Abstract
Background
The classification or index of heart failure severity in patients with acute myocardial infarction (AMI) was proposed by Killip and Kimball aiming at assessing the risk of in-hospital death and the potential benefit of specific management of care provided in Coronary Care Units (CCU) during the decade of 60.
Objective
To validate the risk stratification of Killip classification in the long-term mortality and compare the prognostic value in patients with non-ST-segment elevation MI (NSTEMI) relative to patients with ST-segment elevation MI (STEMI), in the era of reperfusion and modern antithrombotic therapies.
Methods
We evaluated 1906 patients with documented AMI and admitted to the CCU, from 1995 to 2011, with a mean follow-up of 05 years to assess total mortality. Kaplan-Meier (KM) curves were developed for comparison between survival distributions according to Killip class and NSTEMI versus STEMI. Cox proportional regression models were developed to determine the independent association between Killip class and mortality, with sensitivity analyses based on type of AMI. Results: The proportions of deaths and the KM survival distributions were significantly different across Killip class >1 (p <0.001) and with a similar pattern between patients with NSTEMI and STEMI. Cox models identified the Killip classification as a significant, sustained, consistent predictor and independent of relevant covariables (Wald χ2 16.5 [p = 0.001], NSTEMI) and (Wald χ2 11.9 [p = 0.008], STEMI).
Conclusion
The Killip and Kimball classification performs relevant prognostic role in mortality at mean follow-up of 05 years post-AMI, with a similar pattern between NSTEMI and STEMI patients.
Keywords: Severity of Illness Index, Heart failure / mortality, Myocardial Infarction / mortality, Prognosis
Introduction
Classification of heart failure severity in acute myocardial infarction
The classification proposed by Thomas Killip III and John T. Kimball1 in 1967 involved bedside stratification. This stratification was based on the physical examination of patients with possible acute myocardial infarction (AMI), and it was used to identify those at the highest risk of death and the potential benefits of specialized care in coronary care units (CCUs). This study described 250 cases with suspected AMI admitted to the CCU of a university hospital in the United States. There were no objective clinical outcomes nor systematic collection of data or adjustments for confounding factors; moreover, there were no validations in an independent series of patients. The cases were stratified into the following classes:
Killip I: 81 (33%) with no clinical signs of heart failure,
Killip II: 96 (38%) with rales in the lungs, third heart sound (S3), and elevated jugular venous pressure,
Killip III: 26 (10%) with acute pulmonary edema (APE), and
Killip IV: 47 (19%) with cardiogenic shock or arterial hypotension (measured as systolic blood pressure < 90 mmHg), and evidence of peripheral vasoconstriction (oliguria, cyanosis, and diaphoresis), with mortality rates of 6%, 17%, 38%, and 81%, respectively.
Although originally described in the pre-reperfusion era, the use of this classification in ST-segment elevation myocardial infarction (STEMI) was further studied in the post-reperfusion era2,3. In contrast, the prognostic value of this classification in non-ST-segment elevation myocardial infarction (NSTEMI) is not well established, primarily because it has not yet been validated in patients who were not selected from randomized clinical trial databases4 and considering the paucity of data on late follow-up after AMI.
In fact, although recommended as a part of the initial risk stratification in the IV Guidelines of the Brazilian Cardiology Society5 for management of STEMI, the Killip-Kimball classification has not been cited in the society's most recent guideline for management of NSTEMI6.
Therefore, this study aimed to validate the Killip-Kimball classification for total mortality in long-term clinical follow-up and compare its prognostic value in patients with NSTEMI and STEMI in the era of post-reperfusion and modern antithrombotic therapy.
Method
Study Design
This study comprised two designs7,8: 1) analytical cross-sectional study to determine the clinical characteristics including the Killip-Kimball classification based on the first physical examination on admission, history and previous treatments, as well as diagnostic and therapeutic procedures during hospital stay in patients with a confirmed diagnosis of AMI with or without ST-segment elevation and admitted to the CCU of the Dante Pazzanese Institute of Cardiology (IDPC); 2) after hospital admission, patients were recruited and followed prospectively, even for in-hospital clinical events (prospective cohort), in a database between 1995 and 2011, with systematic data collection via electronic datasheets.
Data collection
Information pertaining to the date of the last evaluation of each living patient, medication used 48 h before the admission and at discharge, and on deaths during hospitalization or long-term clinical follow-up were collected by actively searching the patient's electronic records, electronic data management systems of the institute, and medical records, as well as via telephone.
Sampling
We used non-probability sampling considering the paucity of studies that have validated the Killip-Kimball classification to estimate the risk of mortality in patients with AMI in the Brazilian population. It is notable that our sample size was considerably greater than that in the 1967 study, which included 250 patients with a suspected diagnosis of AMI.
Eligibility criteria and analyzed variables
Patients with AMI with or without ST-segment elevation of both genders, aged >18 years, and admitted to the CCU of the IDPC between 1995 and 2011 were included. The study excluded patients with unstable angina. The criteria used for AMI diagnosis was based on the recommendations of the guidelines avaliable between 1995 and 2011. In addition to the standard clinical criteria, those with the presence of persistent ST-segment elevation of ≥1.0 mm in limb leads or through V4-V6, of ≥ 2.0 mm in leads V1-V3, or new or presumably new left bundle branch block were diagnosed with AMI with persistent ST-segment elevation. This condition was confirmed by increased levels of myocardial necrosis biomarkers at the time of AMI (between 1995 and 2001), i.e., creatine kinase MB (CKMB) and total CK activity that were at least twice the upper limit of normal of these markers or CKMB mass and cardiac troponin I (cTnI) after 2001 (according to the cutoff levels advised by the kits used at the institute, based on the 99th percentile).
When the ECG showed ST-segment depression, T-wave inversion, or nonspecific findings in serial tracings along with the increased levels of myocardial necrosis biomarkers, AMI diagnosis without persistent ST-segment elevation was confirmed.
In this study, we analyzed demographic variables (age, gender, and ethnicity), cardiovascular risk factors and comorbidities, physical examination information for the Killip-Kimball classification, simple hemodynamic parameters (heart rate and systolic and diastolic blood pressure), previous treatments and procedures, and angiographic aspects [affected artery, TIMI flow, extent and severity of coronary artery disease (CAD) in those undergoing coronary angiography]. We defined total mortality as the clinical outcome of interest, with landmark analysis at day 30 and at the end of the follow-up period.
Distributions of continuous variables are expressed as mean ± standard deviation or median with interquartile range (IQR), and comparisons between groups were calculated using Student's t-test or nonparametric tests (Kruskal-Wallis test for significance and Jonckheere-Terpstra for trend). The distributions of discrete (or categorical) variables are expressed as frequencies and percentages, and comparisons were calculated using chi-square or Fisher's exact test. Analysis of the clinical outcome was based on the time to occurrence of death, according to the cumulative Kaplan-Meier survival curves and depending on the Killip class. Univariate Cox regression analysis included all demographic, clinical, and angiographic variables. However, only univariate predictors with p < 0.10 and clinically significant variables were included in the Cox proportional hazards regression models to determine if the Killip class would be an independent risk predictor, both in the group with as well as without ST-segment elevation. The backward stepwise procedure enabled the identification of the independent variables for the risk of death, according to AMI type. The results are expressed as hazard ratio with 95% confidence intervals (CI), and the discriminatory ability of the models are expressed as the c-statistic (or index c). We used two-tailed tests with a significance level of α = 0.05. Data were analyzed in conjunction with the Laboratory of Statistics and Epidemiology, IDPC, using the statistical package IBM® SPSS Statistics version 19.0.
Results
Patient characteristics
The main general characteristics of 1906 patients with AMI are described below as well as shown in Table 1, according to the Killip class. Overall, the median age (IQR) was 64 (55.73) years, ranging from 19 to 94 years; 71.3% were male, median body weight was 71.5 kg, and 81.4% were Caucasians. The prevalence of cardiovascular risk factors was significant; 70% hypertension, 36.4% diabetes, 78% dyslipidemia, 7.0% stroke, and 7.7% peripheral arterial disease (PAD); and 34% were smokers. As for the ECG, 4.5% patients had bundle branch block on admission, 61.9% had persistent ST-segment elevation, 13.8% had ST-segment depression, 3.8% had Q-waves, and 8.4% had T-wave inversion; 3.5% were considered normal, and 4.1% had uncharacteristic findings. The proportion of patients evaluated and categorized in each Killip class (I, II, III and IV) were 84.3%, 11.0%, 2.4%, 2.3%, respectively. Diagnosis identified 64% of the patients with STEMI and 36% with NSTEMI.
Table 1.
Clinical characteristics according to the Killip–Kimball
| Patient characteristics | Killip I | Killip II | Killip III | Killip IV | p |
|---|---|---|---|---|---|
| Age | 62 (53, 70) | 66 (58, 77) | 65 (54, 73) | 63 (54, 74) | < 0.0001 |
| Female gender | 27.6% | 34% | 39.1% | 32.6% | 0.031 |
| Caucasian | 80.9% | 84.2% | 84.8% | 79.1% | 0.63 |
| Weight, kg | 73 (65, 82) | 72 (64, 80) | 71 (62, 77) | 70 (65, 80) | 0.54 |
| SBP, mmHg | 132 (120,160) | 130 (110, 150) | 137 (122, 154) | 93 (62, 122) | < 0.0001 |
| DBP, mmHg | 80 (70, 100) | 80 (70, 93) | 80 (70, 100) | 60 (32, 77) | < 0.0001 |
| HR, bpm | 76 (67, 88) | 84 (70, 96) | 91 (80, 105) | 84 (57, 105) | < 0.0001 |
| PAD | 4.6% | 6.2% | 10.9% | 9.3% | 0.06 |
| Diabetes mellitus | 28.2% | 32.5% | 47.8% | 37.2% | 0.01 |
| History of AMI | 31.2% | 24% | 37% | 37.2% | 0.09 |
| History of PCI | 19.4% | 14.8% | 8.7% | 16.3% | 0.12 |
| CABG surgery | 16.1% | 12% | 19.6% | 14% | 0.13 |
| History of stroke (CVA) | 5.2% | 9.6% | 8.7% | 4.7% | 0.05 |
| Smoking: current status | 36.8% | 33% | 32.6% | 34.9% | 0.70 |
| History of angina | 26.9% | 29.7% | 26.1% | 37.2% | 0.41 |
| Hypertension | 72.3% | 74.6% | 60.9% | 72.1% | 0.31 |
| HF | 4% | 14.4% | 19.6% | 14% | <0.001 |
CVA: cerebrovascular accident; PAD: peripheral arterial disease; HR: Heart rate; HF: heart failure; PCI: percutaneous coronary intervention; DBP: diastolic blood pressure; SBP: systolic blood pressure; CABG: Coronary artery bypass graft; AMI: acute myocardial infarction.
Compared with patients in Killip class I, those in class ≥2 had higher median age, PAD, history of heart failure (HF), stroke, diabetes mellitus, ECG with ST segment deviation or bundle branch blocks, higher heart rate (HR), and lower systolic and diastolic blood pressures, and most were predominantly females.
Pharmacological treatment and invasive stratification
Regarding the use of medications during the first 48h of hospitalization, aspirin was used in 96% patients, other antiplatelet medications in 68%, beta-blockers in 61%, ACE inhibitors in 60%, statins in 40%, and anticoagulation with antithrombotic agents, i.e., unfractioned heparin or low-molecular-weight heparin in 83% (data not shown). Glycoprotein IIb/IIIa inhibitors were used intravenously in 7.3% patients. Regarding the use of medications during hospitalization and until discharge, aspirin was used in 98% patients, clopidogrel in 75%, beta-blockers in 89.5%, ACE inhibitors in 88.5%, and statins in 98.2%; the rates of use were similar among patients with or without ST-segment elevation.
Invasive strategy was performed in 85% of patients. CAD was considered significant with luminal stenosis of ≥50% observed in at least one vessel in 83% of patients. Percutaneous coronary intervention was performed in 54% patients undergoing diagnostic angiography, with 77% receiving 1 coronary stent implant and 23% receiving >1 stent implant; 91% stents were conventional (non-pharmacological) stents. Coronary artery bypass surgery was performed in 15% patients, with 98.4% use of the left internal thoracic artery.
Reperfusion strategies and adjuvant therapy
Among the 1219 patients with AMI and persistent ST-segment elevation, 69% received some type of reperfusion therapy. The remaining did not receive the therapy due to prolonged delay between the onset of symptoms and hospital admission (>12 h), because they were referred from other hospitals in which fibrinolytic agents were not administered, or because they were referred to the IDPC after this time interval. Of the 845 patients treated using reperfusion therapy, 70% were submitted to mechanical reperfusion with primary percutaneous transluminal coronary angioplasty (90% received at least 1 stent implant) and the remaining 30% were treated using fibrinolysis in other hospitals before being transferred.
With respect to patients with cardiogenic shock (Killip IV), 70% received mechanical circulatory support with intra-aortic balloon along with the use of usual inotropic medications, predominantly dobutamine.
Clinical follow-up and total mortality
Patients were followed since hospital admission during treatment at the CCU and until the last evaluation in the institution to determine their vital status (or until death, if applicable).
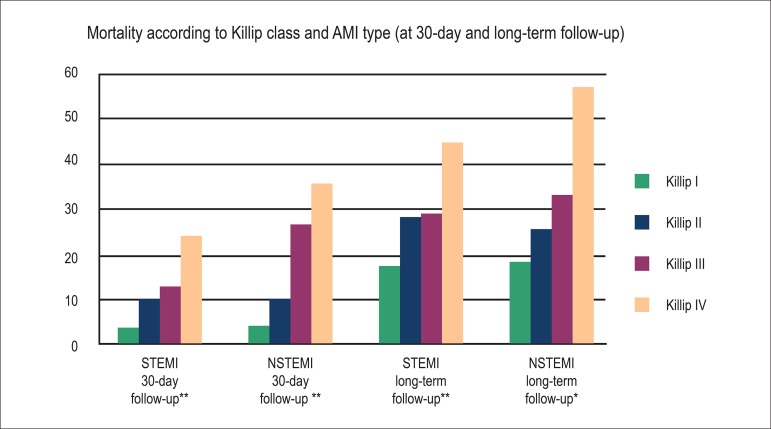
The maximum follow-up time was 6699 days; the average follow-up time was five years, achieved in 99.6% patients. The primary outcome of total mortality was observed in 378 patients (i.e., 19.8% of 1906). The frequencies of death, according to the Killip class, in total long-term clinical follow-up were as follows: Killip class I, 17.7%; II, 27.3%; III, 30.4%; and IV, 48.8% (p < 0.0001). In the analysis according to AMI type, we observed a similar pattern between the NSTEMI and STEMI groups. The same was observed in the period up to 30 days (Figure 1).
Figure 1.
Mortality (%) according to Killip class and AMI type (at 30-day and long-term follow-up). **p<0.0001, *p<0.001; NSTEMI: With non-ST-segment elevation MI; STEMI: With ST-segment elevation MI.
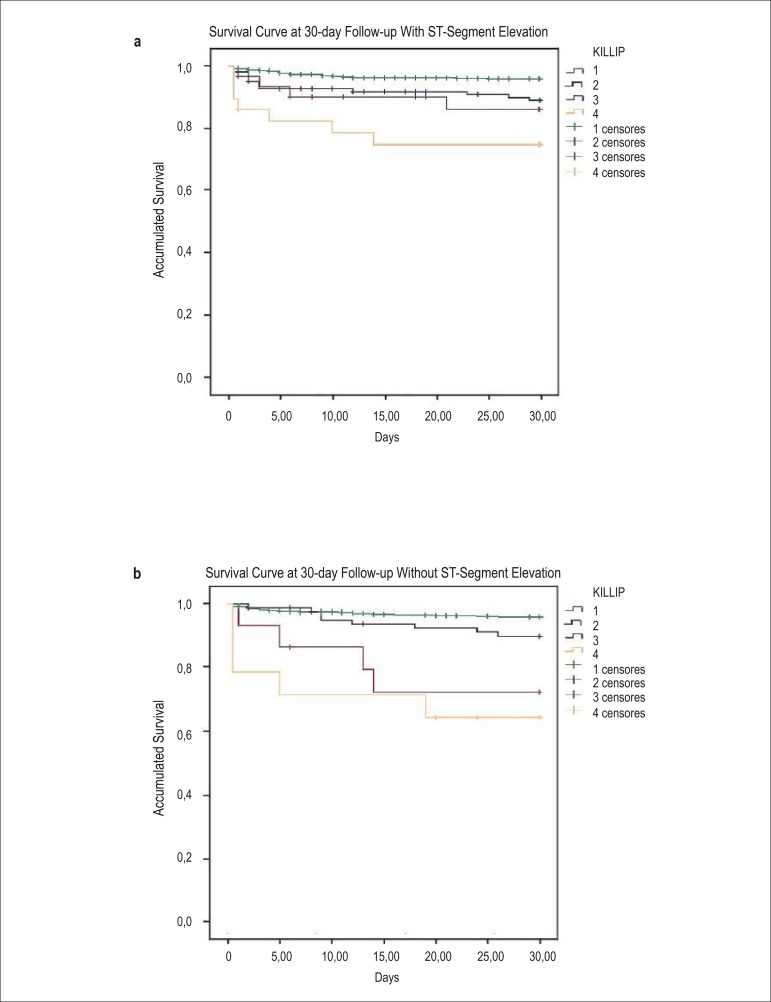
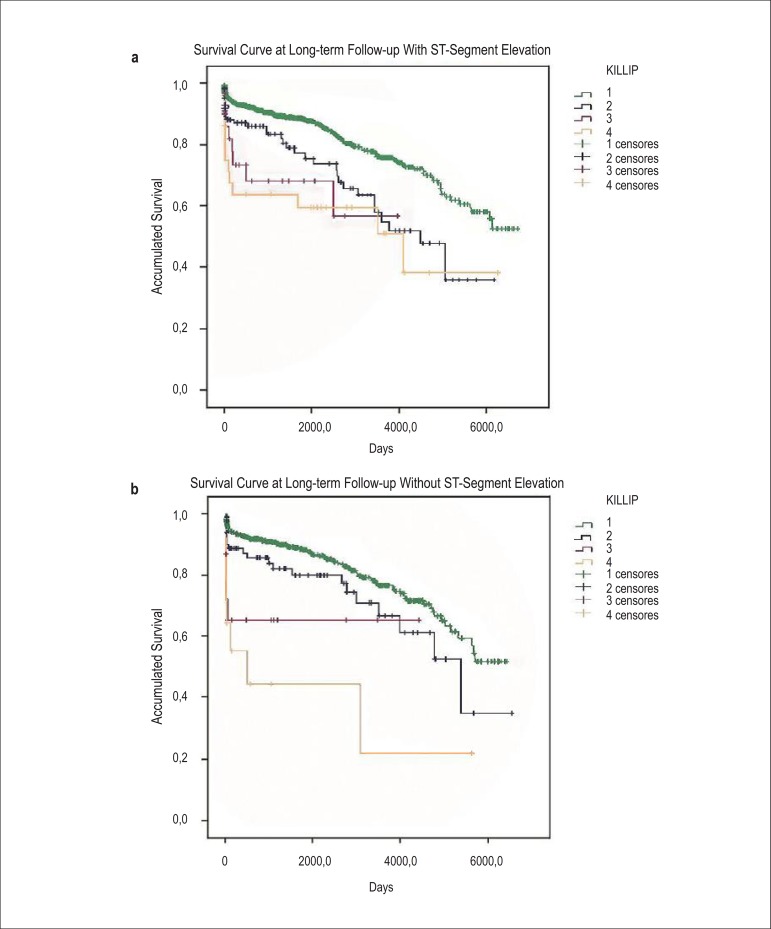
When separately analyzed, as a part of the pre-specified analysis, survival at 30 days was similar between those with and without ST-segment elevation; this progressively increased across Killip classes (p < 0.0001 by log-rank test; Figures 2a and 2b). A similar pattern was observed in the total follow-up time (p < 0.0001 by log-rank test; Figures 3a and 3b).
Figure 2a, 2b.
Kaplan–Meier curves for accumulated survival according to Killip class in patients diagnosed with STEMI (a) vs. those diagnosed with NSTEMI (b) at 30-day follow-up.
Figures 3a, 3b.
Kaplan–Meier curves for accumulated survival according to Killip class in patients diagnosed with STEMI (a) vs. those diagnosed with NSTEMI (b) at long-term follow-up.
Cox proportional hazards model
The risk models included clinical characteristics such as age, gender, cardiovascular risk factors, physical examination and hemodynamic findings, history, treatments and procedures performed previously and during hospitalization, Killip-Kimball classification, and AMI type. The variables that showed significant association with mortality were selected. Four models were constructed to explore the association between the Killip class, AMI type, and risk of death using clinical variables on admission and in-hospital (Tables 2-5). Consistently, the Killip-Kimball classification was an independent predictor of increased risk of mortality.
Table 2.
Cox model with initial data on hospital admission and predictors of mortality in the total follow-up of patients with STEMI
| B | SE | Wald | p | HR | IC 95% | ||
|---|---|---|---|---|---|---|---|
| Age | 0.032 | 0.006 | 24.986 | 0.000 | 1.032 | 1.019 | 1.045 |
| CABG surgery | 0.390 | 0.173 | 5.088 | 0.024 | 1.477 | 1.052 | 2.073 |
| History of HF | 0.496 | 0.246 | 4.049 | 0.044 | 1.641 | 1.013 | 2.660 |
| DM | 0.315 | 0.146 | 4.686 | 0.030 | 1.370 | 1.030 | 1.822 |
| HR | 0.008 | 0.003 | 5.366 | 0.021 | 1.008 | 1.001 | 1.014 |
| Weight | -0.012 | 0.006 | 4.251 | 0.039 | 0.988 | 0.977 | 0.999 |
| Killip I | 10.424 | 0.015 | |||||
| Killip II | 0.386 | 0.200 | 3.728 | 0.054 | 1.472 | 0.994 | 2.178 |
| Killip III | 0.658 | 0.379 | 3.007 | 0.083 | 1.930 | 0.918 | 4.058 |
| Killip IV | 0.843 | 0.338 | 6.220 | 0.013 | 2.323 | 1.198 | 4.504 |
B: coefficient B; DM: diabetes mellitus; HR: heart rate; HR: hazard ratio; HF: history of heart failure; CABG: Coronary artery bypass graft; SE: standard error. *Age, HR, and weight are represented as increments of one unit.
Table 3.
Cox model with initial data on hospital admission and predictors of mortality in the total follow-up of patients with NSTEMI
| B | SE | Wald | p | HR | IC 95% | ||
|---|---|---|---|---|---|---|---|
| Age | 0.042 | 0.008 | 27.885 | 0.000 | 1.043 | 1.027 | 1.060 |
| CABG surgery | 0.120 | 0.241 | 0.248 | 0.619 | 1.127 | 0.703 | 1.808 |
| History of HF | 0.473 | 0.366 | 1.674 | 0.196 | 1.605 | 0.784 | 3.286 |
| DM | 0.219 | 0.192 | 1.302 | 0.254 | 1.244 | 0.855 | 1.811 |
| HR | 0.012 | 0.004 | 8.715 | 0.003 | 1.012 | 1.004 | 1.020 |
| Weight | -0.010 | 0.008 | 1.733 | 0.188 | 0.990 | 0.975 | 1.005 |
| Killip I | 12.197 | 0.007 | |||||
| Killip II | 0.163 | 0.259 | 0.398 | 0.528 | 1.177 | 0.709 | 1.956 |
| Killip III | 1.054 | 0.470 | 5.025 | 0.025 | 2.870 | 1.142 | 7.217 |
| Killip IV | 1.354 | 0.481 | 7.915 | 0.005 | 3.874 | 1.508 | 9.952 |
B: coefficient B; DM: diabetes mellitus; HR: heart rate; HR: hazard ratio; HF: heart failure; CABG: Coronary artery bypass graft; SE: standard error. *Age, HR, and weight are represented as increments of one unit.
Table 4.
Cox model with in-hospital data and predictors of mortality in the total follow-up of patients with STEMI
| B | SE | Wald | p | HR | IC 95% | ||
|---|---|---|---|---|---|---|---|
| Age | 0.034 | 0.006 | 27.812 | 0.000 | 1.034 | 1.022 | 1.048 |
| CABG surgery | 0.498 | 0.174 | 8.159 | 0.004 | 1.646 | 1.169 | 2.317 |
| HF | 0.552 | 0.246 | 5.024 | 0.025 | 1.737 | 1.072 | 2.814 |
| DM | 0.254 | 0.149 | 2.914 | 0.088 | 1.289 | .963 | 1.725 |
| HR | 0.008 | 0.003 | 5.866 | 0.015 | 1.008 | 1.001 | 1.014 |
| Weight | -0.016 | 0.006 | 7.270 | 0.007 | 0.984 | 0.973 | 0.996 |
| Killip I | 11.939 | 0.008 | |||||
| Killip II | 0.295 | 0.201 | 2.153 | 0.142 | 1.343 | 0.906 | 1.992 |
| Killip III | 0.622 | 0.380 | 2.676 | 0.102 | 1.863 | 0.884 | 3.928 |
| Killip IV | 1.046 | 0.342 | 9.362 | 0.002 | 2.847 | 1.457 | 5.566 |
| Statin | 0.335 | 0.150 | 4.996 | 0.025 | 1.398 | 1.042 | 1.876 |
| CRA | 0.852 | 0.351 | 5.888 | 0.015 | 2.344 | 1.178 | 4.663 |
| Shock | 1.025 | 0.207 | 24.444 | 0.000 | 2.787 | 1.856 | 4.184 |
| ARF | 0.922 | 0.232 | 15.792 | 0.000 | 2.514 | 1.596 | 3.962 |
| ASA | -0.360 | 0.177 | 4.137 | 0.042 | 0.697 | 0.493 | 0.987 |
ASA: acetylsalicylic acid/aspirin; B: coefficient B; DM: diabetes mellitus; HR: heart rate; HR: hazard ratio; HF: heart failure; ARF: acute renal failure; CRA: cardiorespiratory arrest; CABG: Coronary artery bypass graft; SE: standard error. *Age, HR, and weight are represented as increments of one unit.
Table 5.
Cox model with in-hospital data and predictors of mortality in the total follow-up of patients with NSTEMI
| B | SE | Wald | p. | HR | IC 95% | ||
|---|---|---|---|---|---|---|---|
| Age | 0.044 | 0.008 | 28.213 | 0.000 | 1.045 | 1.028 | 1.062 |
| CABG surgery | 0.095 | 0.244 | 0.151 | 0.697 | 1.100 | 0.681 | 1.774 |
| HF | 0.436 | 0.371 | 1.382 | 0.240 | 1.546 | 0.748 | 3.198 |
| DM | 0.196 | 0.193 | 1.033 | 0.309 | 1.216 | 0.834 | 1.775 |
| HR | 0.011 | 0.004 | 7.410 | 0.006 | 1.011 | 1.003 | 1.020 |
| Weight | -0.008 | 0.008 | 1.029 | 0.310 | 0.992 | 0.977 | 1.007 |
| Killip I | 16.558 | 0.001 | |||||
| Killip II | 0.147 | 0.261 | 0.315 | 0.574 | 1.158 | 0.694 | 1.932 |
| Killip III | 1.265 | 0.477 | 7.041 | 0.008 | 3.544 | 1.392 | 9.023 |
| Killip IV | 1.608 | 0.487 | 10.890 | 0.001 | 4.993 | 1.921 | 12.974 |
| Statin | 0.176 | 0.197 | 0.797 | 0.372 | 1.192 | 0.811 | 1.753 |
| CRA | 0.033 | 0.729 | 0.002 | 0.964 | 1.033 | 0.247 | 4.316 |
| Shock | 0.694 | 0.336 | 4.268 | 0.039 | 2.001 | 1.036 | 3.864 |
| ARF | 0.634 | 0.278 | 5.177 | 0.023 | 1.884 | 1.092 | 3.252 |
| ASA | -0.545 | 0.259 | 4.427 | 0.035 | 0.580 | 0.349 | 0.963 |
ASA: acetylsalicylic acid/aspirin; B: coefficient B; DM: diabetes mellitus; HR: heart rate; HR: hazard ratio; HF: heart failure; ARF: acute renal failure; CRA: cardiorespiratory arrest; CABG: Coronary artery bypass graft; SE: standard error. *Age, HR, and weight are represented as increments of one unit.
Discussion
In this Brazilian cohort of patients hospitalized with AMI and in long-term clinical follow-up post-AMI, determination of the presence and severity of HF on admission using the Killip-Kimball classification was an independent prognostic factor for mortality, with similar and significant impact on both NSTEMI and STEMI patients. To date and to the best of our knowledge, this study introduces three important aspects: 1) the first validation of the HF severity index proposed by Killip-Kimball in a Brazilian AMI cohort, particularly in the era of reperfusion and modern antithrombotic therapy; 2) the first validation of this index in Brazilian NSTEMI patients; and 3) the first exploratory analysis of the prognostic impact of this classification in very late follow-up post-AMI.
We emphasize that in this study, the Killip classification was an important independent predictor of mortality, even after adjustment for important covariates such as clinical, laboratory, electrocardiographic, and angiographic characteristics related with the risk of mortality in patients with AMI, as well as of the occurrence of relevant complications independently associated with the risk of death, including cardiac arrest during hospitalization and acute renal failure9,10.
Moreover, this analysis highlights the clinical utility of physical examination as a simple tool (easy to apply and without any sophisticated technological requirements) to identify signs and symptoms of HF on admission. It provides relevant information on the early risk stratification of mortality in patients with NSTEMI, similar to that in patients with STEMI11-14. In association with age, the Killip-Kimball classification accounted for approximately 67% and 80% of the total prognostic value on admission for patients with STEMI and NSTEMI, respectively (data not shown). In all Cox proportional hazards models, the variables independently associated with the risk of mortality were consistently maintained at the end of the stepwise procedure, particularly age, emphasizing that the Killip classification is a robust predictor of mortality.
We detected a direct, significant, and independent association between the Killip classification and risk of death during late follow-up post-AMI. In fact, there was consistent risk stratification at 30-day, 5-year, and total follow-up time post-AMI. Khot et al4 evaluated patients with unstable angina and NSTEMI from the database of the randomized clinical trials GUSTO-IIb, PURSUIT, PARAGON-A, and PARAGON-B in order to determine the prognostic value of the Killip classification. Mortality was assessed at 30 days and at 6 months. They identified an independent association with total mortality during these time periods; however, they used only the variables on admission; they did not adjust for in-hospital treatments, and the data were derived from those included in randomized clinical trials. Our study, in contrast, has some important differences. We included patients recruited from daily clinical practice; they were not randomized; therefore, they had characteristics with higher severity, such as more comorbidities and older age, implying a higher representativeness and applicability to "real world" settings. In contrast to a previous study15, our Cox models were adjusted for the use of pharmacological therapies and in-hospital procedures, with noticeable impact on survival. This potential interaction was evaluated in the models, and despite the possible attenuation of the association with risk for the reason described earlier, the Killip classification significantly and independently remained associated with mortality.
The distribution pattern of the survival curves at 30-day and long-term follow-up, according to the Killip class, probably reflected the high intrinsic risk of acute coronary event, particularly in those who developed cardiogenic shock, mainly in the STEMI group, with distinct separation between the curves. In the NSTEMI group, Killip classes III and IV curves showed a similar pattern. We emphasize that the proportionately smaller numbers of patients with poor prognosis in these classes did not allow the determination of whether the behavior is similar or different from a visual perspective only. We also emphasize the pronounced decrease in survival in the first days after AMI for the highest Killip classes. Furthermore, it is notable that the differences in survival distributions at 30-day and long-term follow-up were statistically significant; this observation was similar for the two AMI groups. Moreover, as the cumulative number of deaths increases with long-term follow-up, the Kaplan-Meier survival curves reflect the distributions according to the risk inherent to the Killip class. Patients in Killip class I have an excellent prognosis, both in short- and long-term, whereas those in class III or IV have larger areas of necrosis, left ventricular remodeling and systolic dysfunction, and probably a greater extent of CAD. Thus, STEMI patients with higher HF severity classes survived the initial stage possibly because the AMI-related artery was treated using an artery reperfusion strategy; moreover, they may have been at a lower risk of new events due to CAD, mostly unilateral, or at a younger age. On the other hand, NSTEMI patients with more extensive CAD, probably older, and having survived the initial stage may have been more susceptible to new, recurrent thrombotic events, including AMI and ischemic cardiomyopathy; this may explain the increased risk of death in this group.
In terms of biological plausibility and emphasizing the negative impact on survival, the associations of the Killip-Kimball classification with increased risk of death were consistent with physical examination variables. These are representative of the hemodynamic status of patients on admission, i.e., SBP, DBP, and HR at rest, and clinical characteristics related to higher fragility and attenuated capacity of organs to respond to an acute myocardial ischemic event, i.e., older age, lower body weight, and comorbidities such as diabetes and prior CABG, denoting advanced coronary atherosclerosis. In fact, the Killip-Kimball classification maintained a significant association with the risk of death even after adjusting for these variables, with biological and statistical impact. Importantly, the results of this study identified the impact of these aspects on prognosis, both in NSTEMI and STEMI patients.
The mortality rates at 6 months in the study by Khot et al4 were as follows: Killip class I, 5%; II, 14.7%; and III/IV, 23%. We emphasize that these data refer only to the NSTEMI population, which was analyzed by the authors, and only for 6 months of follow-up. Comparing this data with our data (Killip class I, 17.7%; II, 27.3%; III, 30.4%; and IV, 48.8%) and also with the initial data from Killip-Kimball, we observed that the implementation of pharmacoinvasive and reperfusion therapies, available in the recent decades, according to the types of AMI notably contributed to the reduction in the number of deaths and severe HF post-AMI.
There were some limitations of this study. The accuracy, concordance, and inter/intra-observer variability for signs of HF detectable on physical examination on admission could not be determined for obvious temporal and practical reasons. Moreover, as the Killip-Kimball classification criteria were designed to be easily implemented and the datasheets of the patients were reviewed for consistency (even with some disagreement), the association with risk would have been reduced or nulled and the hypothesis would not have been confirmed, which was not the case. Another aspect is the non-comparison with other diagnostic tests for left ventricular dysfunction, such as transthoracic echocardiography, in order to determine left ventricular ejection fraction and measurement of the natriuretic peptide NT-pro-BNP. However, from the perspective of clinical applicability and generalization of the results, the use of these additional tests would result in additional costs and logistical difficulties to the objectives and hypotheses of this research. Other limitations, as in other observational studies, could include possible selection biases and not elucidating confounding factors, resulting in a non-ideal fit in the Cox proportional hazards models. However, systematic efforts were implemented to minimize these aspects, including standardized data collection of patients with AMI, inclusion of clinical information in a prospective, standardized manner and with blind evaluation of the outcome (mortality), as well as a follow-up of approximately 100% patients and adjustment of the Cox models for important covariates.
Conclusions
This study emphasizes the prognostic importance of determining the presence and severity of HF in patients with AMI and specifically validates its relevance and applicability in the setting of NSTEMI in a similar manner to STEMI. Moreover, in terms of scientific and clinical relevance, this study adds evidence to the available information on the Killip-Kimball classification in terms of prognostic value for mortality in very late follow-up post-AMI. The Killip-Kimball classification demonstrates a discriminatory capacity of the risk of total mortality, even after adjusting for clinical covariates that are relevant in the contemporary era.
Acknowledgments
Acknowledgments
This study received statistical support from João Ítalo Dias França, the Laboratory of Statistics and Epidemiology, IDPC.
Footnotes
Author contributions
Conception and design of the research: Oliveira GBF; Acquisition of data: Mello BHG, Oliveira GBF, Ramos RF, Lopes BBC, Barros CBS, Carvalho EO, Teixeira FBP, Arruda GDS, Revelo MSC, Piegas LS; Analysis and interpretation of the data and Critical revision of the manuscript for intellectual content: Oliveira GBF, Ramos RF, Piegas LS; Statistical analysis: Oliveira GBF, Arruda GDS, Revelo MSC; Writing of the manuscript: Mello BHG, Oliveira GBF, Lopes BBC, Barros CBS, Carvalho EO, Teixeira FBP, Arruda GDS, Revelo MSC.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any thesis or dissertation work.
References
- 1.Killip 3rd T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20(4):457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 2.Lee KL, Woodlief LH, Topol EJ, Weaver WD, Betriu A, Col J, et al. Predictors of 30-day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41,021 patients. GUSTO-I Investigators. Circulation. 1995;91(6):1659–1668. doi: 10.1161/01.cir.91.6.1659. [DOI] [PubMed] [Google Scholar]
- 3.DeGeare VS, Boura JA, Grines LL, O'Neill WW, Grines CL. Predictive value of the Killip classification in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Am J Cardiol. 2001;87(9):1035–1038. doi: 10.1016/s0002-9149(01)01457-6. [DOI] [PubMed] [Google Scholar]
- 4.Khot UN, Jia G, Moliterno DJ, Lincoff AM, Khot MB, Harrington RA, et al. Prognostic importance of physical examination for heart failure in non-ST-elevation acute coronary syndromes: the enduring value of Killip classification. JAMA. 2003;290(16):2174–2181. doi: 10.1001/jama.290.16.2174. [DOI] [PubMed] [Google Scholar]
- 5.Piegas LS, Feitosa G, Mattos LA, JC Nicolau, Rossi JM, Neto, Timerman A, et al. Sociedade Brasileira de Cardiologia Diretriz da Sociedade Brasileira de Cardiologia sobre Tratamento do Infarto Agudo do Miocárdio com Supradesnível do Segmento ST. Arq Bras Cardiol. 2009;93(6) supl. 2:e179–e264. [PubMed] [Google Scholar]
- 6.Nicolau JC, Timerman A, Piegas LS, Marin-Neto JA, Rassi A., Jr Guidelines for Unstable Angina and Non-ST-Segment Elevation Myocardial Infarction of the Brazilian Society of Cardiology (II Edition, 2007) Arq Bras Cardiol. 2007;89(4):e89–e131. doi: 10.1590/s0066-782x2007001600015. [DOI] [PubMed] [Google Scholar]
- 7.Haddad N. Metodologia de estudos em ciências da saúde. São Paulo: Roca; 2004. [Google Scholar]
- 8.Hennekens CH, Julie E. Epidemiology in medicine. Philadelphia: Lippincott Williams & Wilkins; 1987. [Google Scholar]
- 9.Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Global Registry of Acute Coronary Events Investigators Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 10.Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndromes: prospective multinational observational study (GRACE) BMJ. 2006;333(7578):1091–1094. doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mangione S, Nieman LZ. Cardiac auscultatory skills of internal medicine and family practice trainees: a comparison of diagnostic proficiency. JAMA. 1997;278(9):717–722. Erratum in JAMA. 1998;279(18):1444. [PubMed] [Google Scholar]
- 12.Tavel ME. Cardiac auscultation: a glorious past - but does it have a future? Circulation. 1996;93(6):1250–1253. doi: 10.1161/01.cir.93.6.1250. [DOI] [PubMed] [Google Scholar]
- 13.Schneiderman H. Cardiac auscultation and teaching rounds: how can auscultation be resuscitated? Am J Med. 2001;110(3):233–235. doi: 10.1016/s0002-9343(00)00736-1. [DOI] [PubMed] [Google Scholar]
- 14.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345(8):574–581. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 15.Fuster V. 50th anniversary historical article: myocardial Infarction and coronary care units. J Am Coll Cardiol. 1999;34(7):1851–1853. doi: 10.1016/s0735-1097(99)00496-9. [DOI] [PubMed] [Google Scholar]