pharmacol. 2008;66:396–404. doi: 10.1111/j.1365-2125.2008.03250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmer A.R., Valentin J.-P., Pollard C.E. On the relationship between block of the cardiac Na+ channel and drug-induced prolongation of the QRS complex. Br J Pharmacol. 2011;164:260–273. doi: 10.1111/j.1476-5381.2011.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antzelevitch C., Brugada P., Borggrefe M. Brugada syndrome: report of the second consensus conference. Circulation. 2005;111:650–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 19.Veldkamp M.W., Viswanathan P.C., Bezzina C., Baartscheer A., Wilde A.A., Balser J.R. Two distinct congenital arrhythmias evoked by a multidysfunctional Na+ channel. Circ Res. 2000;86:e91–e97. doi: 10.1161/01.res.86.9.e91. [DOI] [PubMed] [Google Scholar]
- 20.Wang D.W., Makita N., Kitabatake A., Balser J.R., George A.L. Enhanced Na+ channel intermediate inactivation in Brugada syndrome. Circ Res. 2000;87:e37–e43. doi: 10.1161/01.res.87.8.e37. [DOI] [PubMed] [Google Scholar]
- 21.Casini S., Tan L.H., Bhuiyan Z.A. Characterization of a novel SCN5A mutation associated with Brugada syndrome reveals involvement of DIIIS4–S5 linker in slow inactivation. Cardiovasc Res. 2007;76:418–429. doi: 10.1016/j.cardiores.2007.08.005. [DOI] [PubMed] [Google Scholar]


